Разбор слова «углерод»: для переноса, на слоги, по составу
Объяснение правил деление (разбивки) слова «углерод» на слоги для переноса.
Онлайн словарь Soosle.ru поможет: фонетический и морфологический разобрать слово «углерод» по составу, правильно делить на слоги по провилам русского языка, выделить части слова, поставить ударение, укажет значение, синонимы, антонимы и сочетаемость к слову «углерод».
Содержимое:
- 1 Слоги в слове «углерод» деление на слоги
- 2 Как перенести слово «углерод»
- 3 Морфологический разбор слова «углерод»
- 4 Разбор слова «углерод» по составу
- 5 Сходные по морфемному строению слова «углерод»
- 6 Синонимы слова «углерод»
- 7 Ударение в слове «углерод»
- 8 Фонетическая транскрипция слова «углерод»
- 9 Фонетический разбор слова «углерод» на буквы и звуки (Звуко-буквенный)
- 10 Предложения со словом «углерод»
- 11 Сочетаемость слова «углерод»
- 12 Значение слова «углерод»
- 13 Как правильно пишется слово «углерод»
- 14 Ассоциации к слову «углерод»
Слоги в слове «углерод» деление на слоги
Количество слогов: 3
По слогам: у-гле-род
По правилам школьной программы слово «углерод» можно поделить на слоги разными способами. Допускается вариативность, то есть все варианты правильные. Например, такой:
уг-ле-род
По программе института слоги выделяются на основе восходящей звучности:
у-гле-род
Ниже перечислены виды слогов и объяснено деление с учётом программы института и школ с углублённым изучением русского языка.
г примыкает к этому слогу, а не к предыдущему, так как не является сонорной (непарной звонкой согласной)
Как перенести слово «углерод»
уг—лерод
угле—род
Морфологический разбор слова «углерод»
Часть речи:
Имя существительное
Грамматика:
часть речи: имя существительное;
одушевлённость: неодушевлённое;
род: мужской;
число: единственное;
падеж: именительный, винительный;
отвечает на вопрос: (есть) Что?, (вижу/виню) Что?
Разбор слова «углерод» по составу
| углерод | корень |
| ø | нулевое окончание |
углерод
Сходные по морфемному строению слова «углерод»
Сходные по морфемному строению слова
Синонимы слова «углерод»
1. элемент
2. органоген
3. радиоуглерод
4. техуглерод
5. неметалл
Ударение в слове «углерод»
углеро́д — ударение падает на 3-й слог
Фонетическая транскрипция слова «углерод»
[угл’ир`от]
Фонетический разбор слова «углерод» на буквы и звуки (Звуко-буквенный)
| Буква | Звук | Характеристики звука | Цвет |
|---|---|---|---|
| у | [у] | гласный, безударный | у |
| г | [г] | согласный, звонкий парный, твёрдый, шумный | г |
| л | [л’] | согласный, звонкий непарный (сонорный), мягкий | л |
| е | [и] | гласный, безударный | е |
| р | [р] | согласный, звонкий непарный (сонорный), твёрдый | р |
| о | [`о] | гласный, ударный | о |
| д | [т] | согласный, глухой парный, твёрдый, шумный | д |
Число букв и звуков:
На основе сделанного разбора делаем вывод, что в слове 7 букв и 7 звуков.
Буквы: 3 гласных буквы, 4 согласных букв.
Звуки: 3 гласных звука, 4 согласных звука.
Предложения со словом «углерод»
Тогда у приёмника должно быть в распоряжении достаточно атомов углерода, достаточно атомов железа, достаточно атомов кальция и так далее — и всё наготове.
Источник: Дэйв Голдберг, Вселенная! Курс выживания среди черных дыр, временных парадоксов, квантовой неопределенности, 2010.
Смертельная доза 2, 5 мг окиси углерода на 1 л воздуха.
Источник: Илья Мельников, Грузовые автомобили. Охрана труда, 2013.
Название связано с тем, что первые из известных науке углеводов по существу были соединениями углерода и воды.
Источник: А. А. Синельникова, Еда, которая Вам действительно нужна, 2013.
Сочетаемость слова «углерод»
1. радиоактивный углерод
2. чистый углерод
3. лишний углерод
4. окись углерода
5. двуокись углерода
6. атомы углерода
7. состоять из углерода
8. содержать углерод
9. (полная таблица сочетаемости)
Значение слова «углерод»
УГЛЕРО́Д , -а, м. Химический элемент, важнейшая составная часть всех органических веществ в природе. (Малый академический словарь, МАС)
Как правильно пишется слово «углерод»
Правильно слово пишется: углеро́д
Нумерация букв в слове
Номера букв в слове «углерод» в прямом и обратном порядке:
- 7
у
1 - 6
г
2 - 5
л
3 - 4
е
4 - 3
р
5 - 2
о
6 - 1
д
7
Ассоциации к слову «углерод»
-
Азот
-
Водород
-
Атом
-
Кислород
-
Молекула
-
Примесь
-
Соединение
-
Кислота
-
Отравление
-
Выброс
-
Синтез
-
Концентрация
-
Электрон
-
Алмаз
-
Вещество
-
Газ
-
Выделение
-
Газа
-
Атмосфера
-
Сплав
-
Поглощение
-
Железа
-
Содержание
-
Элемент
-
Бактерия
-
Разложение
-
Температура
-
Спирт
-
Раствор
-
Охлаждение
-
Титан
-
Загрязнение
-
Железо
-
Уголь
-
Частица
-
Сера
-
Йод
-
Формула
-
Алмазов
-
Отложение
-
Очистки
-
Цикл
-
Ядро
-
Присоединение
-
Химик
-
Известняк
-
Переработка
-
Топливо
-
Химия
-
Избыток
-
Соотношение
-
Уран
-
Бор
-
Волокно
-
Медь
-
Конфигурация
-
Превращение
-
Фильтр
-
Решётка
-
Смесь
-
Реакция
-
Органический
-
Неорганический
-
Кристаллический
-
Радиоактивный
-
Молекулярный
-
Атмосферный
-
Химический
-
Производный
-
Растворимый
-
Газовый
-
Угольный
-
Кислородный
-
Горючий
-
Насыщенный
-
Избыточный
-
Рудый
-
Кубический
-
Стабильный
-
Растительный
-
Водяной
-
Жидкий
-
Удельный
-
Ископаемый
-
Твёрдый
-
Первичный
-
Разлагаться
-
Смесить
-
Взаимодействовать
-
Содержать
-
Соединяться
-
Растворить
-
Выделять
-
Восстанавливаться
-
Образоваться
-
Поглощать
-
Растворяться
-
Образовывать
-
Содержаться
-
Повышаться
1.
… стабильно… А кости превратятся в углерод. Все придет, так сказать, к первоначальному! Доктор поднял стек и …
Орлов Алекс. Охотники за головами
2.
… узнали, что алмазы суть кристаллы углерода, в эпоху, когда всему находят объяснение, когда полиция привлекла бы …
Оноре де Бальзак. Шагреневая кожа
3.
… я, кажется, нашел средство кристаллизовать углерод — вещество, из которого состоит алмаз… Дорогая жена, через несколько дней … твердое, но не металл, а углерод. Напротив, неорганическая природа, столь мало разнообразная, лишенная движения и чувства … алмаз, эта слеза кристаллизовавшегося чистого углерода, последней субстанцией, какую только можно создать? Старые алхимики, считавшие золото … началом, общим трем газам и углероду. Средство должно быть началом, общим электричеству отрицательному и электричеству положительному … Клаас совсем угорел от своего углерода и о детях уже не заботится; если я попрошу у …
Оноре де Бальзак. Поиски Абсолюта
4.
… Михаил Никифорович, произошла утечка четыреххлористого углерода, вечером Михаила Никифоровича стало рвать, температура пошла под сорок, «скорая … самое уважительное. Но утек четыреххлористый углерод. Не по вине Михаила Никифоровича утек. Однако дышал им Михаил … что при нем утек четыреххлористый углерод? Это ведь нехорошо… — Но я… — начала Любовь Николаевна. И не … и от них утек четыреххлористый углерод. Начальник смены Пигулин и не имел права допустить Михаила Никифоровича …
Орлов Владимир. Аптекарь
5.
… озер и резком повышении окиси углерода в атмосфере…» — Да мы в этих Штатах ни разу не …
Орлов Алекс. Тютюнин 1-2
6.
… стабильно… А кости превратятся в углерод. Все придет, так сказать, к первоначальному! Доктор поднял стек и …
Орлов Алекс. Тени войны 1-14
7.
… м. Бесцветный горючий газ, соединение углерода с водородом. || прил. ацетиленовый, -ая, -ое. АЦЕТОН, -а, м. Бесцветная … КАРБИД, -а, м. Химическое соединение углерода с металлами и нек-рыми неметаллами. || прил. карбидный, -ая, -ое … связям. Сухой лед — твердая двуокись углерода. || уменьш. ледок, -дка (-дку),м. || прил. ледяной, -ая, -ое и … без цвета и запаха, соединение углерода с водородом. || прил. метановый, -ая,-ое. МЕТАСТАЗ, -а, м. (спец … знач.) — группа из одного атома углерода и трех атомов водорода. || прил. метиловый, -ая, -ое. М. спирт …
Ожегов С., Шведова Н. Толковый словарь русского языка
8.
… телам: кислороду, азоту, водороду и углероду; {* Вот сие любопытное, доныне едва ли замеченное место у Платона … оно состоит из кислорода, водорода, углерода и, может быть, азота; какое же его действие, спросите у … невозможно отождествлять кислород, азот, водород, углерод с четырьмя стихиями древних мыслителей (огонь, воздух, вода, земля). 36 …
Одоевский В.Ф.. Русские ночи
9.
… готов к неудаче. Но нет, углерод сгустился не в рыхлый графит, а как надо: на ладони …
О’Санчес. Нечисть
10.
… выделяться пузырьки газа. Это диоксид углерода СО_2 (он же двуокись углерода, углекислый газ). Его добавляют в воду, чтобы она была вкуснее … пропускать через известковую воду диоксид углерода. Некоторое время спустя раствор опять станет прозрачным, потому что диоксид углерода вступает в реакцию с карбонатом кальция, превращая его в другую … использовать для опытов с диоксидом углерода. Следующий опыт с газами можно ставить только при хорошей вентиляции … диоксиде серы будет примесь диоксида углерода, но опыту это не помешает. ОКИСЛЕНИЕ-ВОССТАНОВЛЕНИЕ Опыт с диоксидом … к опыту с получением диоксида углерода (углекислого газа). Заполните этим газом две пробирки, причем в одну …
Ольгин Олег. Опыты без взрывов
11.
… треть мировых загрязнений атмосферы двуокисью углерода в результате промышленной деятельности, а если еще учесть деятельность американских …
Олег Платонов — Почему погибнет Америка
12.
… ближнего боя, гарроту из мономолевого углерода, аптечку химических средств для допроса с пристрастием. Он проклял свою … любого вида жизни, основанного на углероде. Ему не хотелось даже думать, на что похоже дно этой …
Олдридж Рэй. Освободитель 1-3
13.
… представляет собой цепочку из атомов углерода, к каждому из которых, как лапки сороконожки, прикреплены два атома … твердыми. Цепочка из шести атомов углерода может быть замкнута в кольцо — это циклогексан, у него нет … лапки» — по два на атом углерода. В природном газе обычно больше всего метана. Это «цепочка», состоящая … из одного звена — 1 атом углерода и 4 водорода. Метан легче воздуха. А вот уже этан (2 атома углерода и 6 водорода) тяжелее. Смесь метана и этана горит у …
Паршев Андрей. Почему Америка наступает
14.
… по-особому сложившиеся молекулы чистого углерода. Прошли еще миллионы лет, и магма где-то прорвала земную …
Пальман В.. Кратер Эршота
15.
… они и разлагают углекислоту на углерод и кислород и умеют использовать незначительное количество азота. Может быть … белки состоят не только из углерода, кислорода и азота, в их состав входят еще водород, сера … животными добываемыми ими кислородом и углеродом. Если же это симбиоз, то он предполагает взаимную пользу. Но …
Палей Абрам. В простор планетный
16.
… наслаивалась ледяная кора — смесь двуокиси углерода и аммиака. Местами лед достигал толщины полутора метров. Сколь бы … интересного. Кристаллы аммиака, воды, двуокиси углерода — вот и все, ничего более. Движущиеся атмосферные газы наэлектризовали их … пахучей травки. Воздух, обогащенный двуокисью углерода, слегка светился. Серебристые своды купола оранжереи источали ультрафиолетовые лучи. На …
Пеев Димитр. Рассказы
17.
… Не горит. Видно, здесь двуокись углерода… Дышать стало совсем тяжело. Джеррард почувствовал легкое головокружение — это был …
Педлер Кит, Дэвис Дж. Мутант-59
18.
… раствор дибензилфосфорил-хлорида в четыреххлористом углероде объемом 30 мл, который был свежеприготовлен из 8,3 г … жидкого клея на основе четыреххлористого углерода, в пластиковую сумку. Опустите голову в сумку и вдохните. Эффект …
Пауэлл Уильям. Поваренная книга анархиста
19.
… вносит метил, радиоактивно меченный по углероду. Выделенная и очищенная из таких бактерий тРНК метилирована (радиоактивно) собственными … часовой стадии, были помечены радиоактивным углеродом (14С). Продукты двух реакций присоединения одной и той же аминокислоты … независимо радиоактивности трития и 14С-углерода. Условия хроматографического разделения очевидно тождественны для обоих тРНК, взятых из …
Остерман Лев. Течению наперекор
20.
… болтаний неважный, с низким насыщением углеродом, и потребители чугуна позволяли себе задерживать платежи. Якобы появлялся Черный …
Орлов Владимир. Шеврикука, или Любовь к привидению
21.
… болтаний неважный, с низким насыщением углеродом, и потребители чугуна позволяли себе задерживать платежи. Якобы появлялся Черный …
Орлов Владимир. Останкинские истории 1-2
22.
… кислота. При гипервентиляции уровень диоксида углерода падает, и их кровь становится щелочной. «Респераторный алкалозис,» — говорит он …
Паланик Чак. Уцелевший
23.
… она состоит из водорода и углерода, но там есть несколько атомов титана. Если это именно то …
Пайпер Генри. Пушистики 1-2
24.
… некоей совокупности простых стабильных соединений углерода, водорода, кислорода и азота бороться миллионы лет, чтобы организоваться вкакого … превратят его в простую совокупность углерода, кислорода и водорода и азота, кальция, фосфора и небольшое количество … в качестве основного орудия, является углерод. Вс„ живое содержит углерод, и вс„ же исследование свойств атома углерода свидетельствует о том, что за исключением чрезвычайной твердости одной из … только одно физическое свойство делает углерод уникальным: он самый легкий и самый активный атом из группы … собственной группы. А группа, содержащая углерод находится на полпути между металлами и неметаллами, так что иногда углерод соединяется с металлами, иногда с неметаллами, а иногда не соединяется …
Пёрсинг М. Р.. Лайла. Исследование морали
25.
… лишенная кислорода и богатая двуокисью углерода, прилила к его лицу. Губы и ногти тоже посинели. Он …
О’Рейли Виктор. Правила охоты
26.
… говоря совсем уже точно, молекула углерода с четверкой ее валентностей — эта главная химическая компонента мозга, машинально …
Набоков Владимир. Пнин
27.
… вымерзала углекислота. Смерзшиеся частицы двуокиси углерода медленно опускались вниз и, достигая более низких и теплых слоев … ответ знаю заранее. Из двуокиси углерода свободный кислород восстанавливается не полностью, а с потерей примерно десяти …
На суше и на море. Сборник приключений и фантастики.
28.
… кислота carbon noun 1) chem. углерод 2) electr. уголь, угольный электрод 3) хими- чески чистый уголь 4 … carbonaceous adj. chem. углистый; содержащий углерод carbonari it. noun collect.; hist. карбонарии carbonate noun 1) chem … угольная кислота carbonic oxide окись углерода carboniferous adj. 1) угленосный 2) каменноугольный (о периоде, системе, формации … v. chem. карбюрировать, соединять с углеродом carburet(t)or noun tech. карбюратор carburetter noun tech. карбюратор … radiocarbon noun chem. радиоактивный изотоп углерода; радиоуглерод radiogenic adj. phys. радиогенный; радиоактивного происхождени radiogram noun 1 …
Мюллер, ред.. Англо-русский словарь
29.
… Бэдлонга? Но графит — это чистый углерод. Так же, как бриллиант. Он любовно положил оба предмета на …
Найт Даймонд. Рассказы
30.
… состав входят азот и двуокись углерода, но оно совершенно безвредное. — Дойль, убирайся отсюда! — воскликнул Карл. — Нет …
Най Джоди Линн. Прикладная мифология
Если вы нашли ошибку, пожалуйста, выделите фрагмент текста и нажмите Ctrl+Enter.

|
||
|
||
| Names | ||
|---|---|---|
Other names
|
||
| Identifiers | ||
|
CAS Number |
|
|
|
3D model (JSmol) |
|
|
| 3DMet |
|
|
|
Beilstein Reference |
1900390 | |
| ChEBI |
|
|
| ChEMBL |
|
|
| ChemSpider |
|
|
| ECHA InfoCard | 100.004.271 |
|
| EC Number |
|
|
| E number | E290 (preservatives) | |
|
Gmelin Reference |
989 | |
| KEGG |
|
|
| MeSH | Carbon+dioxide | |
|
PubChem CID |
|
|
| RTECS number |
|
|
| UNII |
|
|
| UN number | 1013 (gas), 1845 (solid) | |
|
CompTox Dashboard (EPA) |
|
|
|
InChI
|
||
|
SMILES
|
||
| Properties | ||
|
Chemical formula |
CO2 | |
| Molar mass | 44.009 g·mol−1 | |
| Appearance | Colorless gas | |
| Odor |
|
|
| Density |
|
|
| Critical point (T, P) | 304.128(15) K[2] (30.978(15) °C), 7.3773(30) MPa[2] (72.808(30) atm) | |
|
Sublimation |
194.6855(30) K (−78.4645(30) °C) at 1 atm (0.101325 MPa) | |
|
Solubility in water |
1.45 g/L at 25 °C (77 °F), 100 kPa (0.99 atm) | |
| Vapor pressure | 5.7292(30) MPa, 56.54(30) atm (20 °C (293.15 K)) | |
| Acidity (pKa) | 6.35, 10.33 | |
|
Magnetic susceptibility (χ) |
−20.5·10−6 cm3/mol | |
| Thermal conductivity | 0.01662 W·m−1·K−1 (300 K (27 °C; 80 °F))[3] | |
|
Refractive index (nD) |
1.00045 | |
| Viscosity |
|
|
|
Dipole moment |
0 D | |
| Structure | ||
|
Crystal structure |
Trigonal | |
|
Molecular shape |
Linear | |
| Thermochemistry | ||
|
Heat capacity (C) |
37.135 J/K·mol | |
|
Std molar |
214 J·mol−1·K−1 | |
|
Std enthalpy of |
−393.5 kJ·mol−1 | |
| Pharmacology | ||
|
ATC code |
V03AN02 (WHO) | |
| Hazards | ||
| NFPA 704 (fire diamond) |
[7][8] 2 0 0 SA |
|
| Lethal dose or concentration (LD, LC): | ||
|
LCLo (lowest published) |
90,000 ppm (human, 5 min)[6] | |
| NIOSH (US health exposure limits): | ||
|
PEL (Permissible) |
TWA 5000 ppm (9000 mg/m3)[5] | |
|
REL (Recommended) |
TWA 5000 ppm (9000 mg/m3), ST 30,000 ppm (54,000 mg/m3)[5] | |
|
IDLH (Immediate danger) |
40,000 ppm[5] | |
| Safety data sheet (SDS) | Sigma-Aldrich | |
| Related compounds | ||
|
Other anions |
|
|
|
Other cations |
|
|
|
Related carbon oxides |
|
|
|
Related compounds |
|
|
| Supplementary data page | ||
| Carbon dioxide (data page) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Carbon dioxide (chemical formula CO2) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth’s atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm.[9][10] Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.[11] Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. When carbon dioxide dissolves in water, it forms carbonate and mainly bicarbonate (HCO−
3), which causes ocean acidification as atmospheric CO2 levels increase.[12]
As the source of available carbon in the carbon cycle, atmospheric CO2 is the primary carbon source for life on Earth. Its concentration in Earth’s pre-industrial atmosphere since late in the Precambrian has been regulated by organisms and geological phenomena. Plants, algae and cyanobacteria use energy from sunlight to synthesize carbohydrates from carbon dioxide and water in a process called photosynthesis, which produces oxygen as a waste product.[13] In turn, oxygen is consumed and CO2 is released as waste by all aerobic organisms when they metabolize organic compounds to produce energy by respiration.[14] CO2 is released from organic materials when they decay or combust, such as in forest fires. Since plants require CO2 for photosynthesis, and humans and animals depend on plants for food, CO2 is necessary for the survival of life on earth.
Carbon dioxide is 53% more dense than dry air, but is long lived and thoroughly mixes in the atmosphere. About half of excess CO2 emissions to the atmosphere are absorbed by land and ocean carbon sinks.[15] These sinks can become saturated and are volatile, as decay and wildfires result in the CO2 being released back into the atmosphere.[16] CO2 is eventually sequestered (stored for the long term) in rocks and organic deposits like coal, petroleum and natural gas. Sequestered CO2 is released into the atmosphere through burning fossil fuels or naturally by volcanoes, hot springs, geysers, and when carbonate rocks dissolve in water or react with acids.
CO2 is a versatile industrial material, used, for example, as an inert gas in welding and fire extinguishers, as a pressurizing gas in air guns and oil recovery, and as a supercritical fluid solvent in decaffeination of coffee and supercritical drying.[17] It is a byproduct of fermentation of sugars in bread, beer and wine making, and is added to carbonated beverages like seltzer and beer for effervescence. It has a sharp and acidic odor and generates the taste of soda water in the mouth,[18] but at normally encountered concentrations it is odorless.[1]
Chemical and physical properties
Structure, bonding and molecular vibrations
The symmetry of a carbon dioxide molecule is linear and centrosymmetric at its equilibrium geometry. The length of the carbon-oxygen bond in carbon dioxide is 116.3 pm, noticeably shorter than the roughly 140-pm length of a typical single C–O bond, and shorter than most other C–O multiply-bonded functional groups such as carbonyls.[19] Since it is centrosymmetric, the molecule has no electric dipole moment.
Stretching and bending oscillations of the CO2 carbon dioxide molecule. Upper left: symmetric stretching. Upper right: antisymmetric stretching. Lower line: degenerate pair of bending modes.
As a linear triatomic molecule, CO2 has four vibrational modes as shown in the diagram. In the symmetric and the antisymmetric stretching modes, the atoms move along the axis of the molecule. There are two bending modes, which are degenerate, meaning that they have the same frequency and same energy, because of the symmetry of the molecule. When a molecule touches a surface or touches another molecule, the two bending modes can differ in frequency because the interaction is different for the two modes. Some of the vibrational modes are observed in the infrared (IR) spectrum: the antisymmetric stretching mode at wavenumber 2349 cm−1 (wavelength 4.25 μm) and the degenerate pair of bending modes at 667 cm−1 (wavelength 15 μm). The symmetric stretching mode does not create an electric dipole so is not observed in IR spectroscopy, but it is detected in by Raman spectroscopy at 1388 cm−1 (wavelength 7.2 μm).[20]
In the gas phase, carbon dioxide molecules undergo significant vibrational motions and do not keep a fixed structure. However, in a Coulomb explosion imaging experiment, an instantaneous image of the molecular structure can be deduced. Such an experiment[21] has been performed for carbon dioxide.
The result of this experiment, and the conclusion of theoretical calculations[22] based on an ab initio potential energy surface of the molecule, is that none of the
molecules in the gas phase are ever exactly linear. This counter-intuitive result is trivially due to
the fact that the nuclear motion volume element vanishes for linear geometries.[22]
This is so for all molecules (except diatomics!).
In aqueous solution
Carbon dioxide is soluble in water, in which it reversibly forms H2CO3 (carbonic acid), which is a weak acid since its ionization in water is incomplete.
The hydration equilibrium constant of carbonic acid is, at 25 °C:
Hence, the majority of the carbon dioxide is not converted into carbonic acid, but remains as CO2 molecules, not affecting the pH.
The relative concentrations of CO2, H2CO3, and the deprotonated forms HCO−3 (bicarbonate) and CO2−3(carbonate) depend on the pH. As shown in a Bjerrum plot, in neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater. In very alkaline water (pH > 10.4), the predominant (>50%) form is carbonate. The oceans, being mildly alkaline with typical pH = 8.2–8.5, contain about 120 mg of bicarbonate per liter.
Being diprotic, carbonic acid has two acid dissociation constants, the first one for the dissociation into the bicarbonate (also called hydrogen carbonate) ion (HCO−3):
- Ka1 = 2.5×10−4 mol/L; pKa1 = 3.6 at 25 °C.[19]
This is the true first acid dissociation constant, defined as
where the denominator includes only covalently bound H2CO3 and does not include hydrated CO2(aq). The much smaller and often-quoted value near 4.16×10−7 is an apparent value calculated on the (incorrect) assumption that all dissolved CO2 is present as carbonic acid, so that
Since most of the dissolved CO2remains as CO2 molecules, Ka1(apparent) has a much larger denominator and a much smaller value than the true Ka1.[23]
The bicarbonate ion is an amphoteric species that can act as an acid or as a base, depending on pH of the solution. At high pH, it dissociates significantly into the carbonate ion (CO2−3):
- Ka2 = 4.69×10−11 mol/L; pKa2 = 10.329
In organisms carbonic acid production is catalysed by the enzyme, carbonic anhydrase.
Chemical reactions of CO2
CO2 is a potent electrophile having an electrophilic reactivity that is comparable to benzaldehyde or strong α,β-unsaturated carbonyl compounds. However, unlike electrophiles of similar reactivity, the reactions of nucleophiles with CO2 are thermodynamically less favored and are often found to be highly reversible.[24] Only very strong nucleophiles, like the carbanions provided by Grignard reagents and organolithium compounds react with CO2 to give carboxylates:
- where M = Li or Mg Br and R = alkyl or aryl.
In metal carbon dioxide complexes, CO2 serves as a ligand, which can facilitate the conversion of CO2 to other chemicals.[25]
The reduction of CO2 to CO is ordinarily a difficult and slow reaction:
Photoautotrophs (i.e. plants and cyanobacteria) use the energy contained in sunlight to photosynthesize simple sugars from CO2 absorbed from the air and water:
The redox potential for this reaction near pH 7 is about −0.53 V versus the standard hydrogen electrode. The nickel-containing enzyme carbon monoxide dehydrogenase catalyses this process.[26]
Physical properties
Pellets of «dry ice», a common form of solid carbon dioxide
Carbon dioxide is colorless. At low concentrations the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor.[1] At standard temperature and pressure, the density of carbon dioxide is around 1.98 kg/m3, about 1.53 times that of air.[27]
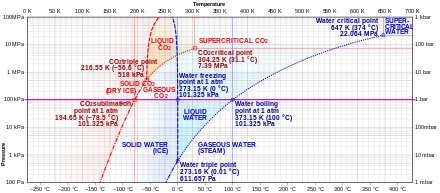
Carbon dioxide has no liquid state at pressures below 0.51795(10) MPa[2] (5.11177(99) atm). At a pressure of 1 atm (0.101325 MPa), the gas deposits directly to a solid at temperatures below 194.6855(30) K[2] (−78.4645(30) °C) and the solid sublimes directly to a gas above this temperature. In its solid state, carbon dioxide is commonly called dry ice.
Pressure–temperature phase diagram of carbon dioxide. Note that it is a log-lin chart.
Liquid carbon dioxide forms only at pressures above 0.51795(10) MPa[2] (5.11177(99) atm); the triple point of carbon dioxide is 216.592(3) K[2] (−56.558(3) °C) at 0.51795(10) MPa[2] (5.11177(99) atm) (see phase diagram). The critical point is 304.128(15) K[2] (30.978(15) °C) at 7.3773(30) MPa[2] (72.808(30) atm). Another form of solid carbon dioxide observed at high pressure is an amorphous glass-like solid.[28] This form of glass, called carbonia, is produced by supercooling heated CO2 at extreme pressures (40–48 GPa, or about 400,000 atmospheres) in a diamond anvil. This discovery confirmed the theory that carbon dioxide could exist in a glass state similar to other members of its elemental family, like silicon dioxide (silica glass) and germanium dioxide. Unlike silica and germania glasses, however, carbonia glass is not stable at normal pressures and reverts to gas when pressure is released.
At temperatures and pressures above the critical point, carbon dioxide behaves as a supercritical fluid known as supercritical carbon dioxide.
Table of thermal and physical properties of saturated liquid carbon dioxide:[29][30]
| Temperature (°C) | Density (kg/m^3) | Specific heat (kJ/kg K) | Kinematic viscosity (m^2/s) | Conductivity (W/m K) | Thermal diffusivity (m^2/s) | Prandtl Number | Bulk modulus (K^-1) |
| -50 | 1156.34 | 1.84 | 1.19E-07 | 0.0855 | 4.02E-08 | 2.96 | — |
| -40 | 1117.77 | 1.88 | 1.18E-07 | 0.1011 | 4.81E-08 | 2.46 | — |
| -30 | 1076.76 | 1.97 | 1.17E-07 | 0.1116 | 5.27E-08 | 2.22 | — |
| -20 | 1032.39 | 2.05 | 1.15E-07 | 0.1151 | 5.45E-08 | 2.12 | — |
| -10 | 983.38 | 2.18 | 1.13E-07 | 0.1099 | 5.13E-08 | 2.2 | — |
| 0 | 926.99 | 2.47 | 1.08E-07 | 0.1045 | 4.58E-08 | 2.38 | — |
| 10 | 860.03 | 3.14 | 1.01E-07 | 0.0971 | 3.61E-08 | 2.8 | — |
| 20 | 772.57 | 5 | 9.10E-08 | 0.0872 | 2.22E-08 | 4.1 | 1.40E-02 |
| 30 | 597.81 | 36.4 | 8.00E-08 | 0.0703 | 0.279E-08 | 28.7 | — |
Table of thermal and physical properties of carbon dioxide (CO2) at atmospheric pressure:[29][30]
| Temperature (K) | Density (kg/m^3) | Specific heat (kJ/kg °C) | Dynamic viscosity (kg/m s) | Kinematic viscosity (m^2/s) | Thermal conductivity (W/m °C) | Thermal diffusivity (m^2/s) | Prandtl Number |
| 220 | 2.4733 | 0.783 | 1.11E-05 | 4.49E-06 | 0.010805 | 5.92E-06 | 0.818 |
| 250 | 2.1657 | 0.804 | 1.26E-05 | 5.81E-06 | 0.012884 | 7.40E-06 | 0.793 |
| 300 | 1.7973 | 0.871 | 1.50E-05 | 8.32E-06 | 0.016572 | 1.06E-05 | 0.77 |
| 350 | 1.5362 | 0.9 | 1.72E-05 | 1.12E-05 | 0.02047 | 1.48E-05 | 0.755 |
| 400 | 1.3424 | 0.942 | 1.93E-05 | 1.44E-05 | 0.02461 | 1.95E-05 | 0.738 |
| 450 | 1.1918 | 0.98 | 2.13E-05 | 1.79E-05 | 0.02897 | 2.48E-05 | 0.721 |
| 500 | 1.0732 | 1.013 | 2.33E-05 | 2.17E-05 | 0.03352 | 3.08E-05 | 0.702 |
| 550 | 0.9739 | 1.047 | 2.51E-05 | 2.57E-05 | 0.03821 | 3.75E-05 | 0.685 |
| 600 | 0.8938 | 1.076 | 2.68E-05 | 3.00E-05 | 0.04311 | 4.48E-05 | 0.668 |
| 650 | 0.8143 | 1.1 | 2.88E-05 | 3.54E-05 | 0.0445 | 4.97E-05 | 0.712 |
| 700 | 0.7564 | 1.13E+00 | 3.05E-05 | 4.03E-05 | 0.0481 | 5.63E-05 | 0.717 |
| 750 | 0.7057 | 1.15 | 3.21E-05 | 4.55E-05 | 0.0517 | 6.37E-05 | 0.714 |
| 800 | 0.6614 | 1.17E+00 | 3.37E-05 | 5.10E-05 | 0.0551 | 7.12E-05 | 0.716 |
Biological role
Carbon dioxide is an end product of cellular respiration in organisms that obtain energy by breaking down sugars, fats and amino acids with oxygen as part of their metabolism. This includes all plants, algae and animals and aerobic fungi and bacteria. In vertebrates, the carbon dioxide travels in the blood from the body’s tissues to the skin (e.g., amphibians) or the gills (e.g., fish), from where it dissolves in the water, or to the lungs from where it is exhaled. During active photosynthesis, plants can absorb more carbon dioxide from the atmosphere than they release in respiration.
Photosynthesis and carbon fixation
Carbon fixation is a biochemical process by which atmospheric carbon dioxide is incorporated by plants, algae and (cyanobacteria) into energy-rich organic molecules such as glucose, thus creating their own food by photosynthesis. Photosynthesis uses carbon dioxide and water to produce sugars from which other organic compounds can be constructed, and oxygen is produced as a by-product.
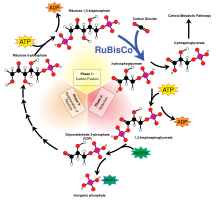
Ribulose-1,5-bisphosphate carboxylase oxygenase, commonly abbreviated to RuBisCO, is the enzyme involved in the first major step of carbon fixation, the production of two molecules of 3-phosphoglycerate from CO2 and ribulose bisphosphate, as shown in the diagram at left.
RuBisCO is thought to be the single most abundant protein on Earth.[31]
Phototrophs use the products of their photosynthesis as internal food sources and as raw material for the biosynthesis of more complex organic molecules, such as polysaccharides, nucleic acids and proteins. These are used for their own growth, and also as the basis of the food chains and webs that feed other organisms, including animals such as ourselves. Some important phototrophs, the coccolithophores synthesise hard calcium carbonate scales.[32] A globally significant species of coccolithophore is Emiliania huxleyi whose calcite scales have formed the basis of many sedimentary rocks such as limestone, where what was previously atmospheric carbon can remain fixed for geological timescales.
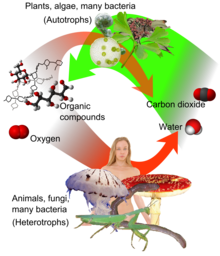
Overview of photosynthesis and respiration. Carbon dioxide (at right), together with water, form oxygen and organic compounds (at left) by photosynthesis, which can be respired to water and (CO2).
Plants can grow as much as 50 percent faster in concentrations of 1,000 ppm CO2 when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients.[33] Elevated CO2 levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated CO2 in FACE experiments.[34][35]
Increased atmospheric CO2 concentrations result in fewer stomata developing on plants[36] which leads to reduced water usage and increased water-use efficiency.[37] Studies using FACE have shown that CO2 enrichment leads to decreased concentrations of micronutrients in crop plants.[38] This may have knock-on effects on other parts of ecosystems as herbivores will need to eat more food to gain the same amount of protein.[39]
The concentration of secondary metabolites such as phenylpropanoids and flavonoids can also be altered in plants exposed to high concentrations of CO2.[40][41]
Plants also emit CO2 during respiration, and so the majority of plants and algae, which use C3 photosynthesis, are only net absorbers during the day. Though a growing forest will absorb many tons of CO2 each year, a mature forest will produce as much CO2 from respiration and decomposition of dead specimens (e.g., fallen branches) as is used in photosynthesis in growing plants.[42] Contrary to the long-standing view that they are carbon neutral, mature forests can continue to accumulate carbon[43] and remain valuable carbon sinks, helping to maintain the carbon balance of Earth’s atmosphere. Additionally, and crucially to life on earth, photosynthesis by phytoplankton consumes dissolved CO2 in the upper ocean and thereby promotes the absorption of CO2 from the atmosphere.[44]
Toxicity
Symptoms of carbon dioxide toxicity, by increasing volume percent in air[45]
Carbon dioxide content in fresh air (averaged between sea-level and 10 kPa level, i.e., about 30 km (19 mi) altitude) varies between 0.036% (360 ppm) and 0.041% (412 ppm), depending on the location.[46][clarification needed]
CO2 is an asphyxiant gas and not classified as toxic or harmful in accordance with Globally Harmonized System of Classification and Labelling of Chemicals standards of United Nations Economic Commission for Europe by using the OECD Guidelines for the Testing of Chemicals. In concentrations up to 1% (10,000 ppm), it will make some people feel drowsy and give the lungs a stuffy feeling.[45] Concentrations of 7% to 10% (70,000 to 100,000 ppm) may cause suffocation, even in the presence of sufficient oxygen, manifesting as dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes to an hour.[47] The physiological effects of acute carbon dioxide exposure are grouped together under the term hypercapnia, a subset of asphyxiation.
Because it is heavier than air, in locations where the gas seeps from the ground (due to sub-surface volcanic or geothermal activity) in relatively high concentrations, without the dispersing effects of wind, it can collect in sheltered/pocketed locations below average ground level, causing animals located therein to be suffocated. Carrion feeders attracted to the carcasses are then also killed. Children have been killed in the same way near the city of Goma by CO2 emissions from the nearby volcano Mount Nyiragongo.[48] The Swahili term for this phenomenon is mazuku.
Adaptation to increased concentrations of CO2 occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification (acidosis). Several studies suggested that 2.0 percent inspired concentrations could be used for closed air spaces (e.g. a submarine) since the adaptation is physiological and reversible, as deterioration in performance or in normal physical activity does not happen at this level of exposure for five days.[49][50] Yet, other studies show a decrease in cognitive function even at much lower levels.[51][52] Also, with ongoing respiratory acidosis, adaptation or compensatory mechanisms will be unable to reverse such condition.
Below 1%
There are few studies of the health effects of long-term continuous CO2 exposure on humans and animals at levels below 1%. Occupational CO2 exposure limits have been set in the United States at 0.5% (5000 ppm) for an eight-hour period.[53] At this CO2 concentration, International Space Station crew experienced headaches, lethargy, mental slowness, emotional irritation, and sleep disruption.[54] Studies in animals at 0.5% CO2 have demonstrated kidney calcification and bone loss after eight weeks of exposure.[55] A study of humans exposed in 2.5 hour sessions demonstrated significant negative effects on cognitive abilities at concentrations as low as 0.1% (1000 ppm) CO2 likely due to CO2 induced increases in cerebral blood flow.[51] Another study observed a decline in basic activity level and information usage at 1000 ppm, when compared to 500 ppm.[52] However a review of the literature found that most studies on the phenomenon of carbon dioxide induced cognitive impairment to have a small effect on high-level decision making and most of the studies were confounded by inadequate study designs, environmental comfort, uncertainties in exposure doses and differing cognitive assessments used.[56] Similarly a study on the effects of the concentration of CO2 in motorcycle helmets has been criticized for having dubious methodology in not noting the self-reports of motorcycle riders and taking measurements using mannequins. Further when normal motorcycle conditions were achieved (such as highway or city speeds) or the visor was raised the concentration of CO2 declined to safe levels (0.2%).[57][58]
Ventilation
Poor ventilation is one of the main causes of excessive CO2 concentrations in closed spaces, leading to poor indoor air quality. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that CO2 concentration has stabilized) are sometimes used to estimate ventilation rates per person.[citation needed] Higher CO2 concentrations are associated with occupant health, comfort and performance degradation.[59][60] ASHRAE Standard 62.1–2007 ventilation rates may result in indoor concentrations up to 2,100 ppm above ambient outdoor conditions. Thus if the outdoor concentration is 400 ppm, indoor concentrations may reach 2,500 ppm with ventilation rates that meet this industry consensus standard. Concentrations in poorly ventilated spaces can be found even higher than this (range of 3,000 or 4,000 ppm).
Miners, who are particularly vulnerable to gas exposure due to insufficient ventilation, referred to mixtures of carbon dioxide and nitrogen as «blackdamp», «choke damp» or «stythe». Before more effective technologies were developed, miners would frequently monitor for dangerous levels of blackdamp and other gases in mine shafts by bringing a caged canary with them as they worked. The canary is more sensitive to asphyxiant gases than humans, and as it became unconscious would stop singing and fall off its perch. The Davy lamp could also detect high levels of blackdamp (which sinks, and collects near the floor) by burning less brightly, while methane, another suffocating gas and explosion risk, would make the lamp burn more brightly.
In February 2020, three people died from suffocation at a party in Moscow when dry ice (frozen CO2) was added to a swimming pool to cool it down.[61] A similar accident occurred in 2018 when a woman died from CO2 fumes emanating from the large amount of dry ice she was transporting in her car.[62]
Outdoor areas with elevated concentrations
Local concentrations of carbon dioxide can reach high values near strong sources, especially those that are isolated by surrounding terrain. At the Bossoleto hot spring near Rapolano Terme in Tuscany, Italy, situated in a bowl-shaped depression about 100 m (330 ft) in diameter, concentrations of CO2 rise to above 75% overnight, sufficient to kill insects and small animals. After sunrise the gas is dispersed by convection.[63] High concentrations of CO2 produced by disturbance of deep lake water saturated with CO2 are thought to have caused 37 fatalities at Lake Monoun, Cameroon in 1984 and 1700 casualties at Lake Nyos, Cameroon in 1986.[64]
Human physiology
Content
| Blood compartment | (kPa) | (mm Hg) | |
|---|---|---|---|
| Venous blood carbon dioxide | 5.5–6.8 | 41–51[65] | 41–51[65] |
| Alveolar pulmonary gas pressures |
4.8 | 36 | 36 |
| Arterial blood carbon dioxide | 4.7–6.0 | 35–45[65] | 35–45[65] |
The body produces approximately 2.3 pounds (1.0 kg) of carbon dioxide per day per person,[66] containing 0.63 pounds (290 g) of carbon. In humans, this carbon dioxide is carried through the venous system and is breathed out through the lungs, resulting in lower concentrations in the arteries. The carbon dioxide content of the blood is often given as the partial pressure, which is the pressure which carbon dioxide would have had if it alone occupied the volume.[67] In humans, the blood carbon dioxide contents is shown in the adjacent table.
Transport in the blood
CO2 is carried in blood in three different ways. (Exact percentages vary between arterial and venous blood).
- Majority (about 70% to 80%) is converted to bicarbonate ions HCO−3 by the enzyme carbonic anhydrase in the red blood cells,[68] by the reaction CO2 + H2O → H2CO3 → H+ + HCO−3.
- 5–10% is dissolved in blood plasma[68]
- 5–10% is bound to hemoglobin as carbamino compounds[68]
Hemoglobin, the main oxygen-carrying molecule in red blood cells, carries both oxygen and carbon dioxide. However, the CO2 bound to hemoglobin does not bind to the same site as oxygen. Instead, it combines with the N-terminal groups on the four globin chains. However, because of allosteric effects on the hemoglobin molecule, the binding of CO2 decreases the amount of oxygen that is bound for a given partial pressure of oxygen. This is known as the Haldane Effect, and is important in the transport of carbon dioxide from the tissues to the lungs. Conversely, a rise in the partial pressure of CO2 or a lower pH will cause offloading of oxygen from hemoglobin, which is known as the Bohr effect.
Regulation of respiration
Carbon dioxide is one of the mediators of local autoregulation of blood supply. If its concentration is high, the capillaries expand to allow a greater blood flow to that tissue.[69]
Bicarbonate ions are crucial for regulating blood pH. A person’s breathing rate influences the level of CO2 in their blood. Breathing that is too slow or shallow causes respiratory acidosis, while breathing that is too rapid leads to hyperventilation, which can cause respiratory alkalosis.[70]
Although the body requires oxygen for metabolism, low oxygen levels normally do not stimulate breathing. Rather, breathing is stimulated by higher carbon dioxide levels. As a result, breathing low-pressure air or a gas mixture with no oxygen at all (such as pure nitrogen) can lead to loss of consciousness without ever experiencing air hunger. This is especially perilous for high-altitude fighter pilots. It is also why flight attendants instruct passengers, in case of loss of cabin pressure, to apply the oxygen mask to themselves first before helping others; otherwise, one risks losing consciousness.[68]
The respiratory centers try to maintain an arterial CO2 pressure of 40 mm Hg. With intentional hyperventilation, the CO2 content of arterial blood may be lowered to 10–20 mm Hg (the oxygen content of the blood is little affected), and the respiratory drive is diminished. This is why one can hold one’s breath longer after hyperventilating than without hyperventilating. This carries the risk that unconsciousness may result before the need to breathe becomes overwhelming, which is why hyperventilation is particularly dangerous before free diving.[71]
Concentrations and role in the environment
Atmosphere
Atmospheric CO2 concentrations measured at Mauna Loa Observatory from 1958 to 2022 (also called the Keeling Curve). Carbon dioxide concentrations have varied widely over the Earth’s 4.54 billion year history. However, in 2013 the daily mean concentration of CO2 in the atmosphere surpassed 400 parts per million (ppmv)[72] — this level has never been reached since the mid-Pliocene, 2 to 4 million years ago.[73]
Carbon dioxide in Earth’s atmosphere is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of several greenhouse gases in Earth’s atmosphere that are contributing to climate change due to increasing emissions of greenhouse gases from human activities. The current global average concentration of CO2 in the atmosphere is 421 ppm as of May 2022.[74] This is an increase of 50% since the start of the Industrial Revolution, up from 280 ppm during the 10,000 years prior to the mid-18th century.[75][74][76] The increase is due to human activity.[77] Burning fossil fuels is the main cause of these increased CO2 concentrations and also the main cause of climate change.[78] Other large anthropogenic sources include cement production, deforestation, and biomass burning.
While transparent to visible light, carbon dioxide is a greenhouse gas, absorbing and emitting infrared radiation at its two infrared-active vibrational frequencies. CO2 absorbs and emits infrared radiation at wavelengths of 4.26 μm (2,347 cm−1) (asymmetric stretching vibrational mode) and 14.99 μm (667 cm−1) (bending vibrational mode). It plays a significant role in influencing Earth’s surface temperature through the greenhouse effect.[79] Light emission from the Earth’s surface is most intense in the infrared region between 200 and 2500 cm−1,[80] as opposed to light emission from the much hotter Sun which is most intense in the visible region. Absorption of infrared light at the vibrational frequencies of atmospheric CO2 traps energy near the surface, warming the surface and the lower atmosphere. Less energy reaches the upper atmosphere, which is therefore cooler because of this absorption.[81]
Increases in atmospheric concentrations of CO2 and other long-lived greenhouse gases such as methane, nitrous oxide and ozone increase the absorption and emission of infrared radiation by the atmosphere, causing the observed rise in average global temperature and ocean acidification. Another direct effect is the CO2 fertilization effect. These changes cause a range of indirect effects of climate change on the physical environment, ecosystems and human societies. Carbon dioxide exerts a larger overall warming influence than all of the other greenhouse gases combined.[76] It has an atmospheric lifetime that increases with the cumulative amount of fossil carbon extracted and burned, due to the imbalance that this activity has imposed on Earth’s fast carbon cycle.[82] This means that some fraction (a projected 20-35%) of the fossil carbon transferred thus far will persist in the atmosphere as elevated CO2 levels for many thousands of years after these carbon transfer activities begin to subside.[83][84][85] The carbon cycle is a biogeochemical cycle in which carbon is exchanged between the Earth’s oceans, soil, rocks and the biosphere. Plants and other photoautotrophs use solar energy to produce carbohydrate from atmospheric carbon dioxide and water by photosynthesis. Almost all other organisms depend on carbohydrate derived from photosynthesis as their primary source of energy and carbon compounds.
The present atmospheric concentration of CO2 is the highest for 14 million years.[86] Concentrations of CO2 in the atmosphere were as high as 4,000 ppm during the Cambrian period about 500 million years ago, when the concentration was 20 times greater than today, and as low as 180 ppm during the Quaternary glaciation of the last two million years.[75] Reconstructed temperature records for the last 420 million years indicate that atmospheric CO2 concentrations peaked at ~2,000 ppm during the Devonian (~400 Ma) period, and again in the Triassic (220–200 Ma) period and was four times current levels during the Jurassic period (201-145 Ma).[87][88]
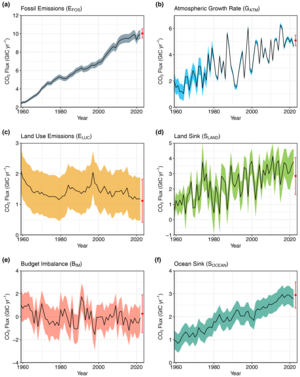
Annual CO2 flows from anthropogenic sources (left) into Earth’s atmosphere, land, and ocean sinks (right) since the 1960s. Units in equivalent gigatonnes carbon per year.[89]
Oceans
Ocean acidification
Carbon dioxide dissolves in the ocean to form carbonic acid (H2CO3), bicarbonate (HCO3−) and carbonate (CO32−). There is about fifty times as much carbon dioxide dissolved in the oceans as exists in the atmosphere. The oceans act as an enormous carbon sink, and have taken up about a third of CO2 emitted by human activity.[90]
Pterapod shell dissolved in seawater adjusted to an ocean chemistry projected for the year 2100
Changes in ocean chemistry can have extensive direct and indirect effects on organisms and their habitats. One of the most important repercussions of increasing ocean acidity relates to the production of shells out of calcium carbonate (CaCO
3).[92] This process is called calcification and is important to the biology and survival of a wide range of marine organisms. Calcification involves the precipitation of dissolved ions into solid CaCO
3 structures, structures for many marine organisms, such as coccolithophores, foraminifera, crustaceans, mollusks, etc. After they are formed, these CaCO
3 structures are vulnerable to dissolution unless the surrounding seawater contains saturating concentrations of carbonate ions (CO32−).
Given the current pH of the ocean (around 8.14), of the extra carbon dioxide added into the ocean, very little remains as dissolved carbon dioxide. The majority dissociates into additional bicarbonate and free hydrogen ions. The increase in hydrogen is larger than the increase in bicarbonate,[93] creating an imbalance in the reaction HCO3− ⇌ CO32− + H+. To maintain chemical equilibrium, some of the carbonate ions already in the ocean combine with some of the hydrogen ions to make further bicarbonate. Thus the ocean’s concentration of carbonate ions is reduced, removing an essential building block for marine organisms to build shells, or calcify: Ca2+ + CO32− ⇌ CaCO3.
Hydrothermal vents
Carbon dioxide is also introduced into the oceans through hydrothermal vents. The Champagne hydrothermal vent, found at the Northwest Eifuku volcano in the Mariana Trench, produces almost pure liquid carbon dioxide, one of only two known sites in the world as of 2004, the other being in the Okinawa Trough.[94] The finding of a submarine lake of liquid carbon dioxide in the Okinawa Trough was reported in 2006.[95]
Production
Biological processes
Carbon dioxide is a by-product of the fermentation of sugar in the brewing of beer, whisky and other alcoholic beverages and in the production of bioethanol. Yeast metabolizes sugar to produce CO2 and ethanol, also known as alcohol, as follows:
All aerobic organisms produce CO2 when they oxidize carbohydrates, fatty acids, and proteins. The large number of reactions involved are exceedingly complex and not described easily. Refer to (cellular respiration, anaerobic respiration and photosynthesis). The equation for the respiration of glucose and other monosaccharides is:
Anaerobic organisms decompose organic material producing methane and carbon dioxide together with traces of other compounds.[96] Regardless of the type of organic material, the production of gases follows well defined kinetic pattern. Carbon dioxide comprises about 40–45% of the gas that emanates from decomposition in landfills (termed «landfill gas»). Most of the remaining 50–55% is methane.[97]
Industrial processes
Carbon dioxide can be obtained by distillation from air, but the method is inefficient. Industrially, carbon dioxide is predominantly an unrecovered waste product, produced by several methods which may be practiced at various scales.[98]
Combustion
The combustion of all carbon-based fuels, such as methane (natural gas), petroleum distillates (gasoline, diesel, kerosene, propane), coal, wood and generic organic matter produces carbon dioxide and, except in the case of pure carbon, water. As an example, the chemical reaction between methane and oxygen:
Iron is reduced from its oxides with coke in a blast furnace, producing pig iron and carbon dioxide:[99]
By-product from hydrogen production
Carbon dioxide is a byproduct of the industrial production of hydrogen by steam reforming and the water gas shift reaction in ammonia production. These processes begin with the reaction of water and natural gas (mainly methane).[100] This is a major source of food-grade carbon dioxide for use in carbonation of beer and soft drinks, and is also used for stunning animals such as poultry. In the summer of 2018 a shortage of carbon dioxide for these purposes arose in Europe due to the temporary shut-down of several ammonia plants for maintenance.[101]
Thermal decomposition of limestone
It is produced by thermal decomposition of limestone, CaCO3 by heating (calcining) at about 850 °C (1,560 °F), in the manufacture of quicklime (calcium oxide, CaO), a compound that has many industrial uses:
Acids liberate CO2 from most metal carbonates. Consequently, it may be obtained directly from natural carbon dioxide springs, where it is produced by the action of acidified water on limestone or dolomite. The reaction between hydrochloric acid and calcium carbonate (limestone or chalk) is shown below:
The carbonic acid (H2CO3) then decomposes to water and CO2:
Such reactions are accompanied by foaming or bubbling, or both, as the gas is released. They have widespread uses in industry because they can be used to neutralize waste acid streams.
Commercial uses
Carbon dioxide is used by the food industry, the oil industry, and the chemical industry.[98]
The compound has varied commercial uses but one of its greatest uses as a chemical is in the production of carbonated beverages; it provides the sparkle in carbonated beverages such as soda water, beer and sparkling wine.
Precursor to chemicals
|
This section needs expansion. You can help by adding to it. (July 2014) |
In the chemical industry, carbon dioxide is mainly consumed as an ingredient in the production of urea, with a smaller fraction being used to produce methanol and a range of other products.[102] Some carboxylic acid derivatives such as sodium salicylate are prepared using CO2 by the Kolbe–Schmitt reaction.[103]
In addition to conventional processes using CO2 for chemical production, electrochemical methods are also being explored at a research level. In particular, the use of renewable energy for production of fuels from CO2 (such as methanol) is attractive as this could result in fuels that could be easily transported and used within conventional combustion technologies but have no net CO2 emissions.[104]
Agriculture
Plants require carbon dioxide to conduct photosynthesis. The atmospheres of greenhouses may (if of large size, must) be enriched with additional CO2 to sustain and increase the rate of plant growth.[105][106] At very high concentrations (100 times atmospheric concentration, or greater), carbon dioxide can be toxic to animal life, so raising the concentration to 10,000 ppm (1%) or higher for several hours will eliminate pests such as whiteflies and spider mites in a greenhouse.[107]
Foods
Carbon dioxide bubbles in a soft drink
Carbon dioxide is a food additive used as a propellant and acidity regulator in the food industry. It is approved for usage in the EU[108] (listed as E number E290), US[109] and Australia and New Zealand[110] (listed by its INS number 290).
A candy called Pop Rocks is pressurized with carbon dioxide gas[111] at about 4,000 kPa (40 bar; 580 psi). When placed in the mouth, it dissolves (just like other hard candy) and releases the gas bubbles with an audible pop.
Leavening agents cause dough to rise by producing carbon dioxide.[112] Baker’s yeast produces carbon dioxide by fermentation of sugars within the dough, while chemical leaveners such as baking powder and baking soda release carbon dioxide when heated or if exposed to acids.
Beverages
Carbon dioxide is used to produce carbonated soft drinks and soda water. Traditionally, the carbonation of beer and sparkling wine came about through natural fermentation, but many manufacturers carbonate these drinks with carbon dioxide recovered from the fermentation process. In the case of bottled and kegged beer, the most common method used is carbonation with recycled carbon dioxide. With the exception of British real ale, draught beer is usually transferred from kegs in a cold room or cellar to dispensing taps on the bar using pressurized carbon dioxide, sometimes mixed with nitrogen.
The taste of soda water (and related taste sensations in other carbonated beverages) is an effect of the dissolved carbon dioxide rather than the bursting bubbles of the gas. Carbonic anhydrase 4 converts to carbonic acid leading to a sour taste, and also the dissolved carbon dioxide induces a somatosensory response.[113]
Winemaking
Dry ice used to preserve grapes after harvest
Carbon dioxide in the form of dry ice is often used during the cold soak phase in winemaking to cool clusters of grapes quickly after picking to help prevent spontaneous fermentation by wild yeast. The main advantage of using dry ice over water ice is that it cools the grapes without adding any additional water that might decrease the sugar concentration in the grape must, and thus the alcohol concentration in the finished wine. Carbon dioxide is also used to create a hypoxic environment for carbonic maceration, the process used to produce Beaujolais wine.
Carbon dioxide is sometimes used to top up wine bottles or other storage vessels such as barrels to prevent oxidation, though it has the problem that it can dissolve into the wine, making a previously still wine slightly fizzy. For this reason, other gases such as nitrogen or argon are preferred for this process by professional wine makers.
Stunning animals
Carbon dioxide is often used to «stun» animals before slaughter.[114] «Stunning» may be a misnomer, as the animals are not knocked out immediately and may suffer distress.[115][116]
Inert gas
Carbon dioxide is one of the most commonly used compressed gases for pneumatic (pressurized gas) systems in portable pressure tools. Carbon dioxide is also used as an atmosphere for welding, although in the welding arc, it reacts to oxidize most metals. Use in the automotive industry is common despite significant evidence that welds made in carbon dioxide are more brittle than those made in more inert atmospheres.[citation needed] When used for MIG welding, CO2 use is sometimes referred to as MAG welding, for Metal Active Gas, as CO2 can react at these high temperatures. It tends to produce a hotter puddle than truly inert atmospheres, improving the flow characteristics. Although, this may be due to atmospheric reactions occurring at the puddle site. This is usually the opposite of the desired effect when welding, as it tends to embrittle the site, but may not be a problem for general mild steel welding, where ultimate ductility is not a major concern.
Carbon dioxide is used in many consumer products that require pressurized gas because it is inexpensive and nonflammable, and because it undergoes a phase transition from gas to liquid at room temperature at an attainable pressure of approximately 60 bar (870 psi; 59 atm), allowing far more carbon dioxide to fit in a given container than otherwise would. Life jackets often contain canisters of pressured carbon dioxide for quick inflation. Aluminium capsules of CO2 are also sold as supplies of compressed gas for air guns, paintball markers/guns, inflating bicycle tires, and for making carbonated water. High concentrations of carbon dioxide can also be used to kill pests. Liquid carbon dioxide is used in supercritical drying of some food products and technological materials, in the preparation of specimens for scanning electron microscopy[117] and in the decaffeination of coffee beans.
Fire extinguisher
Use of a CO2 fire extinguisher
Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for electrical fires, contain liquid carbon dioxide under pressure. Carbon dioxide extinguishers work well on small flammable liquid and electrical fires, but not on ordinary combustible fires, because they do not cool the burning substances significantly, and when the carbon dioxide disperses, they can catch fire upon exposure to atmospheric oxygen. They are mainly used in server rooms.[118]
Carbon dioxide has also been widely used as an extinguishing agent in fixed fire-protection systems for local application of specific hazards and total flooding of a protected space.[119] International Maritime Organization standards recognize carbon-dioxide systems for fire protection of ship holds and engine rooms. Carbon-dioxide-based fire-protection systems have been linked to several deaths, because it can cause suffocation in sufficiently high concentrations. A review of CO2 systems identified 51 incidents between 1975 and the date of the report (2000), causing 72 deaths and 145 injuries.[120]
Supercritical CO2 as solvent
Liquid carbon dioxide is a good solvent for many lipophilic organic compounds and is used to remove caffeine from coffee.[17] Carbon dioxide has attracted attention in the pharmaceutical and other chemical processing industries as a less toxic alternative to more traditional solvents such as organochlorides. It is also used by some dry cleaners for this reason. It is used in the preparation of some aerogels because of the properties of supercritical carbon dioxide.
Medical and pharmacological uses
In medicine, up to 5% carbon dioxide (130 times atmospheric concentration) is added to oxygen for stimulation of breathing after apnea and to stabilize the O2/CO2 balance in blood.
Carbon dioxide can be mixed with up to 50% oxygen, forming an inhalable gas; this is known as Carbogen and has a variety of medical and research uses.
Another medical use are the mofette, dry spas that use carbon dioxide from post-volcanic discharge for therapeutic purposes.
Energy
Supercritical CO2 is used as the working fluid in the Allam power cycle engine.
Fossil fuel recovery
Carbon dioxide is used in enhanced oil recovery where it is injected into or adjacent to producing oil wells, usually under supercritical conditions, when it becomes miscible with the oil. This approach can increase original oil recovery by reducing residual oil saturation by 7–23% additional to primary extraction.[121] It acts as both a pressurizing agent and, when dissolved into the underground crude oil, significantly reduces its viscosity, and changing surface chemistry enabling the oil to flow more rapidly through the reservoir to the removal well.[122] In mature oil fields, extensive pipe networks are used to carry the carbon dioxide to the injection points.
In enhanced coal bed methane recovery, carbon dioxide would be pumped into the coal seam to displace methane, as opposed to current methods which primarily rely on the removal of water (to reduce pressure) to make the coal seam release its trapped methane.[123]
Bio transformation into fuel
It has been proposed that CO2 from power generation be bubbled into ponds to stimulate growth of algae that could then be converted into biodiesel fuel.[124] A strain of the cyanobacterium Synechococcus elongatus has been genetically engineered to produce the fuels isobutyraldehyde and isobutanol from CO2 using photosynthesis.[125]
Researchers have developed a process called electrolysis, using enzymes isolated from bacteria to power the chemical reactions which convert CO2 into fuels.[126][127][128]
Refrigerant
Comparison of the pressure–temperature phase diagrams of carbon dioxide (red) and water (blue) as a log-lin chart with phase transitions points at 1 atmosphere
Liquid and solid carbon dioxide are important refrigerants, especially in the food industry, where they are employed during the transportation and storage of ice cream and other frozen foods. Solid carbon dioxide is called «dry ice» and is used for small shipments where refrigeration equipment is not practical. Solid carbon dioxide is always below −78.5 °C (−109.3 °F) at regular atmospheric pressure, regardless of the air temperature.
Liquid carbon dioxide (industry nomenclature R744 or R-744) was used as a refrigerant prior to the use[citation needed] of dichlorodifluoromethane (R12, a chlorofluorocarbon (CFC) compound). CO2 might enjoy a renaissance because one of the main substitutes to CFCs, 1,1,1,2-tetrafluoroethane (R134a, a hydrofluorocarbon (HFC) compound) contributes to climate change more than CO2 does. CO2 physical properties are highly favorable for cooling, refrigeration, and heating purposes, having a high volumetric cooling capacity. Due to the need to operate at pressures of up to 130 bars (1,900 psi; 13,000 kPa), CO2 systems require highly mechanically resistant reservoirs and components that have already been developed for mass production in many sectors. In automobile air conditioning, in more than 90% of all driving conditions for latitudes higher than 50°, CO2 (R744) operates more efficiently than systems using HFCs (e.g., R134a). Its environmental advantages (GWP of 1, non-ozone depleting, non-toxic, non-flammable) could make it the future working fluid to replace current HFCs in cars, supermarkets, and heat pump water heaters, among others. Coca-Cola has fielded CO2-based beverage coolers and the U.S. Army is interested in CO2 refrigeration and heating technology.[129][130]
Minor uses
Carbon dioxide is the lasing medium in a carbon-dioxide laser, which is one of the earliest type of lasers.
Carbon dioxide can be used as a means of controlling the pH of swimming pools,[131] by continuously adding gas to the water, thus keeping the pH from rising. Among the advantages of this is the avoidance of handling (more hazardous) acids. Similarly, it is also used in the maintaining reef aquaria, where it is commonly used in calcium reactors to temporarily lower the pH of water being passed over calcium carbonate in order to allow the calcium carbonate to dissolve into the water more freely, where it is used by some corals to build their skeleton.
Used as the primary coolant in the British advanced gas-cooled reactor for nuclear power generation.
Carbon dioxide induction is commonly used for the euthanasia of laboratory research animals. Methods to administer CO2 include placing animals directly into a closed, prefilled chamber containing CO2, or exposure to a gradually increasing concentration of CO2. The American Veterinary Medical Association’s 2020 guidelines for carbon dioxide induction state that a displacement rate of 30–70% of the chamber or cage volume per minute is optimal for the humane euthanasia of small rodents.[132]: 5, 31 Percentages of CO2 vary for different species, based on identified optimal percentages to minimize distress.[132]: 22
Carbon dioxide is also used in several related cleaning and surface-preparation techniques.
History of discovery
Carbon dioxide was the first gas to be described as a discrete substance. In about 1640,[133] the Flemish chemist Jan Baptist van Helmont observed that when he burned charcoal in a closed vessel, the mass of the resulting ash was much less than that of the original charcoal. His interpretation was that the rest of the charcoal had been transmuted into an invisible substance he termed a «gas» or «wild spirit» (spiritus sylvestris).[134]
The properties of carbon dioxide were further studied in the 1750s by the Scottish physician Joseph Black. He found that limestone (calcium carbonate) could be heated or treated with acids to yield a gas he called «fixed air». He observed that the fixed air was denser than air and supported neither flame nor animal life. Black also found that when bubbled through limewater (a saturated aqueous solution of calcium hydroxide), it would precipitate calcium carbonate. He used this phenomenon to illustrate that carbon dioxide is produced by animal respiration and microbial fermentation. In 1772, English chemist Joseph Priestley published a paper entitled Impregnating Water with Fixed Air in which he described a process of dripping sulfuric acid (or oil of vitriol as Priestley knew it) on chalk in order to produce carbon dioxide, and forcing the gas to dissolve by agitating a bowl of water in contact with the gas.[135]
Carbon dioxide was first liquefied (at elevated pressures) in 1823 by Humphry Davy and Michael Faraday.[136] The earliest description of solid carbon dioxide (dry ice) was given by the French inventor Adrien-Jean-Pierre Thilorier, who in 1835 opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by the rapid evaporation of the liquid yielded a «snow» of solid CO2.[137][138]
See also
- Arterial blood gas
- Bosch reaction
- Carbon dioxide removal – Removal of atmospheric carbon dioxide (from the atmosphere)
- List of least carbon efficient power stations
- List of countries by carbon dioxide emissions
- Meromictic lake – Permanently stratified lake with layers of water that do not intermix
- Gilbert Plass – Canadian physicist (early work on CO2 and climate change)
- Sabatier reaction – Methanation process of carbon dioxide with hydrogen
- NASA’s Orbiting Carbon Observatory 2
- Greenhouse Gases Observing Satellite – Earth observation satellite
- Soil gas
References
- ^ a b c «Carbon Dioxide» (PDF). Air Products. Archived from the original (PDF) on 29 July 2020. Retrieved 28 April 2017.
- ^ a b c d e f g h i Span R, Wagner W (1 November 1996). «A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple‐Point Temperature to 1100 K at Pressures up to 800 MPa». Journal of Physical and Chemical Reference Data. 25 (6): 1519. Bibcode:1996JPCRD..25.1509S. doi:10.1063/1.555991.
- ^ Touloukian YS, Liley PE, Saxena SC (1970). «Thermophysical properties of matter — the TPRC data series». Thermal Conductivity — Nonmetallic Liquids and Gases. Data book. 3.
- ^ Schäfer M, Richter M, Span R (2015). «Measurements of the viscosity of carbon dioxide at temperatures from (253.15 to 473.15) K with pressures up to 1.2 MPa». The Journal of Chemical Thermodynamics. 89: 7–15. doi:10.1016/j.jct.2015.04.015.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0103». National Institute for Occupational Safety and Health (NIOSH).
- ^ «Carbon dioxide». Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ «Safety Data Sheet – Carbon Dioxide Gas – version 0.03 11/11» (PDF). AirGas.com. 12 February 2018. Archived (PDF) from the original on 4 August 2018. Retrieved 4 August 2018.
- ^ «Carbon dioxide, refrigerated liquid» (PDF). Praxair. p. 9. Archived from the original (PDF) on 29 July 2018. Retrieved 26 July 2018.
- ^ Eggleton T (2013). A Short Introduction to Climate Change. Cambridge University Press. p. 52. ISBN 9781107618763. Archived from the original on 23 July 2021. Retrieved 9 November 2020.
- ^ «Carbon dioxide now more than 50% higher than pre-industrial levels | National Oceanic and Atmospheric Administration». www.noaa.gov. Retrieved 14 June 2022.
- ^ IPCC (2022) Summary for policy makers in Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
- ^ Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean. Washington, DC: National Academies Press. 22 April 2010. pp. 23–24. doi:10.17226/12904. ISBN 978-0-309-15359-1. Archived from the original on 5 February 2016. Retrieved 29 February 2016.
- ^ Kaufman DG, Franz CM (1996). Biosphere 2000: protecting our global environment. Kendall/Hunt Pub. Co. ISBN 978-0-7872-0460-0.
- ^ «Food Factories». www.legacyproject.org. Archived from the original on 12 August 2017. Retrieved 10 October 2011.
- ^ IPCC (2021). «Summary for Policymakers» (PDF). Climate Change 2021: The Physical Science Basis. p. 20. Archived (PDF) from the original on 10 October 2022.
- ^ Myles, Allen (September 2020). «The Oxford Principles for Net Zero Aligned Carbon Offsetting» (PDF). Archived (PDF) from the original on 2 October 2020. Retrieved 10 December 2021.
- ^ a b Tsotsas E, Mujumdar AS (2011). Modern drying technology. Vol. 3: Product quality and formulation. John Wiley & Sons. ISBN 978-3-527-31558-1. Archived from the original on 21 March 2020. Retrieved 3 December 2019.
- ^ Spritzler F (3 November 2019). «Carbonated (Sparkling) Water: Good or Bad?». healthline.com. Archived from the original on 10 May 2020.
- ^ a b Greenwood NN, Earnshaw A (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 305–314. ISBN 978-0-08-037941-8.
- ^ Atkins P, de Paula J (2006). Physical Chemistry (8th ed.). W.H. Freeman. pp. 461, 464. ISBN 978-0-7167-8759-4.
- ^ Siegmann B, Werner U, Lutz HO, Mann R (2002). «Complete Coulomb fragmentation of CO2 in collisions with 5.9 MeV u−1 Xe18+ and Xe43+«. J Phys B Atom Mol Opt Phys. 35 (17): 3755. Bibcode:2002JPhB…35.3755S. doi:10.1088/0953-4075/35/17/311. S2CID 250782825.
- ^ a b
Jensen P, Spanner M, Bunker PR (2020). «The CO2 molecule is never linear−». J Mol Struct. 1212: 128087. Bibcode:2020JMoSt121228087J. doi:10.1016/j.molstruc.2020.128087. hdl:2142/107329. S2CID 216318907. - ^ Jolly WL (1984). Modern Inorganic Chemistry. McGraw-Hill. p. 196. ISBN 978-0-07-032760-3.
- ^ Li Z, Mayer RJ, Ofial AR, Mayr H (May 2020). «From Carbodiimides to Carbon Dioxide: Quantification of the Electrophilic Reactivities of Heteroallenes». Journal of the American Chemical Society. 142 (18): 8383–8402. doi:10.1021/jacs.0c01960. PMID 32338511. S2CID 216557447.
- ^ Aresta M, ed. (2010). Carbon Dioxide as a Chemical Feedstock. Weinheim: Wiley-VCH. ISBN 978-3-527-32475-0.
- ^ Finn C, Schnittger S, Yellowlees LJ, Love JB (February 2012). «Molecular approaches to the electrochemical reduction of carbon dioxide» (PDF). Chemical Communications. 48 (10): 1392–1399. doi:10.1039/c1cc15393e. hdl:20.500.11820/b530915d-451c-493c-8251-da2ea2f50912. PMID 22116300. S2CID 14356014. Archived (PDF) from the original on 19 April 2021. Retrieved 6 December 2019.
- ^ «Gases – Densities». Engineering Toolbox. Archived from the original on 2 March 2006. Retrieved 21 November 2020.
- ^ Santoro M, Gorelli FA, Bini R, Ruocco G, Scandolo S, Crichton WA (June 2006). «Amorphous silica-like carbon dioxide». Nature. 441 (7095): 857–860. Bibcode:2006Natur.441..857S. doi:10.1038/nature04879. PMID 16778885. S2CID 4363092.
- ^ a b Holman, Jack P. (2002). Heat Transfer (9th ed.). New York, NY: McGraw-Hill Companies, Inc. pp. 600–606. ISBN 9780072406559.
- ^ a b Incropera 1 Dewitt 2 Bergman 3 Lavigne 4, Frank P. 1 David P. 2 Theodore L. 3 Adrienne S. 4 (2007). Fundamentals of Heat and Mass Transfer (6th ed.). Hoboken, NJ: John Wiley and Sons, Inc. pp. 941–950. ISBN 9780471457282.
- ^ Dhingra A, Portis AR, Daniell H (April 2004). «Enhanced translation of a chloroplast-expressed RbcS gene restores small subunit levels and photosynthesis in nuclear RbcS antisense plants». Proceedings of the National Academy of Sciences of the United States of America. 101 (16): 6315–6320. Bibcode:2004PNAS..101.6315D. doi:10.1073/pnas.0400981101. PMC 395966. PMID 15067115.
(Rubisco) is the most prevalent enzyme on this planet, accounting for 30–50% of total soluble protein in the chloroplast
- ^ Falkowski P, Knoll AH (1 January 2007). Evolution of primary producers in the sea. Elsevier, Academic Press. ISBN 978-0-12-370518-1. OCLC 845654016.
- ^ Blom TJ, Straver WA, Ingratta FJ, Khosla S, Brown W (December 2002). «Carbon Dioxide In Greenhouses». Archived from the original on 29 April 2019. Retrieved 12 June 2007.
- ^ Ainsworth EA (2008). «Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration» (PDF). Global Change Biology. 14 (7): 1642–1650. Bibcode:2008GCBio..14.1642A. doi:10.1111/j.1365-2486.2008.01594.x. S2CID 19200429. Archived from the original (PDF) on 19 July 2011.
- ^ Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR (June 2006). «Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations» (PDF). Science. 312 (5782): 1918–1921. Bibcode:2006Sci…312.1918L. CiteSeerX 10.1.1.542.5784. doi:10.1126/science.1114722. PMID 16809532. S2CID 2232629. Archived (PDF) from the original on 20 October 2016. Retrieved 27 October 2017.
- ^ Woodward F, Kelly C (1995). «The influence of CO2 concentration on stomatal density». New Phytologist. 131 (3): 311–327. doi:10.1111/j.1469-8137.1995.tb03067.x.
- ^ Drake BG, Gonzalez-Meler MA, Long SP (June 1997). «MORE EFFICIENT PLANTS: A Consequence of Rising Atmospheric CO2?». Annual Review of Plant Physiology and Plant Molecular Biology. 48 (1): 609–639. doi:10.1146/annurev.arplant.48.1.609. PMID 15012276. S2CID 33415877.
- ^ Loladze I (2002). «Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry?». Trends in Ecology & Evolution. 17 (10): 457–461. doi:10.1016/S0169-5347(02)02587-9. S2CID 16074723.
- ^ Coviella CE, Trumble JT (1999). «Effects of Elevated Atmospheric Carbon Dioxide on Insect-Plant Interactions». Conservation Biology. 13 (4): 700–712. doi:10.1046/j.1523-1739.1999.98267.x. JSTOR 2641685. S2CID 52262618.
- ^ Davey MP, Harmens H, Ashenden TW, Edwards R, Baxter R (2007). «Species-specific effects of elevated CO2 on resource allocation in Plantago maritima and Armeria maritima«. Biochemical Systematics and Ecology. 35 (3): 121–129. doi:10.1016/j.bse.2006.09.004.
- ^ Davey MP, Bryant DN, Cummins I, Ashenden TW, Gates P, Baxter R, Edwards R (August 2004). «Effects of elevated CO2 on the vasculature and phenolic secondary metabolism of Plantago maritima». Phytochemistry. 65 (15): 2197–2204. doi:10.1016/j.phytochem.2004.06.016. PMID 15587703.
- ^ «Global Environment Division Greenhouse Gas Assessment Handbook – A Practical Guidance Document for the Assessment of Project-level Greenhouse Gas Emissions». World Bank. Archived from the original on 3 June 2016. Retrieved 10 November 2007.
- ^ Luyssaert S, Schulze ED, Börner A, Knohl A, Hessenmöller D, Law BE, et al. (September 2008). «Old-growth forests as global carbon sinks» (PDF). Nature. 455 (7210): 213–215. Bibcode:2008Natur.455..213L. doi:10.1038/nature07276. PMID 18784722. S2CID 4424430.
- ^ Falkowski P, Scholes RJ, Boyle E, Canadell J, Canfield D, Elser J, et al. (October 2000). «The global carbon cycle: a test of our knowledge of earth as a system». Science. 290 (5490): 291–296. Bibcode:2000Sci…290..291F. doi:10.1126/science.290.5490.291. PMID 11030643. S2CID 1779934.
- ^ a b Friedman D. «Toxicity of Carbon Dioxide Gas Exposure, CO2 Poisoning Symptoms, Carbon Dioxide Exposure Limits, and Links to Toxic Gas Testing Procedures». InspectAPedia. Archived from the original on 28 September 2009.
- ^ «CarbonTracker CT2011_oi (Graphical map of CO2)». esrl.noaa.gov. Archived from the original on 13 February 2021. Retrieved 20 April 2007.
- ^ «Carbon Dioxide as a Fire Suppressant: Examining the Risks». U.S. Environmental Protection Agency. Archived from the original on 2 October 2015.
- ^ «Volcano Under the City». A NOVA Production by Bonne Pioche and Greenspace for WGBH/Boston. Public Broadcasting System. 1 November 2005. Archived from the original on 5 April 2011..
- ^ Glatte Jr HA, Motsay GJ, Welch BE (1967). Carbon Dioxide Tolerance Studies (Report). Brooks AFB, TX School of Aerospace Medicine Technical Report. SAM-TR-67-77. Archived from the original on 9 May 2008. Retrieved 2 May 2008.
{{cite report}}: CS1 maint: unfit URL (link) - ^ Lambertsen CJ (1971). Carbon Dioxide Tolerance and Toxicity (Report). IFEM Report. Environmental Biomedical Stress Data Center, Institute for Environmental Medicine, University of Pennsylvania Medical Center. No. 2-71. Archived from the original on 24 July 2011. Retrieved 2 May 2008.
{{cite report}}: CS1 maint: unfit URL (link) - ^ a b Satish U, Mendell MJ, Shekhar K, Hotchi T, Sullivan D, Streufert S, Fisk WJ (December 2012). «Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance» (PDF). Environmental Health Perspectives. 120 (12): 1671–1677. doi:10.1289/ehp.1104789. PMC 3548274. PMID 23008272. Archived from the original (PDF) on 5 March 2016. Retrieved 11 December 2014.
- ^ a b Allen JG, MacNaughton P, Satish U, Santanam S, Vallarino J, Spengler JD (June 2016). «Associations of Cognitive Function Scores with Carbon Dioxide, Ventilation, and Volatile Organic Compound Exposures in Office Workers: A Controlled Exposure Study of Green and Conventional Office Environments». Environmental Health Perspectives. 124 (6): 805–812. doi:10.1289/ehp.1510037. PMC 4892924. PMID 26502459.
- ^ «Exposure Limits for Carbon Dioxide Gas – CO2 Limits». InspectAPedia.com. Archived from the original on 16 September 2018. Retrieved 19 October 2014.
- ^ Law J, Watkins S, Alexander D (2010). In-Flight Carbon Dioxide Exposures and Related Symptoms: Associations, Susceptibility and Operational Implications (PDF) (Report). NASA Technical Report. TP–2010–216126. Archived from the original (PDF) on 27 June 2011. Retrieved 26 August 2014.
- ^ Schaefer KE, Douglas WH, Messier AA, Shea ML, Gohman PA (1979). «Effect of prolonged exposure to 0.5% CO2 on kidney calcification and ultrastructure of lungs». Undersea Biomedical Research. 6 (Suppl): S155–S161. PMID 505623. Archived from the original on 19 October 2014. Retrieved 19 October 2014.
- ^ Du B, Tandoc MC, Mack ML, Siegel JA (November 2020). «Indoor CO2 concentrations and cognitive function: A critical review». Indoor Air. 30 (6): 1067–1082. doi:10.1111/ina.12706. PMID 32557862. S2CID 219915861.
- ^ Kaplan L (4 June 2019). «Ask the doc: Does my helmet make me stupid? — RevZilla». www.revzilla.com. Archived from the original on 22 May 2021. Retrieved 22 May 2021.
- ^ Brühwiler PA, Stämpfli R, Huber R, Camenzind M (September 2005). «CO2 and O2 concentrations in integral motorcycle helmets». Applied Ergonomics. 36 (5): 625–633. doi:10.1016/j.apergo.2005.01.018. PMID 15893291.
- ^ Allen JG, MacNaughton P, Satish U, Santanam S, Vallarino J, Spengler JD (June 2016). «Associations of Cognitive Function Scores with Carbon Dioxide, Ventilation, and Volatile Organic Compound Exposures in Office Workers: A Controlled Exposure Study of Green and Conventional Office Environments». Environmental Health Perspectives. 124 (6): 805–812. doi:10.1289/ehp.1510037. PMC 4892924. PMID 26502459.
- ^ Romm J (26 October 2015). «Exclusive: Elevated CO2 Levels Directly Affect Human Cognition, New Harvard Study Shows». ThinkProgress. Archived from the original on 9 October 2019. Retrieved 14 October 2019.
- ^ «Three die in dry-ice incident at Moscow pool party». BBC News. 29 February 2020. Archived from the original on 29 February 2020.
The victims were connected to Instagram influencer Yekaterina Didenko.
- ^ Rettner R (2 August 2018). «A Woman Died from Dry Ice Fumes. Here’s How It Can Happen». livescience.com. Archived from the original on 22 May 2021. Retrieved 22 May 2021.
- ^ van Gardingen PR, Grace J, Jeffree CE, Byari SH, Miglietta F, Raschi A, Bettarini I (1997). «Long-term effects of enhanced CO2 concentrations on leaf gas exchange: research opportunities using CO2 springs». In Raschi A, Miglietta F, Tognetti R, van Gardingen PR (eds.). Plant responses to elevated CO2: Evidence from natural springs. Cambridge: Cambridge University Press. pp. 69–86. ISBN 978-0-521-58203-2.
- ^ Martini M (1997). «CO2 emissions in volcanic areas: case histories and hazards». In Raschi A, Miglietta F, Tognetti R, van Gardingen PR (eds.). Plant responses to elevated CO2: Evidence from natural springs. Cambridge: Cambridge University Press. pp. 69–86. ISBN 978-0-521-58203-2.
- ^ a b c d «ABG (Arterial Blood Gas)». Brookside Associates. Archived from the original on 12 August 2017. Retrieved 2 January 2017.
- ^ «How much carbon dioxide do humans contribute through breathing?». EPA.gov. Archived from the original on 2 February 2011. Retrieved 30 April 2009.
- ^ Henrickson C (2005). Chemistry. Cliffs Notes. ISBN 978-0-7645-7419-1.
- ^ a b c d «Carbon dioxide». solarnavigator.net. Archived from the original on 14 September 2008. Retrieved 12 October 2007.
- ^ Battisti-Charbonney, A.; Fisher, J.; Duffin, J. (15 June 2011). «The cerebrovascular response to carbon dioxide in humans». J. Physiol. 589 (12): 3039–3048. doi:10.1113/jphysiol.2011.206052. PMC 3139085. PMID 21521758.
- ^ Patel, S.; Miao, J.H.; Yetiskul, E.; Anokhin, A.; Majmunder, S.H. (2022). «Physiology, Carbon Dioxide Retention». National Library of Medicine. National Center for Biotechnology Information, NIH. PMID 29494063. Retrieved 20 August 2022.
- ^ Wilmshurst, Peter (1998). «ABC of oxygen». BMJ. 317 (7164): 996–999. doi:10.1136/bmj.317.7164.996. PMC 1114047. PMID 9765173.
- ^ Showstack, Randy (2013). «Carbon dioxide tops 400 ppm at Mauna Loa, Hawaii». Eos, Transactions American Geophysical Union. 94 (21): 192. Bibcode:2013EOSTr..94Q.192S. doi:10.1002/2013eo210004. ISSN 0096-3941.
- ^ Montaigne, Fen. «Son of Climate Science Pioneer Ponders A Sobering Milestone». Yale Environment 360. Yale School of Forestry & Environmental Studies. Archived from the original on 8 June 2013. Retrieved 14 May 2013.
- ^ a b «Carbon dioxide now more than 50% higher than pre-industrial levels | National Oceanic and Atmospheric Administration». www.noaa.gov. Retrieved 14 June 2022.
- ^ a b Eggleton, Tony (2013). A Short Introduction to Climate Change. Cambridge University Press. p. 52. ISBN 9781107618763.
- ^ a b «The NOAA Annual Greenhouse Gas Index (AGGI) – An Introduction». NOAA Global Monitoring Laboratory/Earth System Research Laboratories. Archived from the original on 27 November 2020. Retrieved 18 December 2020.
- ^ Etheridge, D.M.; L.P. Steele; R.L. Langenfelds; R.J. Francey; J.-M. Barnola; V.I. Morgan (1996). «Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn». Journal of Geophysical Research. 101 (D2): 4115–28. Bibcode:1996JGR…101.4115E. doi:10.1029/95JD03410. ISSN 0148-0227.
- ^ IPCC (2022) Summary for policy makers in Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
- ^ Petty, G.W. (2004). «A First Course in Atmospheric Radiation». Eos Transactions. 85 (36): 229–51. Bibcode:2004EOSTr..85..341P. doi:10.1029/2004EO360007.
- ^ Atkins P, de Paula J (2006). Atkins’ Physical Chemistry (8th ed.). W. H. Freeman. p. 462. ISBN 978-0-7167-8759-4.
- ^ «Carbon Dioxide Absorbs and Re-emits Infrared Radiation». UCAR Center for Science Education. 2012. Archived from the original on 21 September 2017. Retrieved 9 September 2017.
- ^ Archer D (15 March 2005). «How long will global warming last?». RealClimate. Archived from the original on 4 March 2021. Retrieved 5 March 2021.
- ^ Archer D (2009). «Atmospheric lifetime of fossil fuel carbon dioxide». Annual Review of Earth and Planetary Sciences. 37 (1): 117–34. Bibcode:2009AREPS..37..117A. doi:10.1146/annurev.earth.031208.100206. hdl:2268/12933. Archived from the original on 24 February 2021. Retrieved 7 March 2021.
- ^ Joos F, Roth R, Fuglestvedt JS, Peters GP, Enting IG, Von Bloh W, et al. (2013). «Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis». Atmospheric Chemistry and Physics. 13 (5): 2793–2825. doi:10.5194/acpd-12-19799-2012. Archived from the original on 22 July 2020. Retrieved 7 March 2021.
- ^ «Figure 8.SM.4» (PDF). Intergovernmental Panel on Climate Change Fifth Assessment Report. p. 8SM-16. Archived (PDF) from the original on 24 March 2021. Retrieved 7 March 2021.
- ^ Zhang, Yi Ge; et al. (28 October 2013). «A 40-million-year history of atmospheric CO2«. Philosophical Transactions of the Royal Society A. 371 (2001): 20130096. Bibcode:2013RSPTA.37130096Z. doi:10.1098/rsta.2013.0096. PMID 24043869.
- ^ «Climate and CO2 in the Atmosphere». Archived from the original on 6 October 2018. Retrieved 10 October 2007.
- ^ Berner RA, Kothavala Z (2001). «GEOCARB III: A revised model of atmospheric CO2 over Phanerozoic Time» (PDF). American Journal of Science. 301 (2): 182–204. Bibcode:2001AmJS..301..182B. CiteSeerX 10.1.1.393.582. doi:10.2475/ajs.301.2.182. Archived (PDF) from the original on 4 September 2011. Retrieved 15 February 2008.
- ^ Friedlingstein P, Jones MW, O’sullivan M, Andrew RM, Hauck J, Peters GP, et al. (2019). «Global Carbon Budget 2019». Earth System Science Data. 11 (4): 1783–1838. Bibcode:2019ESSD…11.1783F. doi:10.5194/essd-11-1783-2019..
- ^ Doney SC, Levine NM (29 November 2006). «How Long Can the Ocean Slow Global Warming?». Oceanus. Archived from the original on 4 January 2008. Retrieved 21 November 2007.
- ^ Jacobson, M. Z. (2005). «Studying ocean acidification with conservative, stable numerical schemes for nonequilibrium air-ocean exchange and ocean equilibrium chemistry». Journal of Geophysical Research: Atmospheres. 110: D07302. Bibcode:2005JGRD..11007302J. doi:10.1029/2004JD005220.
- ^ a b «Ocean acidification due to increasing atmospheric carbon dioxide». The Royal Society.
- ^ Mitchell, Mark J.; Jensen, Oliver E.; Cliffe, K. Andrew; Maroto-Valer, M. Mercedes (8 May 2010). «A model of carbon dioxide dissolution and mineral carbonation kinetics». Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 466 (2117): 1265–1290. doi:10.1098/rspa.2009.0349.
- ^ Lupton J, Lilley M, Butterfield D, Evans L, Embley R, Olson E, et al. (2004). «Liquid Carbon Dioxide Venting at the Champagne Hydrothermal Site, NW Eifuku Volcano, Mariana Arc». American Geophysical Union. 2004 (Fall Meeting). V43F–08. Bibcode:2004AGUFM.V43F..08L.
- ^ Inagaki F, Kuypers MM, Tsunogai U, Ishibashi J, Nakamura K, Treude T, et al. (September 2006). «Microbial community in a sediment-hosted CO2 lake of the southern Okinawa Trough hydrothermal system». Proceedings of the National Academy of Sciences of the United States of America. 103 (38): 14164–14169. Bibcode:2006PNAS..10314164I. doi:10.1073/pnas.0606083103. PMC 1599929. PMID 16959888. Videos can be downloaded at «Supporting Information». Archived from the original on 19 October 2018.
- ^ «Collecting and using biogas from landfills». U.S. Energy Information Administration. 11 January 2017. Archived from the original on 11 July 2018. Retrieved 22 November 2015.
- ^ «Facts About Landfill Gas» (PDF). U.S. Environmental Protection Agency. January 2000. Archived (PDF) from the original on 23 September 2015. Retrieved 4 September 2015.
- ^ a b Pierantozzi R (2001). «Carbon Dioxide». Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. doi:10.1002/0471238961.0301180216090518.a01.pub2. ISBN 978-0-471-23896-6.
- ^
Strassburger J (1969). Blast Furnace Theory and Practice. New York: American Institute of Mining, Metallurgical, and Petroleum Engineers. ISBN 978-0-677-10420-1. - ^ Topham S (2000). «Carbon Dioxide». Ullmann’s Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a05_165. ISBN 3527306730.
- ^ «CO2 shortage: Food industry calls for government action». BBC. 21 June 2018. Archived from the original on 23 May 2021. Retrieved 24 June 2018.
- ^ «IPCC Special Report on Carbon dioxide Capture and Storage» (PDF). The Intergovernmental Panel on Climate Change. Archived from the original (PDF) on 24 September 2015. Retrieved 4 September 2015.
- ^ Morrison RT, Boyd RN (1983). Organic Chemistry (4th ed.). Allyn and Bacon. pp. 976–977. ISBN 978-0-205-05838-9.
- ^ Badwal SP, Giddey SS, Munnings C, Bhatt AI, Hollenkamp AF (24 September 2014). «Emerging electrochemical energy conversion and storage technologies». Frontiers in Chemistry. 2: 79. Bibcode:2014FrCh….2…79B. doi:10.3389/fchem.2014.00079. PMC 4174133. PMID 25309898.
- ^ Whiting D, Roll M, Vickerman L (August 2010). «Plant Growth Factors: Photosynthesis, Respiration, and Transpiration». CMG GardenNotes. Colorado Master Gardener Program. Archived from the original on 2 September 2014. Retrieved 10 October 2011.
- ^ Waggoner PE (February 1994). «Carbon dioxide». How Much Land Can Ten Billion People Spare for Nature?. Archived from the original on 12 October 2011. Retrieved 10 October 2011.
- ^ Stafford N (August 2007). «Future crops: the other greenhouse effect». Nature. 448 (7153): 526–528. Bibcode:2007Natur.448..526S. doi:10.1038/448526a. PMID 17671477. S2CID 9845813.
- ^ UK Food Standards Agency: «Current EU approved additives and their E Numbers». Archived from the original on 7 October 2010. Retrieved 27 October 2011.
- ^ US Food and Drug Administration: «Food Additive Status List». Food and Drug Administration. Archived from the original on 4 November 2017. Retrieved 13 June 2015.
- ^ Australia New Zealand Food Standards Code«Standard 1.2.4 – Labelling of ingredients». Archived from the original on 19 January 2012. Retrieved 27 October 2011.
- ^ Futurific Leading Indicators Magazine. Vol. 1. CRAES LLC. ISBN 978-0-9847670-1-4. Archived from the original on 15 August 2021. Retrieved 9 November 2020.
- ^ Vijay GP (25 September 2015). Indian Breads: A Comprehensive Guide to Traditional and Innovative Indian Breads. Westland. ISBN 978-93-85724-46-6.
- ^ «Scientists Discover Protein Receptor For Carbonation Taste». ScienceDaily. 16 October 2009. Archived from the original on 29 March 2020. Retrieved 29 March 2020.
- ^ Coghlan A (3 February 2018). «A more humane way of slaughtering chickens might get EU approval». New Scientist. Archived from the original on 24 June 2018. Retrieved 24 June 2018.
- ^ «What is CO2 stunning?». RSPCA. Archived from the original on 9 April 2014.
- ^ Campbell A (10 March 2018). «Humane execution and the fear of the tumbril». New Scientist. Archived from the original on 24 June 2018. Retrieved 24 June 2018.
- ^ Nordestgaard BG, Rostgaard J (February 1985). «Critical-point drying versus freeze drying for scanning electron microscopy: a quantitative and qualitative study on isolated hepatocytes». Journal of Microscopy. 137 (Pt 2): 189–207. doi:10.1111/j.1365-2818.1985.tb02577.x. PMID 3989858. S2CID 32065173.
- ^ «Types of Fire Extinguishers». The Fire Safety Advice Centre. Archived from the original on 28 June 2021. Retrieved 28 June 2021.
- ^ National Fire Protection Association Code 12.
- ^ Carbon Dioxide as a Fire Suppressant: Examining the Risks, US EPA. 2000.
- ^ «Appendix A: CO2 for use in enhanced oil recovery (EOR)». Accelerating the uptake of CCS: industrial use of captured carbon dioxide. Global CCS Institute. 20 December 2011. Archived from the original on 28 April 2017. Retrieved 2 January 2017.
- ^ Austell JM (2005). «CO2 for Enhanced Oil Recovery Needs – Enhanced Fiscal Incentives». Exploration & Production: The Oil & Gas Review. Archived from the original on 7 February 2012. Retrieved 28 September 2007.
- ^ «Enhanced coal bed methane recovery». ETH Zurich. 31 August 2006. Archived from the original on 6 July 2011.
- ^ Clayton M (11 January 2006). «Algae – like a breath mint for smokestacks». The Christian Science Monitor. Archived from the original on 14 September 2008. Retrieved 11 October 2007.
- ^ Atsumi S, Higashide W, Liao JC (December 2009). «Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde». Nature Biotechnology. 27 (12): 1177–1180. doi:10.1038/nbt.1586. PMID 19915552. S2CID 1492698.
- ^ Cobb S, Badiani V, Dharani A, Wagner A, Zacarias S, Oliveira AR, et al. (28 February 2022). «Fast CO2 hydration kinetics impair heterogeneous but improve enzymatic CO2 reduction catalysis». Nature Chemistry. 14 (4): 417–424. Bibcode:2022NatCh..14..417C. doi:10.1038/s41557-021-00880-2. ISSN 1755-4349. PMC 7612589. PMID 35228690. S2CID 247160910.
- ^ Edwardes Moore E, Cobb SJ, Coito AM, Oliveira AR, Pereira IA, Reisner E (January 2022). «Understanding the local chemical environment of bioelectrocatalysis». Proceedings of the National Academy of Sciences of the United States of America. 119 (4): e2114097119. Bibcode:2022PNAS..11914097E. doi:10.1073/pnas.2114097119. PMC 8795565. PMID 35058361.
- ^ «Clean Way To Turn CO2 Into Fuel Inspired by Nature». Applied Sciences from Technology Networks. 1 March 2022. Retrieved 2 March 2022.
- ^ «The Coca-Cola Company Announces Adoption of HFC-Free Insulation in Refrigeration Units to Combat Global Warming». The Coca-Cola Company. 5 June 2006. Archived from the original on 1 November 2013. Retrieved 11 October 2007.
- ^ «Modine reinforces its CO2 research efforts». R744.com. 28 June 2007. Archived from the original on 10 February 2008.
- ^ TCE, the Chemical Engineer. Institution of Chemical Engineers. 1990. Archived from the original on 17 August 2021. Retrieved 2 June 2020.
- ^ a b «AVMA guidelines for the euthanasia of animals: 2020 Edition» (PDF). American Veterinary Medical Association. 2020. Archived (PDF) from the original on 1 February 2014. Retrieved 13 August 2021.
- ^ Harris D (September 1910). «The Pioneer in the Hygiene of Ventilation». The Lancet. 176 (4542): 906–908. doi:10.1016/S0140-6736(00)52420-9. Archived from the original on 17 March 2020. Retrieved 6 December 2019.
- ^ Almqvist E (2003). History of industrial gases. Springer. p. 93. ISBN 978-0-306-47277-0.
- ^ Priestley J, Hey W (1772). «Observations on Different Kinds of Air». Philosophical Transactions. 62: 147–264. doi:10.1098/rstl.1772.0021. S2CID 186210131. Archived from the original on 7 June 2010. Retrieved 11 October 2007.
- ^ Davy H (1823). «On the Application of Liquids Formed by the Condensation of Gases as Mechanical Agents». Philosophical Transactions. 113: 199–205. doi:10.1098/rstl.1823.0020. JSTOR 107649.
- ^ Thilorier AJ (1835). «Solidification de l’Acide carbonique». Comptes Rendus. 1: 194–196. Archived from the original on 2 September 2017. Retrieved 1 September 2017.
- ^ Thilorier AJ (1836). «Solidification of carbonic acid». The London and Edinburgh Philosophical Magazine. 8 (48): 446–447. doi:10.1080/14786443608648911. Archived from the original on 2 May 2016. Retrieved 15 November 2015.
External links
- Current global map of carbon dioxide concentration
- CDC – NIOSH Pocket Guide to Chemical Hazards – Carbon Dioxide
- Trends in Atmospheric Carbon Dioxide (NOAA)

|
||
|
||
| Names | ||
|---|---|---|
Other names
|
||
| Identifiers | ||
|
CAS Number |
|
|
|
3D model (JSmol) |
|
|
| 3DMet |
|
|
|
Beilstein Reference |
1900390 | |
| ChEBI |
|
|
| ChEMBL |
|
|
| ChemSpider |
|
|
| ECHA InfoCard | 100.004.271 |
|
| EC Number |
|
|
| E number | E290 (preservatives) | |
|
Gmelin Reference |
989 | |
| KEGG |
|
|
| MeSH | Carbon+dioxide | |
|
PubChem CID |
|
|
| RTECS number |
|
|
| UNII |
|
|
| UN number | 1013 (gas), 1845 (solid) | |
|
CompTox Dashboard (EPA) |
|
|
|
InChI
|
||
|
SMILES
|
||
| Properties | ||
|
Chemical formula |
CO2 | |
| Molar mass | 44.009 g·mol−1 | |
| Appearance | Colorless gas | |
| Odor |
|
|
| Density |
|
|
| Critical point (T, P) | 304.128(15) K[2] (30.978(15) °C), 7.3773(30) MPa[2] (72.808(30) atm) | |
|
Sublimation |
194.6855(30) K (−78.4645(30) °C) at 1 atm (0.101325 MPa) | |
|
Solubility in water |
1.45 g/L at 25 °C (77 °F), 100 kPa (0.99 atm) | |
| Vapor pressure | 5.7292(30) MPa, 56.54(30) atm (20 °C (293.15 K)) | |
| Acidity (pKa) | 6.35, 10.33 | |
|
Magnetic susceptibility (χ) |
−20.5·10−6 cm3/mol | |
| Thermal conductivity | 0.01662 W·m−1·K−1 (300 K (27 °C; 80 °F))[3] | |
|
Refractive index (nD) |
1.00045 | |
| Viscosity |
|
|
|
Dipole moment |
0 D | |
| Structure | ||
|
Crystal structure |
Trigonal | |
|
Molecular shape |
Linear | |
| Thermochemistry | ||
|
Heat capacity (C) |
37.135 J/K·mol | |
|
Std molar |
214 J·mol−1·K−1 | |
|
Std enthalpy of |
−393.5 kJ·mol−1 | |
| Pharmacology | ||
|
ATC code |
V03AN02 (WHO) | |
| Hazards | ||
| NFPA 704 (fire diamond) |
[7][8] 2 0 0 SA |
|
| Lethal dose or concentration (LD, LC): | ||
|
LCLo (lowest published) |
90,000 ppm (human, 5 min)[6] | |
| NIOSH (US health exposure limits): | ||
|
PEL (Permissible) |
TWA 5000 ppm (9000 mg/m3)[5] | |
|
REL (Recommended) |
TWA 5000 ppm (9000 mg/m3), ST 30,000 ppm (54,000 mg/m3)[5] | |
|
IDLH (Immediate danger) |
40,000 ppm[5] | |
| Safety data sheet (SDS) | Sigma-Aldrich | |
| Related compounds | ||
|
Other anions |
|
|
|
Other cations |
|
|
|
Related carbon oxides |
|
|
|
Related compounds |
|
|
| Supplementary data page | ||
| Carbon dioxide (data page) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Carbon dioxide (chemical formula CO2) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth’s atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm.[9][10] Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.[11] Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. When carbon dioxide dissolves in water, it forms carbonate and mainly bicarbonate (HCO−
3), which causes ocean acidification as atmospheric CO2 levels increase.[12]
As the source of available carbon in the carbon cycle, atmospheric CO2 is the primary carbon source for life on Earth. Its concentration in Earth’s pre-industrial atmosphere since late in the Precambrian has been regulated by organisms and geological phenomena. Plants, algae and cyanobacteria use energy from sunlight to synthesize carbohydrates from carbon dioxide and water in a process called photosynthesis, which produces oxygen as a waste product.[13] In turn, oxygen is consumed and CO2 is released as waste by all aerobic organisms when they metabolize organic compounds to produce energy by respiration.[14] CO2 is released from organic materials when they decay or combust, such as in forest fires. Since plants require CO2 for photosynthesis, and humans and animals depend on plants for food, CO2 is necessary for the survival of life on earth.
Carbon dioxide is 53% more dense than dry air, but is long lived and thoroughly mixes in the atmosphere. About half of excess CO2 emissions to the atmosphere are absorbed by land and ocean carbon sinks.[15] These sinks can become saturated and are volatile, as decay and wildfires result in the CO2 being released back into the atmosphere.[16] CO2 is eventually sequestered (stored for the long term) in rocks and organic deposits like coal, petroleum and natural gas. Sequestered CO2 is released into the atmosphere through burning fossil fuels or naturally by volcanoes, hot springs, geysers, and when carbonate rocks dissolve in water or react with acids.
CO2 is a versatile industrial material, used, for example, as an inert gas in welding and fire extinguishers, as a pressurizing gas in air guns and oil recovery, and as a supercritical fluid solvent in decaffeination of coffee and supercritical drying.[17] It is a byproduct of fermentation of sugars in bread, beer and wine making, and is added to carbonated beverages like seltzer and beer for effervescence. It has a sharp and acidic odor and generates the taste of soda water in the mouth,[18] but at normally encountered concentrations it is odorless.[1]
Chemical and physical properties
Structure, bonding and molecular vibrations
The symmetry of a carbon dioxide molecule is linear and centrosymmetric at its equilibrium geometry. The length of the carbon-oxygen bond in carbon dioxide is 116.3 pm, noticeably shorter than the roughly 140-pm length of a typical single C–O bond, and shorter than most other C–O multiply-bonded functional groups such as carbonyls.[19] Since it is centrosymmetric, the molecule has no electric dipole moment.
Stretching and bending oscillations of the CO2 carbon dioxide molecule. Upper left: symmetric stretching. Upper right: antisymmetric stretching. Lower line: degenerate pair of bending modes.
As a linear triatomic molecule, CO2 has four vibrational modes as shown in the diagram. In the symmetric and the antisymmetric stretching modes, the atoms move along the axis of the molecule. There are two bending modes, which are degenerate, meaning that they have the same frequency and same energy, because of the symmetry of the molecule. When a molecule touches a surface or touches another molecule, the two bending modes can differ in frequency because the interaction is different for the two modes. Some of the vibrational modes are observed in the infrared (IR) spectrum: the antisymmetric stretching mode at wavenumber 2349 cm−1 (wavelength 4.25 μm) and the degenerate pair of bending modes at 667 cm−1 (wavelength 15 μm). The symmetric stretching mode does not create an electric dipole so is not observed in IR spectroscopy, but it is detected in by Raman spectroscopy at 1388 cm−1 (wavelength 7.2 μm).[20]
In the gas phase, carbon dioxide molecules undergo significant vibrational motions and do not keep a fixed structure. However, in a Coulomb explosion imaging experiment, an instantaneous image of the molecular structure can be deduced. Such an experiment[21] has been performed for carbon dioxide.
The result of this experiment, and the conclusion of theoretical calculations[22] based on an ab initio potential energy surface of the molecule, is that none of the
molecules in the gas phase are ever exactly linear. This counter-intuitive result is trivially due to
the fact that the nuclear motion volume element vanishes for linear geometries.[22]
This is so for all molecules (except diatomics!).
In aqueous solution
Carbon dioxide is soluble in water, in which it reversibly forms H2CO3 (carbonic acid), which is a weak acid since its ionization in water is incomplete.
The hydration equilibrium constant of carbonic acid is, at 25 °C:
Hence, the majority of the carbon dioxide is not converted into carbonic acid, but remains as CO2 molecules, not affecting the pH.
The relative concentrations of CO2, H2CO3, and the deprotonated forms HCO−3 (bicarbonate) and CO2−3(carbonate) depend on the pH. As shown in a Bjerrum plot, in neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater. In very alkaline water (pH > 10.4), the predominant (>50%) form is carbonate. The oceans, being mildly alkaline with typical pH = 8.2–8.5, contain about 120 mg of bicarbonate per liter.
Being diprotic, carbonic acid has two acid dissociation constants, the first one for the dissociation into the bicarbonate (also called hydrogen carbonate) ion (HCO−3):
- Ka1 = 2.5×10−4 mol/L; pKa1 = 3.6 at 25 °C.[19]
This is the true first acid dissociation constant, defined as
where the denominator includes only covalently bound H2CO3 and does not include hydrated CO2(aq). The much smaller and often-quoted value near 4.16×10−7 is an apparent value calculated on the (incorrect) assumption that all dissolved CO2 is present as carbonic acid, so that
Since most of the dissolved CO2remains as CO2 molecules, Ka1(apparent) has a much larger denominator and a much smaller value than the true Ka1.[23]
The bicarbonate ion is an amphoteric species that can act as an acid or as a base, depending on pH of the solution. At high pH, it dissociates significantly into the carbonate ion (CO2−3):
- Ka2 = 4.69×10−11 mol/L; pKa2 = 10.329
In organisms carbonic acid production is catalysed by the enzyme, carbonic anhydrase.
Chemical reactions of CO2
CO2 is a potent electrophile having an electrophilic reactivity that is comparable to benzaldehyde or strong α,β-unsaturated carbonyl compounds. However, unlike electrophiles of similar reactivity, the reactions of nucleophiles with CO2 are thermodynamically less favored and are often found to be highly reversible.[24] Only very strong nucleophiles, like the carbanions provided by Grignard reagents and organolithium compounds react with CO2 to give carboxylates:
- where M = Li or Mg Br and R = alkyl or aryl.
In metal carbon dioxide complexes, CO2 serves as a ligand, which can facilitate the conversion of CO2 to other chemicals.[25]
The reduction of CO2 to CO is ordinarily a difficult and slow reaction:
Photoautotrophs (i.e. plants and cyanobacteria) use the energy contained in sunlight to photosynthesize simple sugars from CO2 absorbed from the air and water:
The redox potential for this reaction near pH 7 is about −0.53 V versus the standard hydrogen electrode. The nickel-containing enzyme carbon monoxide dehydrogenase catalyses this process.[26]
Physical properties
Pellets of «dry ice», a common form of solid carbon dioxide
Carbon dioxide is colorless. At low concentrations the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor.[1] At standard temperature and pressure, the density of carbon dioxide is around 1.98 kg/m3, about 1.53 times that of air.[27]
Carbon dioxide has no liquid state at pressures below 0.51795(10) MPa[2] (5.11177(99) atm). At a pressure of 1 atm (0.101325 MPa), the gas deposits directly to a solid at temperatures below 194.6855(30) K[2] (−78.4645(30) °C) and the solid sublimes directly to a gas above this temperature. In its solid state, carbon dioxide is commonly called dry ice.
Pressure–temperature phase diagram of carbon dioxide. Note that it is a log-lin chart.
Liquid carbon dioxide forms only at pressures above 0.51795(10) MPa[2] (5.11177(99) atm); the triple point of carbon dioxide is 216.592(3) K[2] (−56.558(3) °C) at 0.51795(10) MPa[2] (5.11177(99) atm) (see phase diagram). The critical point is 304.128(15) K[2] (30.978(15) °C) at 7.3773(30) MPa[2] (72.808(30) atm). Another form of solid carbon dioxide observed at high pressure is an amorphous glass-like solid.[28] This form of glass, called carbonia, is produced by supercooling heated CO2 at extreme pressures (40–48 GPa, or about 400,000 atmospheres) in a diamond anvil. This discovery confirmed the theory that carbon dioxide could exist in a glass state similar to other members of its elemental family, like silicon dioxide (silica glass) and germanium dioxide. Unlike silica and germania glasses, however, carbonia glass is not stable at normal pressures and reverts to gas when pressure is released.
At temperatures and pressures above the critical point, carbon dioxide behaves as a supercritical fluid known as supercritical carbon dioxide.
Table of thermal and physical properties of saturated liquid carbon dioxide:[29][30]
| Temperature (°C) | Density (kg/m^3) | Specific heat (kJ/kg K) | Kinematic viscosity (m^2/s) | Conductivity (W/m K) | Thermal diffusivity (m^2/s) | Prandtl Number | Bulk modulus (K^-1) |
| -50 | 1156.34 | 1.84 | 1.19E-07 | 0.0855 | 4.02E-08 | 2.96 | — |
| -40 | 1117.77 | 1.88 | 1.18E-07 | 0.1011 | 4.81E-08 | 2.46 | — |
| -30 | 1076.76 | 1.97 | 1.17E-07 | 0.1116 | 5.27E-08 | 2.22 | — |
| -20 | 1032.39 | 2.05 | 1.15E-07 | 0.1151 | 5.45E-08 | 2.12 | — |
| -10 | 983.38 | 2.18 | 1.13E-07 | 0.1099 | 5.13E-08 | 2.2 | — |
| 0 | 926.99 | 2.47 | 1.08E-07 | 0.1045 | 4.58E-08 | 2.38 | — |
| 10 | 860.03 | 3.14 | 1.01E-07 | 0.0971 | 3.61E-08 | 2.8 | — |
| 20 | 772.57 | 5 | 9.10E-08 | 0.0872 | 2.22E-08 | 4.1 | 1.40E-02 |
| 30 | 597.81 | 36.4 | 8.00E-08 | 0.0703 | 0.279E-08 | 28.7 | — |
Table of thermal and physical properties of carbon dioxide (CO2) at atmospheric pressure:[29][30]
| Temperature (K) | Density (kg/m^3) | Specific heat (kJ/kg °C) | Dynamic viscosity (kg/m s) | Kinematic viscosity (m^2/s) | Thermal conductivity (W/m °C) | Thermal diffusivity (m^2/s) | Prandtl Number |
| 220 | 2.4733 | 0.783 | 1.11E-05 | 4.49E-06 | 0.010805 | 5.92E-06 | 0.818 |
| 250 | 2.1657 | 0.804 | 1.26E-05 | 5.81E-06 | 0.012884 | 7.40E-06 | 0.793 |
| 300 | 1.7973 | 0.871 | 1.50E-05 | 8.32E-06 | 0.016572 | 1.06E-05 | 0.77 |
| 350 | 1.5362 | 0.9 | 1.72E-05 | 1.12E-05 | 0.02047 | 1.48E-05 | 0.755 |
| 400 | 1.3424 | 0.942 | 1.93E-05 | 1.44E-05 | 0.02461 | 1.95E-05 | 0.738 |
| 450 | 1.1918 | 0.98 | 2.13E-05 | 1.79E-05 | 0.02897 | 2.48E-05 | 0.721 |
| 500 | 1.0732 | 1.013 | 2.33E-05 | 2.17E-05 | 0.03352 | 3.08E-05 | 0.702 |
| 550 | 0.9739 | 1.047 | 2.51E-05 | 2.57E-05 | 0.03821 | 3.75E-05 | 0.685 |
| 600 | 0.8938 | 1.076 | 2.68E-05 | 3.00E-05 | 0.04311 | 4.48E-05 | 0.668 |
| 650 | 0.8143 | 1.1 | 2.88E-05 | 3.54E-05 | 0.0445 | 4.97E-05 | 0.712 |
| 700 | 0.7564 | 1.13E+00 | 3.05E-05 | 4.03E-05 | 0.0481 | 5.63E-05 | 0.717 |
| 750 | 0.7057 | 1.15 | 3.21E-05 | 4.55E-05 | 0.0517 | 6.37E-05 | 0.714 |
| 800 | 0.6614 | 1.17E+00 | 3.37E-05 | 5.10E-05 | 0.0551 | 7.12E-05 | 0.716 |
Biological role
Carbon dioxide is an end product of cellular respiration in organisms that obtain energy by breaking down sugars, fats and amino acids with oxygen as part of their metabolism. This includes all plants, algae and animals and aerobic fungi and bacteria. In vertebrates, the carbon dioxide travels in the blood from the body’s tissues to the skin (e.g., amphibians) or the gills (e.g., fish), from where it dissolves in the water, or to the lungs from where it is exhaled. During active photosynthesis, plants can absorb more carbon dioxide from the atmosphere than they release in respiration.
Photosynthesis and carbon fixation
Carbon fixation is a biochemical process by which atmospheric carbon dioxide is incorporated by plants, algae and (cyanobacteria) into energy-rich organic molecules such as glucose, thus creating their own food by photosynthesis. Photosynthesis uses carbon dioxide and water to produce sugars from which other organic compounds can be constructed, and oxygen is produced as a by-product.
Ribulose-1,5-bisphosphate carboxylase oxygenase, commonly abbreviated to RuBisCO, is the enzyme involved in the first major step of carbon fixation, the production of two molecules of 3-phosphoglycerate from CO2 and ribulose bisphosphate, as shown in the diagram at left.
RuBisCO is thought to be the single most abundant protein on Earth.[31]
Phototrophs use the products of their photosynthesis as internal food sources and as raw material for the biosynthesis of more complex organic molecules, such as polysaccharides, nucleic acids and proteins. These are used for their own growth, and also as the basis of the food chains and webs that feed other organisms, including animals such as ourselves. Some important phototrophs, the coccolithophores synthesise hard calcium carbonate scales.[32] A globally significant species of coccolithophore is Emiliania huxleyi whose calcite scales have formed the basis of many sedimentary rocks such as limestone, where what was previously atmospheric carbon can remain fixed for geological timescales.
Overview of photosynthesis and respiration. Carbon dioxide (at right), together with water, form oxygen and organic compounds (at left) by photosynthesis, which can be respired to water and (CO2).
Plants can grow as much as 50 percent faster in concentrations of 1,000 ppm CO2 when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients.[33] Elevated CO2 levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated CO2 in FACE experiments.[34][35]
Increased atmospheric CO2 concentrations result in fewer stomata developing on plants[36] which leads to reduced water usage and increased water-use efficiency.[37] Studies using FACE have shown that CO2 enrichment leads to decreased concentrations of micronutrients in crop plants.[38] This may have knock-on effects on other parts of ecosystems as herbivores will need to eat more food to gain the same amount of protein.[39]
The concentration of secondary metabolites such as phenylpropanoids and flavonoids can also be altered in plants exposed to high concentrations of CO2.[40][41]
Plants also emit CO2 during respiration, and so the majority of plants and algae, which use C3 photosynthesis, are only net absorbers during the day. Though a growing forest will absorb many tons of CO2 each year, a mature forest will produce as much CO2 from respiration and decomposition of dead specimens (e.g., fallen branches) as is used in photosynthesis in growing plants.[42] Contrary to the long-standing view that they are carbon neutral, mature forests can continue to accumulate carbon[43] and remain valuable carbon sinks, helping to maintain the carbon balance of Earth’s atmosphere. Additionally, and crucially to life on earth, photosynthesis by phytoplankton consumes dissolved CO2 in the upper ocean and thereby promotes the absorption of CO2 from the atmosphere.[44]
Toxicity
Symptoms of carbon dioxide toxicity, by increasing volume percent in air[45]
Carbon dioxide content in fresh air (averaged between sea-level and 10 kPa level, i.e., about 30 km (19 mi) altitude) varies between 0.036% (360 ppm) and 0.041% (412 ppm), depending on the location.[46][clarification needed]
CO2 is an asphyxiant gas and not classified as toxic or harmful in accordance with Globally Harmonized System of Classification and Labelling of Chemicals standards of United Nations Economic Commission for Europe by using the OECD Guidelines for the Testing of Chemicals. In concentrations up to 1% (10,000 ppm), it will make some people feel drowsy and give the lungs a stuffy feeling.[45] Concentrations of 7% to 10% (70,000 to 100,000 ppm) may cause suffocation, even in the presence of sufficient oxygen, manifesting as dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes to an hour.[47] The physiological effects of acute carbon dioxide exposure are grouped together under the term hypercapnia, a subset of asphyxiation.
Because it is heavier than air, in locations where the gas seeps from the ground (due to sub-surface volcanic or geothermal activity) in relatively high concentrations, without the dispersing effects of wind, it can collect in sheltered/pocketed locations below average ground level, causing animals located therein to be suffocated. Carrion feeders attracted to the carcasses are then also killed. Children have been killed in the same way near the city of Goma by CO2 emissions from the nearby volcano Mount Nyiragongo.[48] The Swahili term for this phenomenon is mazuku.
Adaptation to increased concentrations of CO2 occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification (acidosis). Several studies suggested that 2.0 percent inspired concentrations could be used for closed air spaces (e.g. a submarine) since the adaptation is physiological and reversible, as deterioration in performance or in normal physical activity does not happen at this level of exposure for five days.[49][50] Yet, other studies show a decrease in cognitive function even at much lower levels.[51][52] Also, with ongoing respiratory acidosis, adaptation or compensatory mechanisms will be unable to reverse such condition.
Below 1%
There are few studies of the health effects of long-term continuous CO2 exposure on humans and animals at levels below 1%. Occupational CO2 exposure limits have been set in the United States at 0.5% (5000 ppm) for an eight-hour period.[53] At this CO2 concentration, International Space Station crew experienced headaches, lethargy, mental slowness, emotional irritation, and sleep disruption.[54] Studies in animals at 0.5% CO2 have demonstrated kidney calcification and bone loss after eight weeks of exposure.[55] A study of humans exposed in 2.5 hour sessions demonstrated significant negative effects on cognitive abilities at concentrations as low as 0.1% (1000 ppm) CO2 likely due to CO2 induced increases in cerebral blood flow.[51] Another study observed a decline in basic activity level and information usage at 1000 ppm, when compared to 500 ppm.[52] However a review of the literature found that most studies on the phenomenon of carbon dioxide induced cognitive impairment to have a small effect on high-level decision making and most of the studies were confounded by inadequate study designs, environmental comfort, uncertainties in exposure doses and differing cognitive assessments used.[56] Similarly a study on the effects of the concentration of CO2 in motorcycle helmets has been criticized for having dubious methodology in not noting the self-reports of motorcycle riders and taking measurements using mannequins. Further when normal motorcycle conditions were achieved (such as highway or city speeds) or the visor was raised the concentration of CO2 declined to safe levels (0.2%).[57][58]
Ventilation
Poor ventilation is one of the main causes of excessive CO2 concentrations in closed spaces, leading to poor indoor air quality. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that CO2 concentration has stabilized) are sometimes used to estimate ventilation rates per person.[citation needed] Higher CO2 concentrations are associated with occupant health, comfort and performance degradation.[59][60] ASHRAE Standard 62.1–2007 ventilation rates may result in indoor concentrations up to 2,100 ppm above ambient outdoor conditions. Thus if the outdoor concentration is 400 ppm, indoor concentrations may reach 2,500 ppm with ventilation rates that meet this industry consensus standard. Concentrations in poorly ventilated spaces can be found even higher than this (range of 3,000 or 4,000 ppm).
Miners, who are particularly vulnerable to gas exposure due to insufficient ventilation, referred to mixtures of carbon dioxide and nitrogen as «blackdamp», «choke damp» or «stythe». Before more effective technologies were developed, miners would frequently monitor for dangerous levels of blackdamp and other gases in mine shafts by bringing a caged canary with them as they worked. The canary is more sensitive to asphyxiant gases than humans, and as it became unconscious would stop singing and fall off its perch. The Davy lamp could also detect high levels of blackdamp (which sinks, and collects near the floor) by burning less brightly, while methane, another suffocating gas and explosion risk, would make the lamp burn more brightly.
In February 2020, three people died from suffocation at a party in Moscow when dry ice (frozen CO2) was added to a swimming pool to cool it down.[61] A similar accident occurred in 2018 when a woman died from CO2 fumes emanating from the large amount of dry ice she was transporting in her car.[62]
Outdoor areas with elevated concentrations
Local concentrations of carbon dioxide can reach high values near strong sources, especially those that are isolated by surrounding terrain. At the Bossoleto hot spring near Rapolano Terme in Tuscany, Italy, situated in a bowl-shaped depression about 100 m (330 ft) in diameter, concentrations of CO2 rise to above 75% overnight, sufficient to kill insects and small animals. After sunrise the gas is dispersed by convection.[63] High concentrations of CO2 produced by disturbance of deep lake water saturated with CO2 are thought to have caused 37 fatalities at Lake Monoun, Cameroon in 1984 and 1700 casualties at Lake Nyos, Cameroon in 1986.[64]
Human physiology
Content
| Blood compartment | (kPa) | (mm Hg) | |
|---|---|---|---|
| Venous blood carbon dioxide | 5.5–6.8 | 41–51[65] | 41–51[65] |
| Alveolar pulmonary gas pressures |
4.8 | 36 | 36 |
| Arterial blood carbon dioxide | 4.7–6.0 | 35–45[65] | 35–45[65] |
The body produces approximately 2.3 pounds (1.0 kg) of carbon dioxide per day per person,[66] containing 0.63 pounds (290 g) of carbon. In humans, this carbon dioxide is carried through the venous system and is breathed out through the lungs, resulting in lower concentrations in the arteries. The carbon dioxide content of the blood is often given as the partial pressure, which is the pressure which carbon dioxide would have had if it alone occupied the volume.[67] In humans, the blood carbon dioxide contents is shown in the adjacent table.
Transport in the blood
CO2 is carried in blood in three different ways. (Exact percentages vary between arterial and venous blood).
- Majority (about 70% to 80%) is converted to bicarbonate ions HCO−3 by the enzyme carbonic anhydrase in the red blood cells,[68] by the reaction CO2 + H2O → H2CO3 → H+ + HCO−3.
- 5–10% is dissolved in blood plasma[68]
- 5–10% is bound to hemoglobin as carbamino compounds[68]
Hemoglobin, the main oxygen-carrying molecule in red blood cells, carries both oxygen and carbon dioxide. However, the CO2 bound to hemoglobin does not bind to the same site as oxygen. Instead, it combines with the N-terminal groups on the four globin chains. However, because of allosteric effects on the hemoglobin molecule, the binding of CO2 decreases the amount of oxygen that is bound for a given partial pressure of oxygen. This is known as the Haldane Effect, and is important in the transport of carbon dioxide from the tissues to the lungs. Conversely, a rise in the partial pressure of CO2 or a lower pH will cause offloading of oxygen from hemoglobin, which is known as the Bohr effect.
Regulation of respiration
Carbon dioxide is one of the mediators of local autoregulation of blood supply. If its concentration is high, the capillaries expand to allow a greater blood flow to that tissue.[69]
Bicarbonate ions are crucial for regulating blood pH. A person’s breathing rate influences the level of CO2 in their blood. Breathing that is too slow or shallow causes respiratory acidosis, while breathing that is too rapid leads to hyperventilation, which can cause respiratory alkalosis.[70]
Although the body requires oxygen for metabolism, low oxygen levels normally do not stimulate breathing. Rather, breathing is stimulated by higher carbon dioxide levels. As a result, breathing low-pressure air or a gas mixture with no oxygen at all (such as pure nitrogen) can lead to loss of consciousness without ever experiencing air hunger. This is especially perilous for high-altitude fighter pilots. It is also why flight attendants instruct passengers, in case of loss of cabin pressure, to apply the oxygen mask to themselves first before helping others; otherwise, one risks losing consciousness.[68]
The respiratory centers try to maintain an arterial CO2 pressure of 40 mm Hg. With intentional hyperventilation, the CO2 content of arterial blood may be lowered to 10–20 mm Hg (the oxygen content of the blood is little affected), and the respiratory drive is diminished. This is why one can hold one’s breath longer after hyperventilating than without hyperventilating. This carries the risk that unconsciousness may result before the need to breathe becomes overwhelming, which is why hyperventilation is particularly dangerous before free diving.[71]
Concentrations and role in the environment
Atmosphere
Atmospheric CO2 concentrations measured at Mauna Loa Observatory from 1958 to 2022 (also called the Keeling Curve). Carbon dioxide concentrations have varied widely over the Earth’s 4.54 billion year history. However, in 2013 the daily mean concentration of CO2 in the atmosphere surpassed 400 parts per million (ppmv)[72] — this level has never been reached since the mid-Pliocene, 2 to 4 million years ago.[73]
Carbon dioxide in Earth’s atmosphere is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of several greenhouse gases in Earth’s atmosphere that are contributing to climate change due to increasing emissions of greenhouse gases from human activities. The current global average concentration of CO2 in the atmosphere is 421 ppm as of May 2022.[74] This is an increase of 50% since the start of the Industrial Revolution, up from 280 ppm during the 10,000 years prior to the mid-18th century.[75][74][76] The increase is due to human activity.[77] Burning fossil fuels is the main cause of these increased CO2 concentrations and also the main cause of climate change.[78] Other large anthropogenic sources include cement production, deforestation, and biomass burning.
While transparent to visible light, carbon dioxide is a greenhouse gas, absorbing and emitting infrared radiation at its two infrared-active vibrational frequencies. CO2 absorbs and emits infrared radiation at wavelengths of 4.26 μm (2,347 cm−1) (asymmetric stretching vibrational mode) and 14.99 μm (667 cm−1) (bending vibrational mode). It plays a significant role in influencing Earth’s surface temperature through the greenhouse effect.[79] Light emission from the Earth’s surface is most intense in the infrared region between 200 and 2500 cm−1,[80] as opposed to light emission from the much hotter Sun which is most intense in the visible region. Absorption of infrared light at the vibrational frequencies of atmospheric CO2 traps energy near the surface, warming the surface and the lower atmosphere. Less energy reaches the upper atmosphere, which is therefore cooler because of this absorption.[81]
Increases in atmospheric concentrations of CO2 and other long-lived greenhouse gases such as methane, nitrous oxide and ozone increase the absorption and emission of infrared radiation by the atmosphere, causing the observed rise in average global temperature and ocean acidification. Another direct effect is the CO2 fertilization effect. These changes cause a range of indirect effects of climate change on the physical environment, ecosystems and human societies. Carbon dioxide exerts a larger overall warming influence than all of the other greenhouse gases combined.[76] It has an atmospheric lifetime that increases with the cumulative amount of fossil carbon extracted and burned, due to the imbalance that this activity has imposed on Earth’s fast carbon cycle.[82] This means that some fraction (a projected 20-35%) of the fossil carbon transferred thus far will persist in the atmosphere as elevated CO2 levels for many thousands of years after these carbon transfer activities begin to subside.[83][84][85] The carbon cycle is a biogeochemical cycle in which carbon is exchanged between the Earth’s oceans, soil, rocks and the biosphere. Plants and other photoautotrophs use solar energy to produce carbohydrate from atmospheric carbon dioxide and water by photosynthesis. Almost all other organisms depend on carbohydrate derived from photosynthesis as their primary source of energy and carbon compounds.
The present atmospheric concentration of CO2 is the highest for 14 million years.[86] Concentrations of CO2 in the atmosphere were as high as 4,000 ppm during the Cambrian period about 500 million years ago, when the concentration was 20 times greater than today, and as low as 180 ppm during the Quaternary glaciation of the last two million years.[75] Reconstructed temperature records for the last 420 million years indicate that atmospheric CO2 concentrations peaked at ~2,000 ppm during the Devonian (~400 Ma) period, and again in the Triassic (220–200 Ma) period and was four times current levels during the Jurassic period (201-145 Ma).[87][88]
Annual CO2 flows from anthropogenic sources (left) into Earth’s atmosphere, land, and ocean sinks (right) since the 1960s. Units in equivalent gigatonnes carbon per year.[89]
Oceans
Ocean acidification
Carbon dioxide dissolves in the ocean to form carbonic acid (H2CO3), bicarbonate (HCO3−) and carbonate (CO32−). There is about fifty times as much carbon dioxide dissolved in the oceans as exists in the atmosphere. The oceans act as an enormous carbon sink, and have taken up about a third of CO2 emitted by human activity.[90]
Pterapod shell dissolved in seawater adjusted to an ocean chemistry projected for the year 2100
Changes in ocean chemistry can have extensive direct and indirect effects on organisms and their habitats. One of the most important repercussions of increasing ocean acidity relates to the production of shells out of calcium carbonate (CaCO
3).[92] This process is called calcification and is important to the biology and survival of a wide range of marine organisms. Calcification involves the precipitation of dissolved ions into solid CaCO
3 structures, structures for many marine organisms, such as coccolithophores, foraminifera, crustaceans, mollusks, etc. After they are formed, these CaCO
3 structures are vulnerable to dissolution unless the surrounding seawater contains saturating concentrations of carbonate ions (CO32−).
Given the current pH of the ocean (around 8.14), of the extra carbon dioxide added into the ocean, very little remains as dissolved carbon dioxide. The majority dissociates into additional bicarbonate and free hydrogen ions. The increase in hydrogen is larger than the increase in bicarbonate,[93] creating an imbalance in the reaction HCO3− ⇌ CO32− + H+. To maintain chemical equilibrium, some of the carbonate ions already in the ocean combine with some of the hydrogen ions to make further bicarbonate. Thus the ocean’s concentration of carbonate ions is reduced, removing an essential building block for marine organisms to build shells, or calcify: Ca2+ + CO32− ⇌ CaCO3.
Hydrothermal vents
Carbon dioxide is also introduced into the oceans through hydrothermal vents. The Champagne hydrothermal vent, found at the Northwest Eifuku volcano in the Mariana Trench, produces almost pure liquid carbon dioxide, one of only two known sites in the world as of 2004, the other being in the Okinawa Trough.[94] The finding of a submarine lake of liquid carbon dioxide in the Okinawa Trough was reported in 2006.[95]
Production
Biological processes
Carbon dioxide is a by-product of the fermentation of sugar in the brewing of beer, whisky and other alcoholic beverages and in the production of bioethanol. Yeast metabolizes sugar to produce CO2 and ethanol, also known as alcohol, as follows:
All aerobic organisms produce CO2 when they oxidize carbohydrates, fatty acids, and proteins. The large number of reactions involved are exceedingly complex and not described easily. Refer to (cellular respiration, anaerobic respiration and photosynthesis). The equation for the respiration of glucose and other monosaccharides is:
Anaerobic organisms decompose organic material producing methane and carbon dioxide together with traces of other compounds.[96] Regardless of the type of organic material, the production of gases follows well defined kinetic pattern. Carbon dioxide comprises about 40–45% of the gas that emanates from decomposition in landfills (termed «landfill gas»). Most of the remaining 50–55% is methane.[97]
Industrial processes
Carbon dioxide can be obtained by distillation from air, but the method is inefficient. Industrially, carbon dioxide is predominantly an unrecovered waste product, produced by several methods which may be practiced at various scales.[98]
Combustion
The combustion of all carbon-based fuels, such as methane (natural gas), petroleum distillates (gasoline, diesel, kerosene, propane), coal, wood and generic organic matter produces carbon dioxide and, except in the case of pure carbon, water. As an example, the chemical reaction between methane and oxygen:
Iron is reduced from its oxides with coke in a blast furnace, producing pig iron and carbon dioxide:[99]
By-product from hydrogen production
Carbon dioxide is a byproduct of the industrial production of hydrogen by steam reforming and the water gas shift reaction in ammonia production. These processes begin with the reaction of water and natural gas (mainly methane).[100] This is a major source of food-grade carbon dioxide for use in carbonation of beer and soft drinks, and is also used for stunning animals such as poultry. In the summer of 2018 a shortage of carbon dioxide for these purposes arose in Europe due to the temporary shut-down of several ammonia plants for maintenance.[101]
Thermal decomposition of limestone
It is produced by thermal decomposition of limestone, CaCO3 by heating (calcining) at about 850 °C (1,560 °F), in the manufacture of quicklime (calcium oxide, CaO), a compound that has many industrial uses:
Acids liberate CO2 from most metal carbonates. Consequently, it may be obtained directly from natural carbon dioxide springs, where it is produced by the action of acidified water on limestone or dolomite. The reaction between hydrochloric acid and calcium carbonate (limestone or chalk) is shown below:
The carbonic acid (H2CO3) then decomposes to water and CO2:
Such reactions are accompanied by foaming or bubbling, or both, as the gas is released. They have widespread uses in industry because they can be used to neutralize waste acid streams.
Commercial uses
Carbon dioxide is used by the food industry, the oil industry, and the chemical industry.[98]
The compound has varied commercial uses but one of its greatest uses as a chemical is in the production of carbonated beverages; it provides the sparkle in carbonated beverages such as soda water, beer and sparkling wine.
Precursor to chemicals
|
This section needs expansion. You can help by adding to it. (July 2014) |
In the chemical industry, carbon dioxide is mainly consumed as an ingredient in the production of urea, with a smaller fraction being used to produce methanol and a range of other products.[102] Some carboxylic acid derivatives such as sodium salicylate are prepared using CO2 by the Kolbe–Schmitt reaction.[103]
In addition to conventional processes using CO2 for chemical production, electrochemical methods are also being explored at a research level. In particular, the use of renewable energy for production of fuels from CO2 (such as methanol) is attractive as this could result in fuels that could be easily transported and used within conventional combustion technologies but have no net CO2 emissions.[104]
Agriculture
Plants require carbon dioxide to conduct photosynthesis. The atmospheres of greenhouses may (if of large size, must) be enriched with additional CO2 to sustain and increase the rate of plant growth.[105][106] At very high concentrations (100 times atmospheric concentration, or greater), carbon dioxide can be toxic to animal life, so raising the concentration to 10,000 ppm (1%) or higher for several hours will eliminate pests such as whiteflies and spider mites in a greenhouse.[107]
Foods
Carbon dioxide bubbles in a soft drink
Carbon dioxide is a food additive used as a propellant and acidity regulator in the food industry. It is approved for usage in the EU[108] (listed as E number E290), US[109] and Australia and New Zealand[110] (listed by its INS number 290).
A candy called Pop Rocks is pressurized with carbon dioxide gas[111] at about 4,000 kPa (40 bar; 580 psi). When placed in the mouth, it dissolves (just like other hard candy) and releases the gas bubbles with an audible pop.
Leavening agents cause dough to rise by producing carbon dioxide.[112] Baker’s yeast produces carbon dioxide by fermentation of sugars within the dough, while chemical leaveners such as baking powder and baking soda release carbon dioxide when heated or if exposed to acids.
Beverages
Carbon dioxide is used to produce carbonated soft drinks and soda water. Traditionally, the carbonation of beer and sparkling wine came about through natural fermentation, but many manufacturers carbonate these drinks with carbon dioxide recovered from the fermentation process. In the case of bottled and kegged beer, the most common method used is carbonation with recycled carbon dioxide. With the exception of British real ale, draught beer is usually transferred from kegs in a cold room or cellar to dispensing taps on the bar using pressurized carbon dioxide, sometimes mixed with nitrogen.
The taste of soda water (and related taste sensations in other carbonated beverages) is an effect of the dissolved carbon dioxide rather than the bursting bubbles of the gas. Carbonic anhydrase 4 converts to carbonic acid leading to a sour taste, and also the dissolved carbon dioxide induces a somatosensory response.[113]
Winemaking
Dry ice used to preserve grapes after harvest
Carbon dioxide in the form of dry ice is often used during the cold soak phase in winemaking to cool clusters of grapes quickly after picking to help prevent spontaneous fermentation by wild yeast. The main advantage of using dry ice over water ice is that it cools the grapes without adding any additional water that might decrease the sugar concentration in the grape must, and thus the alcohol concentration in the finished wine. Carbon dioxide is also used to create a hypoxic environment for carbonic maceration, the process used to produce Beaujolais wine.
Carbon dioxide is sometimes used to top up wine bottles or other storage vessels such as barrels to prevent oxidation, though it has the problem that it can dissolve into the wine, making a previously still wine slightly fizzy. For this reason, other gases such as nitrogen or argon are preferred for this process by professional wine makers.
Stunning animals
Carbon dioxide is often used to «stun» animals before slaughter.[114] «Stunning» may be a misnomer, as the animals are not knocked out immediately and may suffer distress.[115][116]
Inert gas
Carbon dioxide is one of the most commonly used compressed gases for pneumatic (pressurized gas) systems in portable pressure tools. Carbon dioxide is also used as an atmosphere for welding, although in the welding arc, it reacts to oxidize most metals. Use in the automotive industry is common despite significant evidence that welds made in carbon dioxide are more brittle than those made in more inert atmospheres.[citation needed] When used for MIG welding, CO2 use is sometimes referred to as MAG welding, for Metal Active Gas, as CO2 can react at these high temperatures. It tends to produce a hotter puddle than truly inert atmospheres, improving the flow characteristics. Although, this may be due to atmospheric reactions occurring at the puddle site. This is usually the opposite of the desired effect when welding, as it tends to embrittle the site, but may not be a problem for general mild steel welding, where ultimate ductility is not a major concern.
Carbon dioxide is used in many consumer products that require pressurized gas because it is inexpensive and nonflammable, and because it undergoes a phase transition from gas to liquid at room temperature at an attainable pressure of approximately 60 bar (870 psi; 59 atm), allowing far more carbon dioxide to fit in a given container than otherwise would. Life jackets often contain canisters of pressured carbon dioxide for quick inflation. Aluminium capsules of CO2 are also sold as supplies of compressed gas for air guns, paintball markers/guns, inflating bicycle tires, and for making carbonated water. High concentrations of carbon dioxide can also be used to kill pests. Liquid carbon dioxide is used in supercritical drying of some food products and technological materials, in the preparation of specimens for scanning electron microscopy[117] and in the decaffeination of coffee beans.
Fire extinguisher
Use of a CO2 fire extinguisher
Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for electrical fires, contain liquid carbon dioxide under pressure. Carbon dioxide extinguishers work well on small flammable liquid and electrical fires, but not on ordinary combustible fires, because they do not cool the burning substances significantly, and when the carbon dioxide disperses, they can catch fire upon exposure to atmospheric oxygen. They are mainly used in server rooms.[118]
Carbon dioxide has also been widely used as an extinguishing agent in fixed fire-protection systems for local application of specific hazards and total flooding of a protected space.[119] International Maritime Organization standards recognize carbon-dioxide systems for fire protection of ship holds and engine rooms. Carbon-dioxide-based fire-protection systems have been linked to several deaths, because it can cause suffocation in sufficiently high concentrations. A review of CO2 systems identified 51 incidents between 1975 and the date of the report (2000), causing 72 deaths and 145 injuries.[120]
Supercritical CO2 as solvent
Liquid carbon dioxide is a good solvent for many lipophilic organic compounds and is used to remove caffeine from coffee.[17] Carbon dioxide has attracted attention in the pharmaceutical and other chemical processing industries as a less toxic alternative to more traditional solvents such as organochlorides. It is also used by some dry cleaners for this reason. It is used in the preparation of some aerogels because of the properties of supercritical carbon dioxide.
Medical and pharmacological uses
In medicine, up to 5% carbon dioxide (130 times atmospheric concentration) is added to oxygen for stimulation of breathing after apnea and to stabilize the O2/CO2 balance in blood.
Carbon dioxide can be mixed with up to 50% oxygen, forming an inhalable gas; this is known as Carbogen and has a variety of medical and research uses.
Another medical use are the mofette, dry spas that use carbon dioxide from post-volcanic discharge for therapeutic purposes.
Energy
Supercritical CO2 is used as the working fluid in the Allam power cycle engine.
Fossil fuel recovery
Carbon dioxide is used in enhanced oil recovery where it is injected into or adjacent to producing oil wells, usually under supercritical conditions, when it becomes miscible with the oil. This approach can increase original oil recovery by reducing residual oil saturation by 7–23% additional to primary extraction.[121] It acts as both a pressurizing agent and, when dissolved into the underground crude oil, significantly reduces its viscosity, and changing surface chemistry enabling the oil to flow more rapidly through the reservoir to the removal well.[122] In mature oil fields, extensive pipe networks are used to carry the carbon dioxide to the injection points.
In enhanced coal bed methane recovery, carbon dioxide would be pumped into the coal seam to displace methane, as opposed to current methods which primarily rely on the removal of water (to reduce pressure) to make the coal seam release its trapped methane.[123]
Bio transformation into fuel
It has been proposed that CO2 from power generation be bubbled into ponds to stimulate growth of algae that could then be converted into biodiesel fuel.[124] A strain of the cyanobacterium Synechococcus elongatus has been genetically engineered to produce the fuels isobutyraldehyde and isobutanol from CO2 using photosynthesis.[125]
Researchers have developed a process called electrolysis, using enzymes isolated from bacteria to power the chemical reactions which convert CO2 into fuels.[126][127][128]
Refrigerant
Comparison of the pressure–temperature phase diagrams of carbon dioxide (red) and water (blue) as a log-lin chart with phase transitions points at 1 atmosphere
Liquid and solid carbon dioxide are important refrigerants, especially in the food industry, where they are employed during the transportation and storage of ice cream and other frozen foods. Solid carbon dioxide is called «dry ice» and is used for small shipments where refrigeration equipment is not practical. Solid carbon dioxide is always below −78.5 °C (−109.3 °F) at regular atmospheric pressure, regardless of the air temperature.
Liquid carbon dioxide (industry nomenclature R744 or R-744) was used as a refrigerant prior to the use[citation needed] of dichlorodifluoromethane (R12, a chlorofluorocarbon (CFC) compound). CO2 might enjoy a renaissance because one of the main substitutes to CFCs, 1,1,1,2-tetrafluoroethane (R134a, a hydrofluorocarbon (HFC) compound) contributes to climate change more than CO2 does. CO2 physical properties are highly favorable for cooling, refrigeration, and heating purposes, having a high volumetric cooling capacity. Due to the need to operate at pressures of up to 130 bars (1,900 psi; 13,000 kPa), CO2 systems require highly mechanically resistant reservoirs and components that have already been developed for mass production in many sectors. In automobile air conditioning, in more than 90% of all driving conditions for latitudes higher than 50°, CO2 (R744) operates more efficiently than systems using HFCs (e.g., R134a). Its environmental advantages (GWP of 1, non-ozone depleting, non-toxic, non-flammable) could make it the future working fluid to replace current HFCs in cars, supermarkets, and heat pump water heaters, among others. Coca-Cola has fielded CO2-based beverage coolers and the U.S. Army is interested in CO2 refrigeration and heating technology.[129][130]
Minor uses
Carbon dioxide is the lasing medium in a carbon-dioxide laser, which is one of the earliest type of lasers.
Carbon dioxide can be used as a means of controlling the pH of swimming pools,[131] by continuously adding gas to the water, thus keeping the pH from rising. Among the advantages of this is the avoidance of handling (more hazardous) acids. Similarly, it is also used in the maintaining reef aquaria, where it is commonly used in calcium reactors to temporarily lower the pH of water being passed over calcium carbonate in order to allow the calcium carbonate to dissolve into the water more freely, where it is used by some corals to build their skeleton.
Used as the primary coolant in the British advanced gas-cooled reactor for nuclear power generation.
Carbon dioxide induction is commonly used for the euthanasia of laboratory research animals. Methods to administer CO2 include placing animals directly into a closed, prefilled chamber containing CO2, or exposure to a gradually increasing concentration of CO2. The American Veterinary Medical Association’s 2020 guidelines for carbon dioxide induction state that a displacement rate of 30–70% of the chamber or cage volume per minute is optimal for the humane euthanasia of small rodents.[132]: 5, 31 Percentages of CO2 vary for different species, based on identified optimal percentages to minimize distress.[132]: 22
Carbon dioxide is also used in several related cleaning and surface-preparation techniques.
History of discovery
Carbon dioxide was the first gas to be described as a discrete substance. In about 1640,[133] the Flemish chemist Jan Baptist van Helmont observed that when he burned charcoal in a closed vessel, the mass of the resulting ash was much less than that of the original charcoal. His interpretation was that the rest of the charcoal had been transmuted into an invisible substance he termed a «gas» or «wild spirit» (spiritus sylvestris).[134]
The properties of carbon dioxide were further studied in the 1750s by the Scottish physician Joseph Black. He found that limestone (calcium carbonate) could be heated or treated with acids to yield a gas he called «fixed air». He observed that the fixed air was denser than air and supported neither flame nor animal life. Black also found that when bubbled through limewater (a saturated aqueous solution of calcium hydroxide), it would precipitate calcium carbonate. He used this phenomenon to illustrate that carbon dioxide is produced by animal respiration and microbial fermentation. In 1772, English chemist Joseph Priestley published a paper entitled Impregnating Water with Fixed Air in which he described a process of dripping sulfuric acid (or oil of vitriol as Priestley knew it) on chalk in order to produce carbon dioxide, and forcing the gas to dissolve by agitating a bowl of water in contact with the gas.[135]
Carbon dioxide was first liquefied (at elevated pressures) in 1823 by Humphry Davy and Michael Faraday.[136] The earliest description of solid carbon dioxide (dry ice) was given by the French inventor Adrien-Jean-Pierre Thilorier, who in 1835 opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by the rapid evaporation of the liquid yielded a «snow» of solid CO2.[137][138]
See also
- Arterial blood gas
- Bosch reaction
- Carbon dioxide removal – Removal of atmospheric carbon dioxide (from the atmosphere)
- List of least carbon efficient power stations
- List of countries by carbon dioxide emissions
- Meromictic lake – Permanently stratified lake with layers of water that do not intermix
- Gilbert Plass – Canadian physicist (early work on CO2 and climate change)
- Sabatier reaction – Methanation process of carbon dioxide with hydrogen
- NASA’s Orbiting Carbon Observatory 2
- Greenhouse Gases Observing Satellite – Earth observation satellite
- Soil gas
References
- ^ a b c «Carbon Dioxide» (PDF). Air Products. Archived from the original (PDF) on 29 July 2020. Retrieved 28 April 2017.
- ^ a b c d e f g h i Span R, Wagner W (1 November 1996). «A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple‐Point Temperature to 1100 K at Pressures up to 800 MPa». Journal of Physical and Chemical Reference Data. 25 (6): 1519. Bibcode:1996JPCRD..25.1509S. doi:10.1063/1.555991.
- ^ Touloukian YS, Liley PE, Saxena SC (1970). «Thermophysical properties of matter — the TPRC data series». Thermal Conductivity — Nonmetallic Liquids and Gases. Data book. 3.
- ^ Schäfer M, Richter M, Span R (2015). «Measurements of the viscosity of carbon dioxide at temperatures from (253.15 to 473.15) K with pressures up to 1.2 MPa». The Journal of Chemical Thermodynamics. 89: 7–15. doi:10.1016/j.jct.2015.04.015.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0103». National Institute for Occupational Safety and Health (NIOSH).
- ^ «Carbon dioxide». Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ «Safety Data Sheet – Carbon Dioxide Gas – version 0.03 11/11» (PDF). AirGas.com. 12 February 2018. Archived (PDF) from the original on 4 August 2018. Retrieved 4 August 2018.
- ^ «Carbon dioxide, refrigerated liquid» (PDF). Praxair. p. 9. Archived from the original (PDF) on 29 July 2018. Retrieved 26 July 2018.
- ^ Eggleton T (2013). A Short Introduction to Climate Change. Cambridge University Press. p. 52. ISBN 9781107618763. Archived from the original on 23 July 2021. Retrieved 9 November 2020.
- ^ «Carbon dioxide now more than 50% higher than pre-industrial levels | National Oceanic and Atmospheric Administration». www.noaa.gov. Retrieved 14 June 2022.
- ^ IPCC (2022) Summary for policy makers in Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
- ^ Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean. Washington, DC: National Academies Press. 22 April 2010. pp. 23–24. doi:10.17226/12904. ISBN 978-0-309-15359-1. Archived from the original on 5 February 2016. Retrieved 29 February 2016.
- ^ Kaufman DG, Franz CM (1996). Biosphere 2000: protecting our global environment. Kendall/Hunt Pub. Co. ISBN 978-0-7872-0460-0.
- ^ «Food Factories». www.legacyproject.org. Archived from the original on 12 August 2017. Retrieved 10 October 2011.
- ^ IPCC (2021). «Summary for Policymakers» (PDF). Climate Change 2021: The Physical Science Basis. p. 20. Archived (PDF) from the original on 10 October 2022.
- ^ Myles, Allen (September 2020). «The Oxford Principles for Net Zero Aligned Carbon Offsetting» (PDF). Archived (PDF) from the original on 2 October 2020. Retrieved 10 December 2021.
- ^ a b Tsotsas E, Mujumdar AS (2011). Modern drying technology. Vol. 3: Product quality and formulation. John Wiley & Sons. ISBN 978-3-527-31558-1. Archived from the original on 21 March 2020. Retrieved 3 December 2019.
- ^ Spritzler F (3 November 2019). «Carbonated (Sparkling) Water: Good or Bad?». healthline.com. Archived from the original on 10 May 2020.
- ^ a b Greenwood NN, Earnshaw A (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 305–314. ISBN 978-0-08-037941-8.
- ^ Atkins P, de Paula J (2006). Physical Chemistry (8th ed.). W.H. Freeman. pp. 461, 464. ISBN 978-0-7167-8759-4.
- ^ Siegmann B, Werner U, Lutz HO, Mann R (2002). «Complete Coulomb fragmentation of CO2 in collisions with 5.9 MeV u−1 Xe18+ and Xe43+«. J Phys B Atom Mol Opt Phys. 35 (17): 3755. Bibcode:2002JPhB…35.3755S. doi:10.1088/0953-4075/35/17/311. S2CID 250782825.
- ^ a b
Jensen P, Spanner M, Bunker PR (2020). «The CO2 molecule is never linear−». J Mol Struct. 1212: 128087. Bibcode:2020JMoSt121228087J. doi:10.1016/j.molstruc.2020.128087. hdl:2142/107329. S2CID 216318907. - ^ Jolly WL (1984). Modern Inorganic Chemistry. McGraw-Hill. p. 196. ISBN 978-0-07-032760-3.
- ^ Li Z, Mayer RJ, Ofial AR, Mayr H (May 2020). «From Carbodiimides to Carbon Dioxide: Quantification of the Electrophilic Reactivities of Heteroallenes». Journal of the American Chemical Society. 142 (18): 8383–8402. doi:10.1021/jacs.0c01960. PMID 32338511. S2CID 216557447.
- ^ Aresta M, ed. (2010). Carbon Dioxide as a Chemical Feedstock. Weinheim: Wiley-VCH. ISBN 978-3-527-32475-0.
- ^ Finn C, Schnittger S, Yellowlees LJ, Love JB (February 2012). «Molecular approaches to the electrochemical reduction of carbon dioxide» (PDF). Chemical Communications. 48 (10): 1392–1399. doi:10.1039/c1cc15393e. hdl:20.500.11820/b530915d-451c-493c-8251-da2ea2f50912. PMID 22116300. S2CID 14356014. Archived (PDF) from the original on 19 April 2021. Retrieved 6 December 2019.
- ^ «Gases – Densities». Engineering Toolbox. Archived from the original on 2 March 2006. Retrieved 21 November 2020.
- ^ Santoro M, Gorelli FA, Bini R, Ruocco G, Scandolo S, Crichton WA (June 2006). «Amorphous silica-like carbon dioxide». Nature. 441 (7095): 857–860. Bibcode:2006Natur.441..857S. doi:10.1038/nature04879. PMID 16778885. S2CID 4363092.
- ^ a b Holman, Jack P. (2002). Heat Transfer (9th ed.). New York, NY: McGraw-Hill Companies, Inc. pp. 600–606. ISBN 9780072406559.
- ^ a b Incropera 1 Dewitt 2 Bergman 3 Lavigne 4, Frank P. 1 David P. 2 Theodore L. 3 Adrienne S. 4 (2007). Fundamentals of Heat and Mass Transfer (6th ed.). Hoboken, NJ: John Wiley and Sons, Inc. pp. 941–950. ISBN 9780471457282.
- ^ Dhingra A, Portis AR, Daniell H (April 2004). «Enhanced translation of a chloroplast-expressed RbcS gene restores small subunit levels and photosynthesis in nuclear RbcS antisense plants». Proceedings of the National Academy of Sciences of the United States of America. 101 (16): 6315–6320. Bibcode:2004PNAS..101.6315D. doi:10.1073/pnas.0400981101. PMC 395966. PMID 15067115.
(Rubisco) is the most prevalent enzyme on this planet, accounting for 30–50% of total soluble protein in the chloroplast
- ^ Falkowski P, Knoll AH (1 January 2007). Evolution of primary producers in the sea. Elsevier, Academic Press. ISBN 978-0-12-370518-1. OCLC 845654016.
- ^ Blom TJ, Straver WA, Ingratta FJ, Khosla S, Brown W (December 2002). «Carbon Dioxide In Greenhouses». Archived from the original on 29 April 2019. Retrieved 12 June 2007.
- ^ Ainsworth EA (2008). «Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration» (PDF). Global Change Biology. 14 (7): 1642–1650. Bibcode:2008GCBio..14.1642A. doi:10.1111/j.1365-2486.2008.01594.x. S2CID 19200429. Archived from the original (PDF) on 19 July 2011.
- ^ Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR (June 2006). «Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations» (PDF). Science. 312 (5782): 1918–1921. Bibcode:2006Sci…312.1918L. CiteSeerX 10.1.1.542.5784. doi:10.1126/science.1114722. PMID 16809532. S2CID 2232629. Archived (PDF) from the original on 20 October 2016. Retrieved 27 October 2017.
- ^ Woodward F, Kelly C (1995). «The influence of CO2 concentration on stomatal density». New Phytologist. 131 (3): 311–327. doi:10.1111/j.1469-8137.1995.tb03067.x.
- ^ Drake BG, Gonzalez-Meler MA, Long SP (June 1997). «MORE EFFICIENT PLANTS: A Consequence of Rising Atmospheric CO2?». Annual Review of Plant Physiology and Plant Molecular Biology. 48 (1): 609–639. doi:10.1146/annurev.arplant.48.1.609. PMID 15012276. S2CID 33415877.
- ^ Loladze I (2002). «Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry?». Trends in Ecology & Evolution. 17 (10): 457–461. doi:10.1016/S0169-5347(02)02587-9. S2CID 16074723.
- ^ Coviella CE, Trumble JT (1999). «Effects of Elevated Atmospheric Carbon Dioxide on Insect-Plant Interactions». Conservation Biology. 13 (4): 700–712. doi:10.1046/j.1523-1739.1999.98267.x. JSTOR 2641685. S2CID 52262618.
- ^ Davey MP, Harmens H, Ashenden TW, Edwards R, Baxter R (2007). «Species-specific effects of elevated CO2 on resource allocation in Plantago maritima and Armeria maritima«. Biochemical Systematics and Ecology. 35 (3): 121–129. doi:10.1016/j.bse.2006.09.004.
- ^ Davey MP, Bryant DN, Cummins I, Ashenden TW, Gates P, Baxter R, Edwards R (August 2004). «Effects of elevated CO2 on the vasculature and phenolic secondary metabolism of Plantago maritima». Phytochemistry. 65 (15): 2197–2204. doi:10.1016/j.phytochem.2004.06.016. PMID 15587703.
- ^ «Global Environment Division Greenhouse Gas Assessment Handbook – A Practical Guidance Document for the Assessment of Project-level Greenhouse Gas Emissions». World Bank. Archived from the original on 3 June 2016. Retrieved 10 November 2007.
- ^ Luyssaert S, Schulze ED, Börner A, Knohl A, Hessenmöller D, Law BE, et al. (September 2008). «Old-growth forests as global carbon sinks» (PDF). Nature. 455 (7210): 213–215. Bibcode:2008Natur.455..213L. doi:10.1038/nature07276. PMID 18784722. S2CID 4424430.
- ^ Falkowski P, Scholes RJ, Boyle E, Canadell J, Canfield D, Elser J, et al. (October 2000). «The global carbon cycle: a test of our knowledge of earth as a system». Science. 290 (5490): 291–296. Bibcode:2000Sci…290..291F. doi:10.1126/science.290.5490.291. PMID 11030643. S2CID 1779934.
- ^ a b Friedman D. «Toxicity of Carbon Dioxide Gas Exposure, CO2 Poisoning Symptoms, Carbon Dioxide Exposure Limits, and Links to Toxic Gas Testing Procedures». InspectAPedia. Archived from the original on 28 September 2009.
- ^ «CarbonTracker CT2011_oi (Graphical map of CO2)». esrl.noaa.gov. Archived from the original on 13 February 2021. Retrieved 20 April 2007.
- ^ «Carbon Dioxide as a Fire Suppressant: Examining the Risks». U.S. Environmental Protection Agency. Archived from the original on 2 October 2015.
- ^ «Volcano Under the City». A NOVA Production by Bonne Pioche and Greenspace for WGBH/Boston. Public Broadcasting System. 1 November 2005. Archived from the original on 5 April 2011..
- ^ Glatte Jr HA, Motsay GJ, Welch BE (1967). Carbon Dioxide Tolerance Studies (Report). Brooks AFB, TX School of Aerospace Medicine Technical Report. SAM-TR-67-77. Archived from the original on 9 May 2008. Retrieved 2 May 2008.
{{cite report}}: CS1 maint: unfit URL (link) - ^ Lambertsen CJ (1971). Carbon Dioxide Tolerance and Toxicity (Report). IFEM Report. Environmental Biomedical Stress Data Center, Institute for Environmental Medicine, University of Pennsylvania Medical Center. No. 2-71. Archived from the original on 24 July 2011. Retrieved 2 May 2008.
{{cite report}}: CS1 maint: unfit URL (link) - ^ a b Satish U, Mendell MJ, Shekhar K, Hotchi T, Sullivan D, Streufert S, Fisk WJ (December 2012). «Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance» (PDF). Environmental Health Perspectives. 120 (12): 1671–1677. doi:10.1289/ehp.1104789. PMC 3548274. PMID 23008272. Archived from the original (PDF) on 5 March 2016. Retrieved 11 December 2014.
- ^ a b Allen JG, MacNaughton P, Satish U, Santanam S, Vallarino J, Spengler JD (June 2016). «Associations of Cognitive Function Scores with Carbon Dioxide, Ventilation, and Volatile Organic Compound Exposures in Office Workers: A Controlled Exposure Study of Green and Conventional Office Environments». Environmental Health Perspectives. 124 (6): 805–812. doi:10.1289/ehp.1510037. PMC 4892924. PMID 26502459.
- ^ «Exposure Limits for Carbon Dioxide Gas – CO2 Limits». InspectAPedia.com. Archived from the original on 16 September 2018. Retrieved 19 October 2014.
- ^ Law J, Watkins S, Alexander D (2010). In-Flight Carbon Dioxide Exposures and Related Symptoms: Associations, Susceptibility and Operational Implications (PDF) (Report). NASA Technical Report. TP–2010–216126. Archived from the original (PDF) on 27 June 2011. Retrieved 26 August 2014.
- ^ Schaefer KE, Douglas WH, Messier AA, Shea ML, Gohman PA (1979). «Effect of prolonged exposure to 0.5% CO2 on kidney calcification and ultrastructure of lungs». Undersea Biomedical Research. 6 (Suppl): S155–S161. PMID 505623. Archived from the original on 19 October 2014. Retrieved 19 October 2014.
- ^ Du B, Tandoc MC, Mack ML, Siegel JA (November 2020). «Indoor CO2 concentrations and cognitive function: A critical review». Indoor Air. 30 (6): 1067–1082. doi:10.1111/ina.12706. PMID 32557862. S2CID 219915861.
- ^ Kaplan L (4 June 2019). «Ask the doc: Does my helmet make me stupid? — RevZilla». www.revzilla.com. Archived from the original on 22 May 2021. Retrieved 22 May 2021.
- ^ Brühwiler PA, Stämpfli R, Huber R, Camenzind M (September 2005). «CO2 and O2 concentrations in integral motorcycle helmets». Applied Ergonomics. 36 (5): 625–633. doi:10.1016/j.apergo.2005.01.018. PMID 15893291.
- ^ Allen JG, MacNaughton P, Satish U, Santanam S, Vallarino J, Spengler JD (June 2016). «Associations of Cognitive Function Scores with Carbon Dioxide, Ventilation, and Volatile Organic Compound Exposures in Office Workers: A Controlled Exposure Study of Green and Conventional Office Environments». Environmental Health Perspectives. 124 (6): 805–812. doi:10.1289/ehp.1510037. PMC 4892924. PMID 26502459.
- ^ Romm J (26 October 2015). «Exclusive: Elevated CO2 Levels Directly Affect Human Cognition, New Harvard Study Shows». ThinkProgress. Archived from the original on 9 October 2019. Retrieved 14 October 2019.
- ^ «Three die in dry-ice incident at Moscow pool party». BBC News. 29 February 2020. Archived from the original on 29 February 2020.
The victims were connected to Instagram influencer Yekaterina Didenko.
- ^ Rettner R (2 August 2018). «A Woman Died from Dry Ice Fumes. Here’s How It Can Happen». livescience.com. Archived from the original on 22 May 2021. Retrieved 22 May 2021.
- ^ van Gardingen PR, Grace J, Jeffree CE, Byari SH, Miglietta F, Raschi A, Bettarini I (1997). «Long-term effects of enhanced CO2 concentrations on leaf gas exchange: research opportunities using CO2 springs». In Raschi A, Miglietta F, Tognetti R, van Gardingen PR (eds.). Plant responses to elevated CO2: Evidence from natural springs. Cambridge: Cambridge University Press. pp. 69–86. ISBN 978-0-521-58203-2.
- ^ Martini M (1997). «CO2 emissions in volcanic areas: case histories and hazards». In Raschi A, Miglietta F, Tognetti R, van Gardingen PR (eds.). Plant responses to elevated CO2: Evidence from natural springs. Cambridge: Cambridge University Press. pp. 69–86. ISBN 978-0-521-58203-2.
- ^ a b c d «ABG (Arterial Blood Gas)». Brookside Associates. Archived from the original on 12 August 2017. Retrieved 2 January 2017.
- ^ «How much carbon dioxide do humans contribute through breathing?». EPA.gov. Archived from the original on 2 February 2011. Retrieved 30 April 2009.
- ^ Henrickson C (2005). Chemistry. Cliffs Notes. ISBN 978-0-7645-7419-1.
- ^ a b c d «Carbon dioxide». solarnavigator.net. Archived from the original on 14 September 2008. Retrieved 12 October 2007.
- ^ Battisti-Charbonney, A.; Fisher, J.; Duffin, J. (15 June 2011). «The cerebrovascular response to carbon dioxide in humans». J. Physiol. 589 (12): 3039–3048. doi:10.1113/jphysiol.2011.206052. PMC 3139085. PMID 21521758.
- ^ Patel, S.; Miao, J.H.; Yetiskul, E.; Anokhin, A.; Majmunder, S.H. (2022). «Physiology, Carbon Dioxide Retention». National Library of Medicine. National Center for Biotechnology Information, NIH. PMID 29494063. Retrieved 20 August 2022.
- ^ Wilmshurst, Peter (1998). «ABC of oxygen». BMJ. 317 (7164): 996–999. doi:10.1136/bmj.317.7164.996. PMC 1114047. PMID 9765173.
- ^ Showstack, Randy (2013). «Carbon dioxide tops 400 ppm at Mauna Loa, Hawaii». Eos, Transactions American Geophysical Union. 94 (21): 192. Bibcode:2013EOSTr..94Q.192S. doi:10.1002/2013eo210004. ISSN 0096-3941.
- ^ Montaigne, Fen. «Son of Climate Science Pioneer Ponders A Sobering Milestone». Yale Environment 360. Yale School of Forestry & Environmental Studies. Archived from the original on 8 June 2013. Retrieved 14 May 2013.
- ^ a b «Carbon dioxide now more than 50% higher than pre-industrial levels | National Oceanic and Atmospheric Administration». www.noaa.gov. Retrieved 14 June 2022.
- ^ a b Eggleton, Tony (2013). A Short Introduction to Climate Change. Cambridge University Press. p. 52. ISBN 9781107618763.
- ^ a b «The NOAA Annual Greenhouse Gas Index (AGGI) – An Introduction». NOAA Global Monitoring Laboratory/Earth System Research Laboratories. Archived from the original on 27 November 2020. Retrieved 18 December 2020.
- ^ Etheridge, D.M.; L.P. Steele; R.L. Langenfelds; R.J. Francey; J.-M. Barnola; V.I. Morgan (1996). «Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn». Journal of Geophysical Research. 101 (D2): 4115–28. Bibcode:1996JGR…101.4115E. doi:10.1029/95JD03410. ISSN 0148-0227.
- ^ IPCC (2022) Summary for policy makers in Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
- ^ Petty, G.W. (2004). «A First Course in Atmospheric Radiation». Eos Transactions. 85 (36): 229–51. Bibcode:2004EOSTr..85..341P. doi:10.1029/2004EO360007.
- ^ Atkins P, de Paula J (2006). Atkins’ Physical Chemistry (8th ed.). W. H. Freeman. p. 462. ISBN 978-0-7167-8759-4.
- ^ «Carbon Dioxide Absorbs and Re-emits Infrared Radiation». UCAR Center for Science Education. 2012. Archived from the original on 21 September 2017. Retrieved 9 September 2017.
- ^ Archer D (15 March 2005). «How long will global warming last?». RealClimate. Archived from the original on 4 March 2021. Retrieved 5 March 2021.
- ^ Archer D (2009). «Atmospheric lifetime of fossil fuel carbon dioxide». Annual Review of Earth and Planetary Sciences. 37 (1): 117–34. Bibcode:2009AREPS..37..117A. doi:10.1146/annurev.earth.031208.100206. hdl:2268/12933. Archived from the original on 24 February 2021. Retrieved 7 March 2021.
- ^ Joos F, Roth R, Fuglestvedt JS, Peters GP, Enting IG, Von Bloh W, et al. (2013). «Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis». Atmospheric Chemistry and Physics. 13 (5): 2793–2825. doi:10.5194/acpd-12-19799-2012. Archived from the original on 22 July 2020. Retrieved 7 March 2021.
- ^ «Figure 8.SM.4» (PDF). Intergovernmental Panel on Climate Change Fifth Assessment Report. p. 8SM-16. Archived (PDF) from the original on 24 March 2021. Retrieved 7 March 2021.
- ^ Zhang, Yi Ge; et al. (28 October 2013). «A 40-million-year history of atmospheric CO2«. Philosophical Transactions of the Royal Society A. 371 (2001): 20130096. Bibcode:2013RSPTA.37130096Z. doi:10.1098/rsta.2013.0096. PMID 24043869.
- ^ «Climate and CO2 in the Atmosphere». Archived from the original on 6 October 2018. Retrieved 10 October 2007.
- ^ Berner RA, Kothavala Z (2001). «GEOCARB III: A revised model of atmospheric CO2 over Phanerozoic Time» (PDF). American Journal of Science. 301 (2): 182–204. Bibcode:2001AmJS..301..182B. CiteSeerX 10.1.1.393.582. doi:10.2475/ajs.301.2.182. Archived (PDF) from the original on 4 September 2011. Retrieved 15 February 2008.
- ^ Friedlingstein P, Jones MW, O’sullivan M, Andrew RM, Hauck J, Peters GP, et al. (2019). «Global Carbon Budget 2019». Earth System Science Data. 11 (4): 1783–1838. Bibcode:2019ESSD…11.1783F. doi:10.5194/essd-11-1783-2019..
- ^ Doney SC, Levine NM (29 November 2006). «How Long Can the Ocean Slow Global Warming?». Oceanus. Archived from the original on 4 January 2008. Retrieved 21 November 2007.
- ^ Jacobson, M. Z. (2005). «Studying ocean acidification with conservative, stable numerical schemes for nonequilibrium air-ocean exchange and ocean equilibrium chemistry». Journal of Geophysical Research: Atmospheres. 110: D07302. Bibcode:2005JGRD..11007302J. doi:10.1029/2004JD005220.
- ^ a b «Ocean acidification due to increasing atmospheric carbon dioxide». The Royal Society.
- ^ Mitchell, Mark J.; Jensen, Oliver E.; Cliffe, K. Andrew; Maroto-Valer, M. Mercedes (8 May 2010). «A model of carbon dioxide dissolution and mineral carbonation kinetics». Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 466 (2117): 1265–1290. doi:10.1098/rspa.2009.0349.
- ^ Lupton J, Lilley M, Butterfield D, Evans L, Embley R, Olson E, et al. (2004). «Liquid Carbon Dioxide Venting at the Champagne Hydrothermal Site, NW Eifuku Volcano, Mariana Arc». American Geophysical Union. 2004 (Fall Meeting). V43F–08. Bibcode:2004AGUFM.V43F..08L.
- ^ Inagaki F, Kuypers MM, Tsunogai U, Ishibashi J, Nakamura K, Treude T, et al. (September 2006). «Microbial community in a sediment-hosted CO2 lake of the southern Okinawa Trough hydrothermal system». Proceedings of the National Academy of Sciences of the United States of America. 103 (38): 14164–14169. Bibcode:2006PNAS..10314164I. doi:10.1073/pnas.0606083103. PMC 1599929. PMID 16959888. Videos can be downloaded at «Supporting Information». Archived from the original on 19 October 2018.
- ^ «Collecting and using biogas from landfills». U.S. Energy Information Administration. 11 January 2017. Archived from the original on 11 July 2018. Retrieved 22 November 2015.
- ^ «Facts About Landfill Gas» (PDF). U.S. Environmental Protection Agency. January 2000. Archived (PDF) from the original on 23 September 2015. Retrieved 4 September 2015.
- ^ a b Pierantozzi R (2001). «Carbon Dioxide». Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. doi:10.1002/0471238961.0301180216090518.a01.pub2. ISBN 978-0-471-23896-6.
- ^
Strassburger J (1969). Blast Furnace Theory and Practice. New York: American Institute of Mining, Metallurgical, and Petroleum Engineers. ISBN 978-0-677-10420-1. - ^ Topham S (2000). «Carbon Dioxide». Ullmann’s Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a05_165. ISBN 3527306730.
- ^ «CO2 shortage: Food industry calls for government action». BBC. 21 June 2018. Archived from the original on 23 May 2021. Retrieved 24 June 2018.
- ^ «IPCC Special Report on Carbon dioxide Capture and Storage» (PDF). The Intergovernmental Panel on Climate Change. Archived from the original (PDF) on 24 September 2015. Retrieved 4 September 2015.
- ^ Morrison RT, Boyd RN (1983). Organic Chemistry (4th ed.). Allyn and Bacon. pp. 976–977. ISBN 978-0-205-05838-9.
- ^ Badwal SP, Giddey SS, Munnings C, Bhatt AI, Hollenkamp AF (24 September 2014). «Emerging electrochemical energy conversion and storage technologies». Frontiers in Chemistry. 2: 79. Bibcode:2014FrCh….2…79B. doi:10.3389/fchem.2014.00079. PMC 4174133. PMID 25309898.
- ^ Whiting D, Roll M, Vickerman L (August 2010). «Plant Growth Factors: Photosynthesis, Respiration, and Transpiration». CMG GardenNotes. Colorado Master Gardener Program. Archived from the original on 2 September 2014. Retrieved 10 October 2011.
- ^ Waggoner PE (February 1994). «Carbon dioxide». How Much Land Can Ten Billion People Spare for Nature?. Archived from the original on 12 October 2011. Retrieved 10 October 2011.
- ^ Stafford N (August 2007). «Future crops: the other greenhouse effect». Nature. 448 (7153): 526–528. Bibcode:2007Natur.448..526S. doi:10.1038/448526a. PMID 17671477. S2CID 9845813.
- ^ UK Food Standards Agency: «Current EU approved additives and their E Numbers». Archived from the original on 7 October 2010. Retrieved 27 October 2011.
- ^ US Food and Drug Administration: «Food Additive Status List». Food and Drug Administration. Archived from the original on 4 November 2017. Retrieved 13 June 2015.
- ^ Australia New Zealand Food Standards Code«Standard 1.2.4 – Labelling of ingredients». Archived from the original on 19 January 2012. Retrieved 27 October 2011.
- ^ Futurific Leading Indicators Magazine. Vol. 1. CRAES LLC. ISBN 978-0-9847670-1-4. Archived from the original on 15 August 2021. Retrieved 9 November 2020.
- ^ Vijay GP (25 September 2015). Indian Breads: A Comprehensive Guide to Traditional and Innovative Indian Breads. Westland. ISBN 978-93-85724-46-6.
- ^ «Scientists Discover Protein Receptor For Carbonation Taste». ScienceDaily. 16 October 2009. Archived from the original on 29 March 2020. Retrieved 29 March 2020.
- ^ Coghlan A (3 February 2018). «A more humane way of slaughtering chickens might get EU approval». New Scientist. Archived from the original on 24 June 2018. Retrieved 24 June 2018.
- ^ «What is CO2 stunning?». RSPCA. Archived from the original on 9 April 2014.
- ^ Campbell A (10 March 2018). «Humane execution and the fear of the tumbril». New Scientist. Archived from the original on 24 June 2018. Retrieved 24 June 2018.
- ^ Nordestgaard BG, Rostgaard J (February 1985). «Critical-point drying versus freeze drying for scanning electron microscopy: a quantitative and qualitative study on isolated hepatocytes». Journal of Microscopy. 137 (Pt 2): 189–207. doi:10.1111/j.1365-2818.1985.tb02577.x. PMID 3989858. S2CID 32065173.
- ^ «Types of Fire Extinguishers». The Fire Safety Advice Centre. Archived from the original on 28 June 2021. Retrieved 28 June 2021.
- ^ National Fire Protection Association Code 12.
- ^ Carbon Dioxide as a Fire Suppressant: Examining the Risks, US EPA. 2000.
- ^ «Appendix A: CO2 for use in enhanced oil recovery (EOR)». Accelerating the uptake of CCS: industrial use of captured carbon dioxide. Global CCS Institute. 20 December 2011. Archived from the original on 28 April 2017. Retrieved 2 January 2017.
- ^ Austell JM (2005). «CO2 for Enhanced Oil Recovery Needs – Enhanced Fiscal Incentives». Exploration & Production: The Oil & Gas Review. Archived from the original on 7 February 2012. Retrieved 28 September 2007.
- ^ «Enhanced coal bed methane recovery». ETH Zurich. 31 August 2006. Archived from the original on 6 July 2011.
- ^ Clayton M (11 January 2006). «Algae – like a breath mint for smokestacks». The Christian Science Monitor. Archived from the original on 14 September 2008. Retrieved 11 October 2007.
- ^ Atsumi S, Higashide W, Liao JC (December 2009). «Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde». Nature Biotechnology. 27 (12): 1177–1180. doi:10.1038/nbt.1586. PMID 19915552. S2CID 1492698.
- ^ Cobb S, Badiani V, Dharani A, Wagner A, Zacarias S, Oliveira AR, et al. (28 February 2022). «Fast CO2 hydration kinetics impair heterogeneous but improve enzymatic CO2 reduction catalysis». Nature Chemistry. 14 (4): 417–424. Bibcode:2022NatCh..14..417C. doi:10.1038/s41557-021-00880-2. ISSN 1755-4349. PMC 7612589. PMID 35228690. S2CID 247160910.
- ^ Edwardes Moore E, Cobb SJ, Coito AM, Oliveira AR, Pereira IA, Reisner E (January 2022). «Understanding the local chemical environment of bioelectrocatalysis». Proceedings of the National Academy of Sciences of the United States of America. 119 (4): e2114097119. Bibcode:2022PNAS..11914097E. doi:10.1073/pnas.2114097119. PMC 8795565. PMID 35058361.
- ^ «Clean Way To Turn CO2 Into Fuel Inspired by Nature». Applied Sciences from Technology Networks. 1 March 2022. Retrieved 2 March 2022.
- ^ «The Coca-Cola Company Announces Adoption of HFC-Free Insulation in Refrigeration Units to Combat Global Warming». The Coca-Cola Company. 5 June 2006. Archived from the original on 1 November 2013. Retrieved 11 October 2007.
- ^ «Modine reinforces its CO2 research efforts». R744.com. 28 June 2007. Archived from the original on 10 February 2008.
- ^ TCE, the Chemical Engineer. Institution of Chemical Engineers. 1990. Archived from the original on 17 August 2021. Retrieved 2 June 2020.
- ^ a b «AVMA guidelines for the euthanasia of animals: 2020 Edition» (PDF). American Veterinary Medical Association. 2020. Archived (PDF) from the original on 1 February 2014. Retrieved 13 August 2021.
- ^ Harris D (September 1910). «The Pioneer in the Hygiene of Ventilation». The Lancet. 176 (4542): 906–908. doi:10.1016/S0140-6736(00)52420-9. Archived from the original on 17 March 2020. Retrieved 6 December 2019.
- ^ Almqvist E (2003). History of industrial gases. Springer. p. 93. ISBN 978-0-306-47277-0.
- ^ Priestley J, Hey W (1772). «Observations on Different Kinds of Air». Philosophical Transactions. 62: 147–264. doi:10.1098/rstl.1772.0021. S2CID 186210131. Archived from the original on 7 June 2010. Retrieved 11 October 2007.
- ^ Davy H (1823). «On the Application of Liquids Formed by the Condensation of Gases as Mechanical Agents». Philosophical Transactions. 113: 199–205. doi:10.1098/rstl.1823.0020. JSTOR 107649.
- ^ Thilorier AJ (1835). «Solidification de l’Acide carbonique». Comptes Rendus. 1: 194–196. Archived from the original on 2 September 2017. Retrieved 1 September 2017.
- ^ Thilorier AJ (1836). «Solidification of carbonic acid». The London and Edinburgh Philosophical Magazine. 8 (48): 446–447. doi:10.1080/14786443608648911. Archived from the original on 2 May 2016. Retrieved 15 November 2015.
External links
- Current global map of carbon dioxide concentration
- CDC – NIOSH Pocket Guide to Chemical Hazards – Carbon Dioxide
- Trends in Atmospheric Carbon Dioxide (NOAA)
На чтение 1 мин.
Значение слова «Углерод»
— химический элемент, важнейшая составная часть всех органических веществ в природе
Содержание
- Транскрипция слова
- MFA Международная транскрипция
- Цветовая схема слова
Транскрипция слова
[угл’иро́т]
MFA Международная транскрипция
[ʊɡlʲɪˈrot]
| у | [у] | гласный, безударный |
| г | [г] | согласный, звонкий парный, твердый парный |
| л | [л’] | согласный, звонкий непарный (сонорный), мягкий парный |
| е | [и] | гласный, безударный |
| р | [р] | согласный, звонкий непарный (сонорный), твердый парный |
| о | [́о] | гласный, ударный |
| д | [т] | согласный, глухой парный, твердый парный |
Букв: 7 Звуков: 7
Цветовая схема слова
углерод
Как произносится слово «Углерод»
Тег audio не поддерживается вашим браузером.
Как правильно пишется «Углерод»
углеро́д
углеро́д, -а
Как правильно перенести «Углерод»
уг—ле—ро́д
Часть речи
Часть речи слова «углерод» — Имя существительное
Морфологические признаки.
углерод (именительный падеж, единственного числа)
Постоянные признаки:
- нарицательное
- неодушевлённое
- мужской
- 2-e склонение
Непостоянные признаки:
- именительный падеж
- единственного числа
Может относится к разным членам предложения.
Склонение слова «Углерод»
| Падеж | Единственное число | Множественное число |
|---|---|---|
| Именительный Кто? Что? |
углеро́д | углеро́ды |
| Родительный Кого? Чего? |
углеро́да | углеро́дов |
| Дательный Кому? Чему? |
углеро́ду | углеро́дам |
| Винительный (неод.) Кого? Что? |
углеро́д | углеро́ды |
| Творительный Кем? Чем? |
углеро́дом | углеро́дами |
| Предложный О ком? О чём? |
углеро́де | углеро́дах |
Разбор по составу слова «Углерод»
Состав слова «углерод»:
корень — [углерод], нулевое окончание — [ ]
Проверьте свои знания русского языка
Категория: Русский язык
Русский язык
Тест Гласные и согласные звуки 1 класс
1 / 10
Гласная «я» даёт звук А в слове:
2 / 10
Если гласные буквы Е, Я, Ё, Ю стоят в начале слова или после гласных, то они дают:
два звука
один звук
звуки [е], [я], [ё], [ю]
все ответы неправильные
3 / 10
Гласная «ю» даёт звук У в слове:
4 / 10
Гласная «е» даёт звук [Э] в слове:
5 / 10
Если гласные буквы Е, Я, Ё, Ю стоят после согласных, то они дают:
звуки [е], [я], [ё], [ю]
один звук
два звука
не дают никакого звука
6 / 10
Гласная «ё» даёт звук О в слове:
7 / 10
Гласная «ё» даёт звук ЙО в слове:
корёжить
ёжик
мёртвый
лёд
8 / 10
Звук ЙУ в слове:
колючий
брошюра
юг
люстра
9 / 10
Гласная «е» даёт звуки [йэ] в слове:
10 / 10
Гласная «я» даёт звук ЙА в слове:
Каким бывает «углерод»;
Синонимы к слову «углерод»
Ассоциации к слову «углерод»
Предложения со словом «углерод»
- Он знал, разумеется, что молекула двуокиси углерода поглощает энергию в инфракрасном диапазоне и что человечество выпускает эти молекулы в атмосферу в нарастающих количествах.
Иэн Макьюэн, Солнечная
- От сгорания дизельного топлива в атмосферу поступают с выхлопными газами окись углерода, сера, мышьяк, свинец и другие токсические и канцерогенные вещества.
Виктор Алексеев, Основы безопасности жизнедеятельности
- Количество лёгких ионов уменьшается с ухудшением микроклиматических условий в помещениях и с повышением содержания диоксида углерода.
Владимир Николаевич Шилов, Гигиена. Конспект лекций, 2009
Происхождение слова «Углерод»
Из угле- (от уголь) + -род (от родить); первая часть — из праслав. *ǫglь, от кот. в числе прочего произошли:др.-русск. ѹгль, ѹгъль, ст.-слав. ѫгль (др.-греч. ἄνθραξ), болг. въ́гле, ср. р. «уголь», сербохорв. у̏гаљ (род. п. у̏гља), словенск. vọ̑gǝl (род. п. vọ̑gla), чешск. uhel, словацк. uhol, польск. węgiel, в.-луж. wuhl, wuhel, н.-луж. hugel; из праиндоевр. *egni- «огонь»; вторая часть — из праслав. *rodìti, от кот. в числе прочего произошли: др.-русск. родити, рожꙋ, ст.-слав. родити, рождѫ (др.-греч. γεννᾶν, τίκτειν), итер. раждати, русск. родить, рождать (церк.-слав. происхождения), укр. роди́ти, болг. родя́, сербохорв. ро̀дити, словенск. rodíti , чешск. rodit, словацк. rоdiť, польск. rodzić, в.-луж. rodźić, н.-луж. roźiś. Термин углерод, образованный по аналогии с водород, кислород как частичная калька лат. carbo, был введен в обиход Михаилом Соловьёвым в 1824 году.
Общие правила
§ 76. Пишутся слитно:
1. Все сложносокращённые слова, например: колхоз, эсминец, профсоюз, автотракторный.
2. Слова с приставками (включая вне-, после-, сверх-, а-, анти-, архи-, инфра-, контр-, ультра- и др.), а также с начальными составными частями пан-, квази-, псевдо- и др., например: довоенный, внеплановый, подотдел, аморальный, сверхприбыль, архинелепый, инфракрасный, контрудар, ультрафиолетовый, междуведомственный, панамериканизм, квазиученый, псевдоклассический.
Если приставка присоединяется к имени собственному, она пишется через дефис, например: Анти-Дюринг.
О написании с дефисом обер-, унтер-, лейб-, штаб-, вице-, экс- см. § 79, п. 13.
3. Сложные существительные, прилагательные и наречия, первым элементом которых является числительное, написанное буквами, например: пятилетка, трёхтонка, полуторагодовалый, двенадцатибалльный, двухсполовинный, троекратно.
Написание слитное и через дефис сложных иноязычных слов устанавливается в словарном порядке.
О написании через дефис собственных имён см. § 79, п. 6.
§ 77. Пишутся через дефис:
1. Лексические образования, представляющие собой:
а) повторение того же самого слова, например: маленький-маленький, еле-еле, чуть-чуть, постояли-постояли и разошлись (значение ограниченности во времени).
б) повторение того же слова или той же основы, но с разными окончаниями или приставками, например: день-деньской, рад-радёхонек, один-одинёшенек, давным-давно, черным-черно, мало-мальски, мало-помалу, крепко-накрепко, крест-накрест, толстый-претолстый, как-никак, волей-неволей, также один-единственный.
в) сочетание двух синонимических слов, например: нежданно-негаданно, тихо-смирно.
О постановке запятой (а не дефиса) при повторении слова см. § 149.
Примечание. Два одинаковых существительных в усилительном сочетании, из которых одно стоит в им. пад., а другое в твор. пад., пишутся раздельно, например: чудак чудаком, честь честью и т.п.
2. Графические буквенные сокращения сложных прилагательных, пишущихся слитно, для отличия от сокращённо написанных словосочетаний из прилагательного и существительного, например: ж.-д. – железнодорожный, но: ж. д. – железная дорога, с.-х. – сельскохозяйственный, но: с. х. – сельское хозяйство.
Дефис сохраняется в графических буквенных сокращениях слов, пишущихся через дефис, например: с.-д. – социал-демократ и социал-демократический, Ж.-Ж. Руссо – Жан-Жак Руссо.
3. Сложные слова, первым элементом которых является числительное (см. § 76, п. 3), если это числительное написано цифрами, например: 25-процентный, 10-летний, 35-летие.
4. Сложные порядковые числительные, если первая часть их написана цифрами, например: 183-миллионный, 5 1/2-тысячный.
5. Порядковые числительные, если они написаны цифрами с грамматическим окончанием, например: 15-й, 127-го.
6. Специальные термины и наименования, в том числе и аббревиатуры, в состав которых входит отдельная буква алфавита, например β-лучи (бета-лучи), или числительное, написанное цифрами и стоящее на втором месте, например ТУ-104, но: 4000 М (автопогрузчик с ковшом).
Имена существительные
§ 78. Пишутся слитно:
1. Сложные имена существительные, образованные при помощи соединительных гласных, а также все образования с аэро-, авиа-, авто-, мото-, вело-, кино-, фото-, стерео-, метео-, электро-, гидро-, агро-, зоо-, био-, микро-, макро-, нео-, например: водопровод, земледелец, льнозаготовки, паровозоремонт, аэропорт, авиаматка, автопробег, мотогонки, велодром, кинорежиссёр, фоторепортаж, стереотруба, метеосводка, электродвигатель, гидросооружения, агротехника, зоотехник, биостанция, микроснижение, макромир, неоламаркизм, веломотогонки, аэрофотосъёмка.
О написании через дефис имен существительных, образованных при помощи соединительных гласных, см. § 79, п. 3, 4.
2. Названия городов, второй составной частью которых является -град или -город, например, Ленинград, Калининград, Белгород, Ужгород, Ивангород.
3. Склоняемые сложные имена существительные с глагольной первой частью на -и, например: горицвет, держидерево, держиморда, вертишейка, вертихвостка, скопидом, сорвиголова.
§ 79. Пишутся через дефис:
1. Сложные существительные, имеющие значение одного слова и состоящие из двух самостоятельно употребляющихся существительных, соединённых без помощи соединительных гласных о и е, например:
а) жар-птица, бой-баба, дизель-мотор, кафе-ресторан, премьер-министр, генерал-майор, Бурят-Монголия (при склонении изменяется только второе существительное);
б) изба-читальня, купля-продажа, паинька-мальчик, пила-рыба, Москва-река (при склонении изменяются оба существительных).
2. Составные названия политических партий и направлений, а также их сторонников, например: социал-демократия, анархо-синдикализм, социал-демократ, анархо-синдикалист.
3. Сложные единицы измерения, независимо от того, образованы ли они при помощи соединительных гласных или без них, например: человеко-день, тонна-километр, киловатт-час. Слово трудодень пишется слитно.
4. Названия промежуточных стран света, русские и иноязычные, например: северо-восток и т.п., норд-ост и т.п.
5. Сочетания слов, имеющие значение существительных, если в состав таких сочетаний входят: а) глагол в личной форме, например: не-тронь-меня (растение), любишь-не-любишь (цветок); б) союз, например: иван-да-марья (растение); в) предлог, например: Ростов-на-Дону, Комсомольск-на-Амуре, Франкфурт-на-Майне.
6. Составные фамилии, образованные от двух личных наименований, например: Римский-Корсаков, Скворцов-Степанов, Мамин-Сибиряк, Мендельсон-Бартольди, Андерсен-Нексе.
7. Иноязычные составные фамилии с первой частью Сен- и Сент-, например: Сен-Симон, Сен-Жюст, Сен-Санс, Сент-Бёв. Так же пишутся восточные (тюркские, арабские и т.п.) личные наименования с начальной или конечной составной частью, обозначающей родственные отношения, социальное положение и т.д., например: Ибн-Фадлан, Кёр-оглы, Турсун-заде, Измаил-бей, Осман-паша.
Примечание 1. Составные имена с первой частью дон- пишутся через дефис только в тех случаях, когда вторая, основная часть имени в русском литературном языке отдельно не употребляется, например: Дон-Жуан, Дон-Кихот. Но если слово дон употребляется в значении «господин», оно пишется раздельно, например: дон Педро, дон Базилио.
Примечание 2. Артикли и частицы, входящие в состав иноязычных фамилий, пишутся отдельно, без дефиса, например: фон Бисмарк, ле Шапелье, де Костер, де Валера, Леонардо да Винчи, Лопе де Вега, Бодуэн де Куртене, фон дер Гольц. Артикли и частицы, без которых фамилии данного типа не употребляются, пишутся через дефис, например: Ван-Дейк.
В русской передаче некоторых иноязычных фамилий артикли и частицы пишутся слитно, хотя в соответствующих языках они пишутся отдельно, например: Лафонтен, Лагарп, Декандоль, Делиль.
Примечание 3. Не соединяются между собой дефисами имена разных категорий, например римские Гай Юлий Цезарь, подобно соответствующим русским имени, отчеству и фамилии.
Примечание 4. Личные имена и фамилии, соединённые с прозвищами, пишутся с последними раздельно, например: Илья Муромец, Всеволод Третий Большое Гнездо, Ванька Каин, Муравьёв Вешатель.
8. Географические названия, состоящие:
а) из двух существительных, например: Орехово-Зуево, Каменец-Подольск, Сердце-Камень (мыс);
б) из существительного и последующего прилагательного, например: Могилёв-Подольский, Гусь-Хрустальный, Москва-Товарная;
в) из сочетания артикля или частицы с знаменательной частью речи, например: Ле-Крезо (город), Ла-Каролина (город), Де-Кастри (залив).
Примечание. Раздельно пишутся географические названия:
а) состоящие из прилагательного и следующего за ним существительного или из числительного и следующего за ним существительного, например: Белая Церковь, Нижний Тагил, Великие Луки, Ясная Поляна, Семь Братьев;
б) представляющие собой сочетания имени и фамилии, имени и отчества, например: поселок Лев Толстой, станция Ерофей Павлович.
9. Названия населённых пунктов, в состав которых в качестве первой части входят: усть-, соль-, верх- и т.п., а также некоторые названия населенных пунктов с первой частью ново-, старо-, верхне-, нижне- и т.п., кроме тех, слитное написание которых закрепилось в справочных изданиях, на географических картах и т.п., например: Усть-Абакан, Соль-Илецк, Верх-Ирмень, Ново-Вязники, Нижне-Гнилое, но: Новосибирск, Малоархангельск, Старобельск, Новоалексеевка, Верхнеколымск, Нижнедевицк.
10. Составные географические названия, образованные как при помощи соединительной гласной, так и без неё из названий частей данного географического объекта, например: Австро-Венгрия, Эльзас-Лотарингия, но: Чехословакия.
11. Иноязычные словосочетания, являющиеся именами собственными, названиями неодушевлённых предметов, например: Аму-Дарья, Алма-Ата, Па-де-Кале, Булонь-сюр-Мер, Нью-Йорк, Пале-Рояль, Гранд-отель.
Примечание. Данное правило не распространяется на передаваемые русскими буквами составные иноязычные названия литературных произведений, газет, журналов, предприятий и т.п., которые пишутся раздельно, если выделяются в тексте кавычками, например: «Стандарт Ойл», «Коррьеро делла Рома».
12. Пол- (половина) с последующим родительным падежом существительного, если существительное начинается с гласной буквы или согласной л, например: пол-оборота, пол-яблока, пол-лимона, но: полметра, полчаса, полкомнаты; через дефис пишутся также сочетания пол- с последующим именем собственным, например: пол-Москвы, пол-Европы. Слова, начинающиеся с полу-, всегда пишутся слитно, например: в полуверсте от города, полустанок, полукруг.
13. Слова, первой составной частью которых являются иноязычные элементы обер-, унтер-, лейб-, штаб-, вице-, экс-, например: обер-мастер, унтер-офицер, лейб-медик, штаб-квартира, вице-президент, экс-чемпион.
Также пишется через дефис контр-адмирал (здесь контр- имеет не то значение, при котором оно пишется слитно).
14. Определяемое слово со следующим непосредственно за ним однословным приложением, например: мать-старуха, Маша-резвушка, Аника-воин.
Примечание 1. Между определяемым словом и стоящим перед ним однословным приложением, которое может быть приравнено по значению к прилагательному, дефис не пишется, например: красавец сынишка.
Примечание 2. Если определяемое слово или приложение само пишется через дефис, то между ними дефис не пишется, например: социал-демократы меньшевики.
Примечание 3. Дефис не пишется также:
а) в сочетании имени нарицательного со следующим за ним именем собственным, например: город Москва, река Волга, резвушка Маша;
б) в сочетании существительных, из которых первое обозначает родовое, а второе видовое понятие, например: птица зяблик, цветок магнолия;
в) после слов гражданин, товарищ, господин и т.п. в сочетании с существительным, например: гражданин судья, товарищ полковник, господин посол. О выделении приложения запятыми см. § 152.
15. Графические сокращения имён существительных, состоящие из начала и конца слова, например: о-во (общество), д-р (доктор), т-во (товарищество), б-ка (библиотека).
16. Дефис пишется после первой части сложного существительного при сочетании двух сложных существительных с одинаковой второй частью, если в первом из существительных эта общая часть опущена, например: шарико- и роликоподшипники (вместо шарикоподшипники и роликоподшипники), паро-, электро- и тепловозы (вместо паровозы, электровозы и тепловозы), парт- и профорганизации, северо- и юго-восток.
Имена прилагательные
§ 80. Пишутся слитно сложные имена прилагательные:
1. Образованные от слитно пишущихся сложных имён существительных, например: водопроводный (водопровод), земледельческий (земледелец, земледелие), новосибирский (Новосибирск).
2. Образованные из сочетаний слов, по своему значению подчинённых одно другому, например: железнодорожный (железная дорога), народнохозяйственный (народное хозяйство), естественнонаучный (естественные науки), сложноподчинённое (сложное по способу подчинения), рельсопрокатный (прокатывающий рельсы), общенародный (общий для народа), полезащитный (образующий защиту для полей), металлорежущий (режущий металл); сюда же относятся обозначающие единое понятие образования (в том числе и терминологические) из наречия и прилагательного (или причастия), например: малоупотребительный, близлежащий, животрепещущий, глубокоуважаемый, свежеиспеченный, ясновидящий, сильнодействующий, дикорастущий, вечнозелёный, гладкокрашеный.
Примечание. Сложные прилагательные, в состав которых входят наречия, не следует смешивать со словосочетаниями, состоящими из наречия и прилагательного (или причастия) и пишущимися раздельно, например: диаметрально противоположный, прямо противоположный, чисто русский, детски наивный, плохо скрываемый, отчётливо выраженный.
3. Употребляемые в качестве терминов и образованные из двух или трёх основ, независимо от характера последних, например: грудобрюшная (преграда), индоевропейские (языки), древневерхненемецкий (язык), двууглекислый (газ); также – глухонемой.
§ 81. Пишутся через дефис сложные имена прилагательные:
1. Образованные от существительных, пишущихся через дефис, от личных наименований – сочетаний имён и фамилий, а также от названий населённых пунктов, представляющих собой сочетания имён и фамилий, имён и отчеств, например:
дизель-моторный, социал-демократический, бурят-монгольский, северо-восточный, алма-атинский, орехово-зуевский, нижне-масловский, усть-абаканский, ромен-роллановский, вальтер-скоттовский, лев-толстовский, ерофей-павловичский.
Примечание 1. Пишется слитно прилагательное москворецкий.
Примечание 2. Имена прилагательные, образованные от имён собственных, пишущихся через дефис, и имеющие приставку, отсутствующую у существительного, пишутся слитно, например: приамударьинский, заиссыккульский.
2. Образованные из двух или более основ, обозначающих равноправные понятия, например: беспроцентно-выигрышный, выпукло-вогнутый, партийно-комсомольский, садово-огородный, мясо-молочный, англо-японский, русско-немецко-французский (словарь), сине-бело-красный (флаг).
3. Образованные из двух основ и обозначающие:
а) качество с дополнительным оттенком, например: раскатисто-громкий, горько-солёный;
б) оттенки цветов, например: бледно-розовый, ярко-синий, тёмно-русый, чёрно-бурый, синевато-голубой, золотисто-жёлтый, пепельно-серый, бутылочно-зелёный, лимонно-жёлтый, изжелта-красный.
4. Входящие в состав географических собственных имён и начинающиеся с восточно-, западно-, северно- и северо-, южно- и юго-, например: Западно-Казахстанская область, Восточно-Китайское море, Южно-Африканский Союз.
Примечание 1. Прилагательные, образованные из двух или более основ, не подходящие под перечисленные правила, пишутся через дефис, например:
литературно-художественный (альманах), политико-массовая (работа), словарно-технический (отдел), подзолисто-болотный, рыхло-комховато-пылеватый, удлинённо-ланцетовидный.
Примечание 2. Через дефис пишутся также слова, первой составной частью которых являются сам-, сама-, например: сам-друг, сам-третей, сам-пят, сама-пята.
Имена числительные
§ 82. Пишутся слитно во всех падежах:
1. Количественные числительные, последним элементом которых является -десят, -ста, -сот, например: пятьдесят, пятидесяти, триста, трёхсот, семьсот, семисот, но: пять тысяч, три миллиона, семь миллиардов.
2. Порядковые числительные, последним элементом которых является -сотый, -тысячный, -миллионный и т.д., если первая часть их написана буквами, например: восьмисотый, двадцатипятитысячный, стовосьмидесятитрёхмиллионный, но: восемьдесят шестой, тысяча девятьсот сорок первый.
Примечание. В сложных порядковых числительных, в состав которых входят дробные обозначения «с половиной», «с четвертью» и т.п., предпочтительно писать первую составную часть цифрами, например: 5 1/2-тысячное население.
Наречия
§ 83. Пишутся слитно:
1. Наречия, образованные соединением предлогов с наречиями, например: доныне, извне, навсегда, напротив, насквозь, позавчера, послезавтра, донельзя, навряд ли, задаром.
От таких наречий следует отличать пишущиеся раздельно сочетания предлогов с неизменяемыми словами, употребляемыми в этих случаях в значении существительных, например: до завтра, на авось, на нет (свести на нет), на ура.
2. Наречия, образованные соединением предлогов в и на с собирательными числительными, например: вдвое, втрое, вчетверо и т.д., надвое, натрое, но: по двое, по трое.
3. Наречия, образованные соединением предлогов с краткими прилагательными, например: влево, досуха, замертво, издалека, наскоро, понемногу, попусту, потихоньку, сгоряча.
4. Наречия, образованные соединением предлогов с полными прилагательными и местоимениями, например: вкрутую, вплотную, врукопашную, зачастую, напропалую, наудалую, впервые, наверное, вничью, вовсю.
Примечание. Пишутся раздельно наречия этого типа, составленные из предлога в и прилагательного, начинающегося с гласной, например: в открытую.
О наречиях, начинающихся с по- и пишущихся через дефис, см. § 84.
5. Наречия, образованные соединением предлогов с существительными, например: вперёд, сбоку, подчас, воочию, встарь, взапуски, наугад, вдобавок, наоборот, поневоле, всмятку, вприсядку. К наречиям этого типа принадлежат:
а) Слова с различными наречными значениями, имеющие в своём составе такие существительные или такие именные формы, которые в современном литературном языке не употребляются: вблизи, вдоволь, вдогонку, вдребезги, взаймы, взамен, взаперти, взапуски, взасос, взашей, вкось, вкривь, внаймы, внутри, внутрь, воочию, восвояси, вперевалку, вперегонки, впереди, вперемежку, вперемешку, вплавь, вповалку, впопыхах, вприглядку, впроголодь, впросак, впросонках, вразвалку, врасплох, врозь, всерьез, вскачь, вскользь, всмятку, встарь, втихомолку, второпях, втридорога, вчуже, дотла, замуж (от старой формы вин. пад.), запанибрата, изнутри, искони, исподволь, исподлобья, исподтишка, испокон, исполу, исстари, набекрень, наверняка, навзничь, навзрыд, навыворот, назади, наземь, наизусть, наискосок, наискось, наобум, наотмашь, наперегонки, наперекор, наперерез, наперечёт, наповал, напрямик, нарасхват, наружу, насмарку, настежь, настороже, натощак, наугад, наутёк, начеку, наяву, невдомёк, невзначай, невмоготу, невпопад, оземь, поделом, позади, понаслышке, поодаль, поперёк, пополам, пополудни, сдуру, сзади, снаружи, спозаранку, спросонок, спросонья, чересчур и т.п.
б) Слова с различными наречными значениями, если между предлогом (приставкой) и существительным, из которых образовалось наречие, не может быть без изменения смысла вставлено определяющее прилагательное, местоимение, числительное или если к существительному не может быть поставлен падежный вопрос:
вдобавок, вброд, влёт, вволю, вволюшку (наесться), взатяжку (курить), вконец (измучиться), вместе, вмиг, внакидку (носить пальто), внакладе, вновь, воистину, вокруг, вослед, вперебой, вперегиб, вплоть, впору (костюм), вовремя (приехать), впоследствии, вполовину, вправду, вправе (поступить так), впрок, вразбивку, вразброд, вразнобой, вразрез, вразрядку, врастяжку, вряд ли, вскорости, вслух, всухомятку, втайне, въявь, задаром, замужем, зараз, кряду, кстати, набок (надеть шляпу), навстречу, навыкат, навылет, навынос, навыпуск, навырез, навытяжку, наголову (разбить), назло, назубок (выучить), наизнанку, накануне, наконец, налицо, наоборот, наотрез, наперебой, наперевес, наполовину, наперерыв, наперехват, напоказ, напоследок, например, напрокат, напролёт, напролом, нараспашку, нараспев, наряду, насилу, насмерть (стоять; но: не на жизнь, а на смерть), наудачу, наутро (вернуться), начистоту, невмочь, обок (жить), отроду, отчасти, побоку, подряд, подчас, поневоле, поодиночке, поутру, сбоку, слишком, сплеча (рубить), сразу, сроду, сряду.
в) Слова с пространственным и временным значением, имеющие в своём составе существительные верх, низ, перед, зад, высь, даль, век, начало, несмотря на возможность постановки перед некоторыми из них определяющего слова: вверх, вверху, кверху, доверху, наверх, сверху; вниз, внизу, книзу, донизу, снизу; вперёд, наперёд; назад; ввысь; вдаль, вдали, издали; ввек, вовек, вовеки, навек, навеки; вначале, сначала; но при наличии пояснительных слов к соответствующим существительным указанные слова пишутся раздельно, например: на верх горы, в высь поднебесную, в даль степей, в дали голубой, во веки веков, на веки вечные, в начале жизни, с начала учебного года.
6. Однако не всякое сочетание существительного с предлогом, являющееся обстоятельством и близкое по значению к наречию, пишется слитно.
А. Следует писать раздельно сочетания предлогов с существительными:
а) если между предлогом и существительным можно вставить определяющее слово: на миг (на один миг), с маху (со всего маху), в тиши (в лесной тиши), на скаку (на всём скаку), в тупик (попал в такой тупик, что…);
б) если предлог оканчивается на согласную, а существительное начинается с гласной буквы: в обмен, в обрез, в обнимку, в отместку, в обтяжку, в обхват, в одиночку, в охапку, в упор, под уклон;
в) если существительное в определённом (одном) значении сохранило хотя бы некоторые падежные формы в сочетании с предлогами: на корточки, на корточках; на карачки, на карачках; на четвереньки, на четвереньках; за границу, за границей, из-за границы; под спуд, под спудом; на дом, на дому; на память, по памяти; на совесть, по совести; на руку, не с руки; в насмешку, с насмешкой; под мышками, под мышки, под мышкой, под мышку, из-под мышек; на цыпочки, на цыпочках; на поруки, на поруках; на запятки, на запятках.
Б. Раздельно пишутся также:
а) некоторые близкие по значению к наречиям сочетания существительных с предлогами:
без: без оглядки, без просыпу, без разбору, без толку, без удержу, без умолку, без устали;
до: до зарезу, до отвала, до отказа, до смерти, до упаду (но: доверху, донизу, см. выше п. 5, «в»);
на: на бегу, на лету, на ходу, на весу, на виду, на вид, на вес, на вкус, на глаз, на глазок, на грех, на диво, на ощупь, на славу, на смех;
с: с налёту, с разбегу, с разгона, с размаху, с наскока, с ходу;
б) сочетания отрицаний не и ни с предложными формами существительных, например: не в меру, не в зачёт, не под силу, не по вкусу, не к добру, ни на йоту, ни за грош, не к спеху,
в) существительные в предложном падеже множественного числа с предлогами в и на, обозначающие местонахождение, время, состояние (физическое и душевное), например: в головах, в ногах, на часах (стоять), на днях, на рысях, на радостях, в сердцах.
В случаях затруднений в правописании наречий, образованных соединением предлога с именами существительными, следует обращаться к орфографическому словарю.
От слитно пишущихся наречий следует отличать одинаково произносимые сочетания предлогов с существительными, пишущиеся раздельно, например: в пору юности, с боку на бок (переваливается), на голову (свалилось несчастье), на силу (не рассчитывали), на пример (сослался), в ряд (выстроились).
7. Пишутся слитно наречия, образовавшиеся путем слияния предлогов с местоимениями, например: почему, потому, поэтому, посему, отчего, оттого, зачем, затем, почём («по какой цене») и т.п., в отличие от сочетаний предлогов с местоимениями в косвенных падежах («по чему ты стучишь?», т.е. по какому предмету; «за чем ты пошёл?», т.е. за какой вещью ты пошёл).
§ 84. Пишутся через дефис наречия во-первых, во-вторых, в-третьих и т.д., а также наречия, образованные от прилагательных и местоимений, начинающиеся с по- и оканчивающиеся на -ки, -ьи, -ому, -ему, например: по-русски, по-немецки (а также по-латыни), по-птичьи, по-соловьиному, по-иному, по-моему, по-пустому, по-прежнему, по-видимому.
Примечание. Наречия, образованные из предлога по и краткой формы прилагательного, пишутся (согласно § 83, п. 3) слитно.
В наречиях с приставкой по-, образованных от составных имён прилагательных, пишущихся с дефисом, дефис пишется только после по-, например: по-социалдемократически.
Примечание. Пишется через дефис наречие на-гора (технический термин).
Не нашли то, что искали? Воспользуйтесь поиском гугл на сайте:
©2015- 2022 zdamsam.ru Размещенные материалы защищены законодательством РФ.
Всего найдено: 7
Здравствуйте! Подскажите, как пишется «углеродно(-)нейтральный? Спасибо!
Ответ справочной службы русского языка
Корректно раздельное написание.
Здравствуйте! Скажите, пожалуйста, правильно ли расставлены знаки препинания в предложении. При вдохе оксида углерода кровь альвеолярных капилляров, вместо того чтобы обогащаться кислородом, обогащается угарным газом.
Ответ справочной службы русского языка
Знаки препинания расставлены верно.
Можно ли уже, на ваш взгляд, использовать в текстах (журнальных и т. п.) слово «карбон» в значении «углепластик»? Употребляется оно широко и звучит красиво :), но есть сомнения, поскольку имеется значение «каменноугольный период», а в исходном английском carbon – это просто «углерод«. Спасибо!
Ответ справочной службы русского языка
Если читателям журнала понятно, о чем идет речь, то почему бы и нет.
Здравствуйте! Правильно ли писать слитно слово «невиноградного» в словосочетании «углерод невиноградного происхождения»?
Ответ справочной службы русского языка
Слитное написание правильно.
Здравствуйте, не могли бы мне помочь, хотелось бы узнать есть ли в этом предложении запятая после слова свойств. Предложение целиком: Для обеспечения высоких прочностных свойств, роторы крупных турбогенераторов (свыше 500 МВт) изготавливают из цельной поковки высоколегированной стали, обладающей высокими механическими и магнитными свойствами, а роторы турбогенераторов малой мощности (до 100 МВт) — из углеродистой стали
Ответ справочной службы русского языка
Запятая после слова свойств не нужна.
Здравствуйте. Нужна ли запятая после «однако» в данном предложении: однако, исходя из теоретической возможности заполнения атомами углерода междоузлий подрешетки, более верно считать ее модификацией карбида?
Ответ справочной службы русского языка
Знаки препинания расставлены верно.
Здравствуйте, этот дикторский текст для фильма, предназначенный для просмотра широкой аудитории. Как стилистически правильно должен выглядеть текст, чтобы был легко воспринят слушителями?
Первые страницы летописи Федерального центра были написаны в суровые годы войны. Тогда в его опытных цехах было изготовлено более полумиллиона зарядов к реактивным снарядам М-13 и М-З1. С легендарных «Катюш» началось в нашей стране развитие ракетной техники на твердом топливе…
Более полувека предприятие разрабатывает и изготавливает
баллиститные и смесевые твердые ракетные топлива и заряды из них для всех родов войск и вооружений, ракет-носителей и космических объектов.Здесь создана непрерывная технология производства баллиститных порохов и твердых ракетных топлив, а также зарядов различных форм и габаритов с широким спектром управляемых свойств.
Разработана и внедрена безопасная промышленная технология производства смесевых твердых ракетных топлив с высоким уровнем энергомассовых характеристик и экологической частотой продуктов сгорания.Федеральный центр является автором и разработчиком технологии изготовления корпусов твердотопливных ракетных двигателей из современных композиционных материалов, в том числе сверхпрочных цельномотанных корпусов типа «кокон».
За годы работы предприятием сдано в эксплуатацию более 460 номенклатур твердотопливных зарядов различного назначения.
Так для сухопутных войск страны отработаны заряды к 59 ракетным комплексам, в том числе к активно-реактивным снарядам «Ель-2», «Буревестник-2», «Баклан», «Краснополь».
К артиллерийским установкам и комплексам «Б-4М», «Пион», «Гиацинт», «2СЗМ», «Смельчак».
К реактивным системам залпового огня «Град», «Град-1», «Ураган».
Для войск ПВО страны отработаны заряды к 10 ракетным комплексам, среди них комплексы: «С-200», «Печора», «Куб», «Шторм»,
«Волга», «Тунгуска».
Военно-морскому флоту России сданы на вооружение заряды к 35 ракетным системам.
Для военно-воздушных сил страны отработаны заряды к 30 боевым ракетам.
В ракетных комплексам тактического и стратегического назначения «Темп-С», «Ока» и «Ока-У», «Точка» и «Точка-У», «Пионер», «Тополь», также используются заряды отработанные «Салютом».Убедительным признанием его заслуг, в общем, для всех деле укреплении безопасности нашей страны, стало открытие монумента «Создателям ядерного щита России».
Федеральный центр «Салют» – один из ведущих научных центров страны по проблемам долговечности твердотопливных зарядов. По результатам проведенных здесь комплексных исследований продлены сроки эксплуатации зарядов к более чем 50 ракетным комплексам, стоящим на вооружении в России, Емени, Сирии, на Кубе, Замбии, Перу и ряде других стран.
Снятые с вооружения пороха и твердые ракетные топлива, после их утилизации эффективно используются для решения народно-хозяйственных задач, например: в качестве детонирующих зарядов сейсморазведки и для рыхления твердых горных пород, в качестве безкорпусного генератора давления в скважинах с целью изучения притока к ним нефти и газа.С помощью гранипоров – гранулированных взрывчатых веществ на основе баллиститов, ведется разработка полезных ископаемых открытым способом.
Федеральный центр является неизменным участником практически всех космических программ, осуществляемых в России. По этим программам отработаны заряды и двигатели к 24 космическим комплексам. Это система аварийного спасения космонавтов, двигатели мягкой посадки, стабилизации, увода, разделения ступеней, торможения космических объектов для комплексов «Восток», «Восход», «Луна», «Марс», «Союз-ТМ», «Протон», «Морской старт», «Циклон», «Молния» и других.
В начале 90-х годов Федеральных центр приступил к разработке технологий двойного назначения и выпуска на их основе наукоемкой гражданской продукции. Так на базе оборонных технологий были созданы уникальные источники электрической энергии – импульсные МГД-генераторы. Они предназначены для глубинного зондирования земной коры и поиска полезных ископаемых.
Разработаны специальные, аэрозольобразующие составы с ингибирующими добавками для принципиально новых средств объемного пожаротушения. Это огнетушители «Степ» и «Степ2», которые эффективно заменяют хладоновые средства борьбы с огнём.
«Степ» и «Степ2» — надежная защита от пожара электростанций, производственных и складских помещений, всех видов транспорта, объектов добычи и переработки нефти и газа и ряда других объектов.
«Степ» и «Степ2» успешно прошли испытания в России и за рубежом. Сегодня они экспортируются во многие страны мира.На основе использование твердотопливных газогенераторов и емкостей из композиционных материалов, созданы быстродействующие установки жидкостного тушения крупных пожаров. Создана опытно- промышленная установка для синтеза алмазов из специальных углеродосодержащих твердотопливных составов.
Основные области применениям таких алмазов шлифовально- полировальные пасты и алмазно-абразивные инструменты.
На базе технологии производства корпусов ракетных двигателей освоен выпуск различных емкостей: контейнеров, труб и другого химически стойкого оборудования. Разработана технология, налажен выпуск оборудования и материалов для керамической сварки, огнеупорных покрытий высокотемпературных печей.
На основе самораспространяющегося высокотемпературного синтеза, разработана технология переработки металлосодержащих отходов в стойкие неорганические пигменты различных цветов и оттенков.Сегодня Федеральный центр является одним из крупнейших в России изготовителем субстанций для производства сердечно-сосудистых препаратов.
Среди других видов гражданской продукции «Салюта», следует отметить бездымные фейерверочные составы, полимерные магниты, технику и материалы для разметки дорог, лаки и краски.
«Салют» – один из участников проекта «СТАРТ 1». Его основу составляет носитель, созданный на базе многоступенчатой твердотопливной ракеты мобильного грунтового стратегического комплекса.
«СТАРТ 1» предназначен для вывода на околоземные орбиты космических аппаратов с целью развертывания систем спутниковой связи, создании новых материалов, разведки полезных ископаемых, экологического мониторинга и других целей.
Федеральный центр «Салют» приглашает все заинтересованные организации к сотрудничеству в области разработки производства и сбыта своей продукции.
Ответ справочной службы русского языка
К сожалению, мы не имеем возможности ответить на столь объёмный вопрос.
Оксид углерода (II)
1. Строение молекулы и физические свойства
2. Способы получения
3. Химические свойства
3.1. Взаимодействие с кислородом
3.2. Взаимодействие с хлором
3.3. Взаимодействие с водородом
3.4. Взаимодействие с щелочами
3.5. Взаимодействие с оксидами металлов
3.6. Взаимодействие с прочими окислителями
Оксид углерода (II)
Строение молекулы и физические свойства
Оксид углерода (II) («угарный газ») – это газ без цвета и запаха. Сильный яд. Небольшая концентрация угарного газа в воздухе может вызвать сонливость и головокружение. Большие концентрации угарного газа вызывают удушье.
Строение молекулы оксида углерода (II) – линейное. Между атомами углерода и кислорода образуется тройная связь, за счет дополнительной донорно-акцепторной связи:
Способы получения
В лаборатории угарный газ можно получить действием концентрированной серной кислоты на муравьиную или щавелевую кислоты:
НСООН → CO + H2O
H2C2O4 → CO + CO2 + H2O
В промышленности угарный газ получают в газогенераторах при пропускании воздуха через раскаленный уголь:
C + O2 → CO2
CO2 + C → 2CO
Еще один важный промышленный способ получения угарного газа — паровая конверсия метана. При взаимодействии перегретого водяного пара с метаном образуется угарный газ и водород:
СН4 + Н2O → СО + 3Н2
Также возможна паровая конверсия угля:
C0 + H2+O → C+2O + H20
Угарный газ в промышленности также можно получать неполным окислением метана:
2СН4+О2 → 2СО + 4Н2
Химические свойства
Оксид углерода (II) – несолеобразующий оксид. За счет углерода со степенью окисления +2 проявляет восстановительные свойства.
1. Угарный газ горит в атмосфере кислорода. Пламя окрашено в синий цвет:
2СO + O2 → 2CO2
2. Оксид углерода (II) окисляется хлором в присутствии катализатора или под действием света с образованием фосгена. Фосген – ядовитый газ.
CO + Cl2 → COCl2
3. Угарный газ взаимодействует с водородом при повышенном давлении. Смесь угарного газа и водорода называется синтез-газ. В зависимости от условий из синтез-газа можно получить метанол, метан, или другие углеводороды.
Например, под давлением больше 20 атмосфер, при температуре 350°C и под действием катализатора угарный газ реагирует с водородом с образованием метанола:
СО + 2Н2 → СН3ОН
4. Под давлением оксид углерода (II) реагирует с щелочами. При этом образуется формиат – соль муравьиной кислоты.
Например, угарный газ реагирует с гидроксидом натрия с образованием формиата натрия:
CO + NaOH → HCOONa
5. Оксид углерода (II) восстанавливает металлы из оксидов.
Например, оксид углерода (II) реагирует с оксидом железа (III) с образованием железа и углекислого газа:
3CO + Fe2O3 → 2Fe + 3CO2
Оксиды меди (II) и никеля (II) также восстанавливаются угарным газом:
СО + CuO → Cu + CO2
СО + NiO → Ni + CO2
6. Угарный газ окисляется и другими сильными окислителями до углекислого газа или карбонатов.
Например, пероксидом натрия:
CO + Na2O2 → Na2CO3

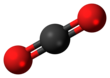




![{displaystyle K_{mathrm {h} }={frac {{ce {[H2CO3]}}}{{ce {[CO2_{(aq)}]}}}}=1.70times 10^{-3}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7b01be634ec0c3d83cbf3aa2c71bbd51b9ce0e26)

![{displaystyle K_{mathrm {a1} }={frac {{ce {[HCO3- ][H+]}}}{{ce {[H2CO3]}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f6013fac05dba06751345e4d824c525ed2c43b12)
![{displaystyle K_{mathrm {a1} }{rm {(apparent)}}={frac {{ce {[HCO3- ][H+]}}}{{ce {[H2CO3] + [CO2_{(aq)}]}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3ae2fa07440c037d6054ce4e7ef95c15c59264e7)