Potassium pearls (in paraffin oil, ~5 mm each) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Potassium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | (pə-TASS-ee-əm) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white, faint bluish-purple hue when exposed to air | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(K) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Potassium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 1: hydrogen and alkali metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | s-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
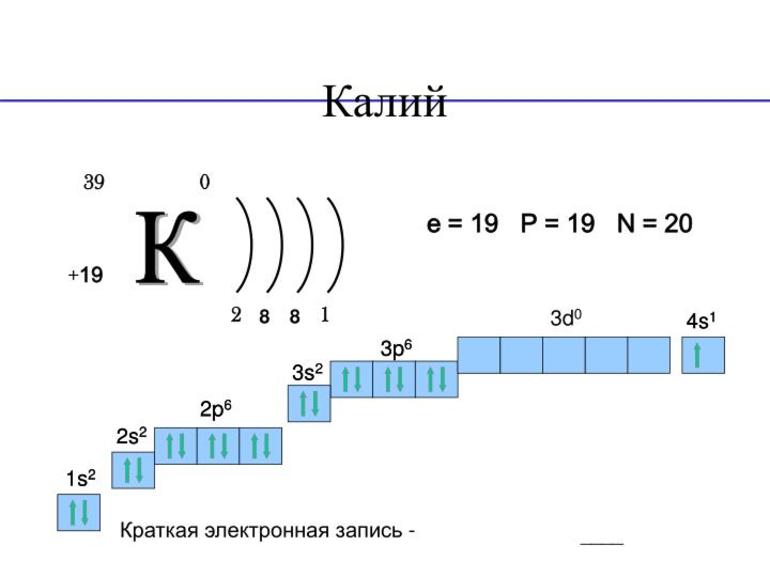
| Electron configuration | [Ar] 4s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 8, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 336.7 K (63.5 °C, 146.3 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1030.793 K (757.643 °C, 1395.757 °F)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 0.89 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 0.82948 g/cm3[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 2223 K, 16 MPa[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.33 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 76.9 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 29.6 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −1, +1 (a strongly basic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 227 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 203±12 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 275 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of potassium |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 2000 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 83.3 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 102.5 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 72 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +20.8×10−6 cm3/mol (298 K)[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 3.53 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 1.3 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 3.1 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 0.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 0.363 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-09-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Humphry Davy (1807) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | «K»: from New Latin kalium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of potassium
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Potassium is the chemical element with the symbol K (from Neo-Latin kalium) and atomic number 19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force.[6] Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight[7][8]), and occurs in many minerals such as orthoclase, a common constituent of granites and other igneous rocks.[9]
Potassium is chemically very similar to sodium, the previous element in group 1 of the periodic table. They have a similar first ionization energy, which allows for each atom to give up its sole outer electron. It was suspected in 1702 that they were distinct elements that combine with the same anions to make similar salts,[10] and this was proven in 1807 through using electrolysis. Naturally occurring potassium is composed of three isotopes, of which 40
K is radioactive. Traces of 40
K are found in all potassium, and it is the most common radioisotope in the human body.
Potassium ions are vital for the functioning of all living cells. The transfer of potassium ions across nerve cell membranes is necessary for normal nerve transmission; potassium deficiency and excess can each result in numerous signs and symptoms, including an abnormal heart rhythm and various electrocardiographic abnormalities. Fresh fruits and vegetables are good dietary sources of potassium. The body responds to the influx of dietary potassium, which raises serum potassium levels, with a shift of potassium from outside to inside cells and an increase in potassium excretion by the kidneys.
Most industrial applications of potassium exploit the high solubility in water of potassium compounds, such as potassium soaps. Heavy crop production rapidly depletes the soil of potassium, and this can be remedied with agricultural fertilizers containing potassium, accounting for 95% of global potassium chemical production.[11]
Etymology
The English name for the element potassium comes from the word potash,[12] which refers to an early method of extracting various potassium salts: placing in a pot the ash of burnt wood or tree leaves, adding water, heating, and evaporating the solution. When Humphry Davy first isolated the pure element using electrolysis in 1807, he named it potassium, which he derived from the word potash.
The symbol K stems from kali, itself from the root word alkali, which in turn comes from Arabic: القَلْيَه al-qalyah ‘plant ashes’. In 1797, the German chemist Martin Klaproth discovered «potash» in the minerals leucite and lepidolite, and realized that «potash» was not a product of plant growth but actually contained a new element, which he proposed calling kali.[13] In 1807, Humphry Davy produced the element via electrolysis: in 1809, Ludwig Wilhelm Gilbert proposed the name Kalium for Davy’s «potassium».[14] In 1814, the Swedish chemist Berzelius advocated the name kalium for potassium, with the chemical symbol K.[15]
The English and French-speaking countries adopted Davy and Gay-Lussac/Thénard’s name Potassium, whereas the Germanic countries adopted Gilbert/Klaproth’s name Kalium.[16] The «Gold Book» of the International Union of Pure and Applied Chemistry has designated the official chemical symbol as K.[17]
Properties
Physical
Potassium is the second least dense metal after lithium. It is a soft solid with a low melting point, and can be easily cut with a knife. Freshly cut potassium is silvery in appearance, but it begins to tarnish toward gray immediately on exposure to air.[18] In a flame test, potassium and its compounds emit a lilac color with a peak emission wavelength of 766.5 nanometers.[19]
Neutral potassium atoms have 19 electrons, one more than the configuration of the noble gas argon. Because of its low first ionization energy of 418.8 kJ/mol, the potassium atom is much more likely to lose the last electron and acquire a positive charge, although negatively charged alkalide K− ions are not impossible.[20] In contrast, the second ionization energy is very high (3052 kJ/mol).
Chemical
Potassium reacts with oxygen, water, and carbon dioxide components in air. With oxygen it forms potassium peroxide. With water potassium forms potassium hydroxide. The reaction of potassium with water can be violently exothermic, especially since the coproduced hydrogen gas can ignite. Because of this, potassium and the liquid sodium-potassium (NaK) alloy are potent desiccants, although they are no longer used as such.[21]
Compounds
Structure of solid potassium superoxide (
KO2).
Four oxides of potassium are well studied: potassium oxide (K2O), potassium peroxide (K2O2), potassium superoxide (KO2)[22] and potassium ozonide (KO3). The binary potassium-oxygen compounds react with water forming potassium hydroxide KOH.
Potassium hydroxide is a strong base. Illustrating its hydrophilic character, as much as 1.21 kg of KOH can dissolve in a single liter of water.[23][24] Anhydrous KOH is rarely encountered. KOH reacts readily with carbon dioxide CO2 to produce potassium carbonate K2CO3, and in principle could be used to remove traces of the gas from air. Like the closely related sodium hydroxide, potassium hydroxide reacts with fats to produce soaps.
In general, potassium compounds are ionic and, owing to the high hydration energy of the K+ ion, have excellent water solubility. The main species in water solution are the aquo complexes [K(H2O)n]+ where n = 6 and 7.[25]
Potassium heptafluorotantalate K2[TaF7] is an intermediate in the purification of tantalum from the otherwise persistent contaminant of niobium.[26]
Organopotassium compounds illustrate nonionic compounds of potassium. They feature highly polar covalent K–C bonds. Examples include benzyl potassium KCH2C6H5. Potassium intercalates into graphite to give a variety of graphite intercalation compounds, including KC8.
Isotopes
There are 25 known isotopes of potassium, three of which occur naturally: 39
K (93.3%), 40
K (0.0117%), and 41
K (6.7%) (by mole fraction). Naturally occurring 40
K has a half-life of 1.250×109 years. It decays to stable 40
Ar by electron capture or positron emission (11.2%) or to stable 40
Ca by beta decay (88.8%).[27] The decay of 40
K to 40
Ar is the basis of a common method for dating rocks. The conventional K-Ar dating method depends on the assumption that the rocks contained no argon at the time of formation and that all the subsequent radiogenic argon (40
Ar) was quantitatively retained. Minerals are dated by measurement of the concentration of potassium and the amount of radiogenic 40
Ar that has accumulated. The minerals best suited for dating include biotite, muscovite, metamorphic hornblende, and volcanic feldspar; whole rock samples from volcanic flows and shallow instrusives can also be dated if they are unaltered.[27][28] Apart from dating, potassium isotopes have been used as tracers in studies of weathering and for nutrient cycling studies because potassium is a macronutrient required for life[29] on Earth.
40
K occurs in natural potassium (and thus in some commercial salt substitutes) in sufficient quantity that large bags of those substitutes can be used as a radioactive source for classroom demonstrations. 40
K is the radioisotope with the largest abundance in the body. In healthy animals and people, 40
K represents the largest source of radioactivity, greater even than 14
C. In a human body of 70 kg, about 4,400 nuclei of 40
K decay per second.[30] The activity of natural potassium is 31 Bq/g.[31]
Cosmic formation and distribution
Potassium is formed in supernovae by nucleosynthesis from lighter atoms. Potassium is principally created in Type II supernovae via an explosive oxygen-burning process.[32] (These are fusion reactions; do not confuse with chemical burning between potassium and oxygen.) 40
K is also formed in s-process nucleosynthesis and the neon burning process.[33]
Potassium is the 20th most abundant element in the solar system and the 17th most abundant element by weight in the Earth. It makes up about 2.6% of the weight of the Earth’s crust and is the seventh most abundant element in the crust.[34] The potassium concentration in seawater is 0.39 g/L[7] (0.039 wt/v%), about one twenty-seventh the concentration of sodium.[35][36]
Potash
Potash is primarily a mixture of potassium salts because plants have little or no sodium content, and the rest of a plant’s major mineral content consists of calcium salts of relatively low solubility in water. While potash has been used since ancient times, its composition was not understood. Georg Ernst Stahl obtained experimental evidence that led him to suggest the fundamental difference of sodium and potassium salts in 1702,[10] and Henri Louis Duhamel du Monceau was able to prove this difference in 1736.[37] The exact chemical composition of potassium and sodium compounds, and the status as chemical element of potassium and sodium, was not known then, and thus Antoine Lavoisier did not include the alkali in his list of chemical elements in 1789.[38][39] For a long time the only significant applications for potash were the production of glass, bleach, soap and gunpowder as potassium nitrate.[40] Potassium soaps from animal fats and vegetable oils were especially prized because they tend to be more water-soluble and of softer texture, and are therefore known as soft soaps.[11] The discovery by Justus Liebig in 1840 that potassium is a necessary element for plants and that most types of soil lack potassium[41] caused a steep rise in demand for potassium salts. Wood-ash from fir trees was initially used as a potassium salt source for fertilizer, but, with the discovery in 1868 of mineral deposits containing potassium chloride near Staßfurt, Germany, the production of potassium-containing fertilizers began at an industrial scale.[42][43][44] Other potash deposits were discovered, and by the 1960s Canada became the dominant producer.[45][46]
Metal
Pieces of potassium metal
Potassium metal was first isolated in 1807 by Humphry Davy, who derived it by electrolysis of molten KOH with the newly discovered voltaic pile. Potassium was the first metal that was isolated by electrolysis.[47] Later in the same year, Davy reported extraction of the metal sodium from a mineral derivative (caustic soda, NaOH, or lye) rather than a plant salt, by a similar technique, demonstrating that the elements, and thus the salts, are different.[38][39][48][49] Although the production of potassium and sodium metal should have shown that both are elements, it took some time before this view was universally accepted.[39]
Because of the sensitivity of potassium to water and air, air-free techniques are normally employed for handling the element. It is unreactive toward nitrogen and saturated hydrocarbons such as mineral oil or kerosene.[50] It readily dissolves in liquid ammonia, up to 480 g per 1000 g of ammonia at 0 °C. Depending on the concentration, the ammonia solutions are blue to yellow, and their electrical conductivity is similar to that of liquid metals. Potassium slowly reacts with ammonia to form KNH
2, but this reaction is accelerated by minute amounts of transition metal salts.[51] Because it can reduce the salts to the metal, potassium is often used as the reductant in the preparation of finely divided metals from their salts by the Rieke method.[52] Illustrative is the preparation of magnesium:
- MgCl2 + 2 K → Mg + 2 KCl
Geology
Elemental potassium does not occur in nature because of its high reactivity. It reacts violently with water (see section Precautions below)[50] and also reacts with oxygen. Orthoclase (potassium feldspar) is a common rock-forming mineral. Granite for example contains 5% potassium, which is well above the average in the Earth’s crust. Sylvite (KCl), carnallite (KCl·MgCl2·6H2O), kainite (MgSO4·KCl·3H2O) and langbeinite (MgSO4·K2SO4) are the minerals found in large evaporite deposits worldwide. The deposits often show layers starting with the least soluble at the bottom and the most soluble on top.[36] Deposits of niter (potassium nitrate) are formed by decomposition of organic material in contact with atmosphere, mostly in caves; because of the good water solubility of niter the formation of larger deposits requires special environmental conditions.[53]
Biological role
Potassium is the eighth or ninth most common element by mass (0.2%) in the human body, so that a 60 kg adult contains a total of about 120 g of potassium.[54] The body has about as much potassium as sulfur and chlorine, and only calcium and phosphorus are more abundant (with the exception of the ubiquitous CHON elements).[55] Potassium ions are present in a wide variety of proteins and enzymes.[56]
Biochemical function
Potassium levels influence multiple physiological processes, including[57][58][59]
- resting cellular-membrane potential and the propagation of action potentials in neuronal, muscular, and cardiac tissue. Due to the electrostatic and chemical properties, K+ ions are larger than Na+ ions, and ion channels and pumps in cell membranes can differentiate between the two ions, actively pumping or passively passing one of the two ions while blocking the other.[60]
- hormone secretion and action
- vascular tone
- systemic blood pressure control
- gastrointestinal motility
- acid–base homeostasis
- glucose and insulin metabolism
- mineralocorticoid action
- renal concentrating ability
- fluid and electrolyte balance
Homeostasis
Potassium homeostasis denotes the maintenance of the total body potassium content, plasma potassium level, and the ratio of the intracellular to extracellular potassium concentrations within narrow limits, in the face of pulsatile intake (meals), obligatory renal excretion, and shifts between intracellular and extracellular compartments.
Plasma levels
Plasma potassium is normally kept at 3.5 to 5.5 millimoles (mmol) [or milliequivalents (mEq)] per liter by multiple mechanisms.[61] Levels outside this range are associated with an increasing rate of death from multiple causes,[62] and some cardiac, kidney,[63] and lung diseases progress more rapidly if serum potassium levels are not maintained within the normal range.
An average meal of 40–50 mmol presents the body with more potassium than is present in all plasma (20–25 mmol). However, this surge causes the plasma potassium to rise only 10% at most as a result of prompt and efficient clearance by both renal and extra-renal mechanisms.[64]
Hypokalemia, a deficiency of potassium in the plasma, can be fatal if severe. Common causes are increased gastrointestinal loss (vomiting, diarrhea), and increased renal loss (diuresis).[65] Deficiency symptoms include muscle weakness, paralytic ileus, ECG abnormalities, decreased reflex response; and in severe cases, respiratory paralysis, alkalosis, and cardiac arrhythmia.[66]
Control mechanisms
Potassium content in the plasma is tightly controlled by four basic mechanisms, which have various names and classifications. The four are 1) a reactive negative-feedback system, 2) a reactive feed-forward system, 3) a predictive or circadian system, and 4) an internal or cell membrane transport system. Collectively, the first three are sometimes termed the «external potassium homeostasis system»;[67] and the first two, the «reactive potassium homeostasis system».
- The reactive negative-feedback system refers to the system that induces renal secretion of potassium in response to a rise in the plasma potassium (potassium ingestion, shift out of cells, or intravenous infusion.)
- The reactive feed-forward system refers to an incompletely understood system that induces renal potassium secretion in response to potassium ingestion prior to any rise in the plasma potassium. This is probably initiated by gut cell potassium receptors that detect ingested potassium and trigger vagal afferent signals to the pituitary gland.
- The predictive or circadian system increases renal secretion of potassium during mealtime hours (e.g. daytime for humans, nighttime for rodents) independent of the presence, amount, or absence of potassium ingestion. It is mediated by a circadian oscillator in the suprachiasmatic nucleus of the brain (central clock), which causes the kidney (peripheral clock) to secrete potassium in this rhythmic circadian fashion.
The action of the sodium-potassium pump is an example of primary active transport. The two carrier proteins embedded in the cell membrane on the left are using ATP to move sodium out of the cell against the concentration gradient; The two proteins on the right are using secondary active transport to move potassium into the cell. This process results in reconstitution of ATP.
- The ion transport system moves potassium across the cell membrane using two mechanisms. One is active and pumps sodium out of, and potassium into, the cell. The other is passive and allows potassium to leak out of the cell. Potassium and sodium cations influence fluid distribution between intracellular and extracellular compartments by osmotic forces. The movement of potassium and sodium through the cell membrane is mediated by the Na⁺/K⁺-ATPase pump.[68] This ion pump uses ATP to pump three sodium ions out of the cell and two potassium ions into the cell, creating an electrochemical gradient and electromotive force across the cell membrane. The highly selective potassium ion channels (which are tetramers) are crucial for hyperpolarization inside neurons after an action potential is triggered, to cite one example. The most recently discovered potassium ion channel is KirBac3.1, which makes a total of five potassium ion channels (KcsA, KirBac1.1, KirBac3.1, KvAP, and MthK) with a determined structure. All five are from prokaryotic species.[69]
Renal filtration, reabsorption, and excretion
Renal handling of potassium is closely connected to sodium handling. Potassium is the major cation (positive ion) inside animal cells [150 mmol/L, (4.8 g)], while sodium is the major cation of extracellular fluid [150 mmol/L, (3.345 g)]. In the kidneys, about 180 liters of plasma is filtered through the glomeruli and into the renal tubules per day.[70] This filtering involves about 600 g of sodium and 33 g of potassium. Since only 1–10 g of sodium and 1–4 g of potassium are likely to be replaced by diet, renal filtering must efficiently reabsorb the remainder from the plasma.
Sodium is reabsorbed to maintain extracellular volume, osmotic pressure, and serum sodium concentration within narrow limits. Potassium is reabsorbed to maintain serum potassium concentration within narrow limits.[71] Sodium pumps in the renal tubules operate to reabsorb sodium. Potassium must be conserved, but because the amount of potassium in the blood plasma is very small and the pool of potassium in the cells is about 30 times as large, the situation is not so critical for potassium. Since potassium is moved passively[72][73] in counter flow to sodium in response to an apparent (but not actual) Donnan equilibrium,[74] the urine can never sink below the concentration of potassium in serum except sometimes by actively excreting water at the end of the processing. Potassium is excreted twice and reabsorbed three times before the urine reaches the collecting tubules.[75] At that point, urine usually has about the same potassium concentration as plasma. At the end of the processing, potassium is secreted one more time if the serum levels are too high.[citation needed]
With no potassium intake, it is excreted at about 200 mg per day until, in about a week, potassium in the serum declines to a mildly deficient level of 3.0–3.5 mmol/L.[76] If potassium is still withheld, the concentration continues to fall until a severe deficiency causes eventual death.[77]
The potassium moves passively through pores in the cell membrane. When ions move through Ion transporters (pumps) there is a gate in the pumps on both sides of the cell membrane and only one gate can be open at once. As a result, approximately 100 ions are forced through per second. Ion channel have only one gate, and there only one kind of ion can stream through, at 10 million to 100 million ions per second.[78] Calcium is required to open the pores,[79] although calcium may work in reverse by blocking at least one of the pores.[80] Carbonyl groups inside the pore on the amino acids mimic the water hydration that takes place in water solution[81] by the nature of the electrostatic charges on four carbonyl groups inside the pore.[82]
Nutrition
Dietary recommendations
The U.S. National Academy of Medicine (NAM), on behalf of both the U.S. and Canada, sets Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs), or Adequate Intakes (AIs) for when there is not sufficient information to set EARs and RDAs. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes.
For both males and females under 9 years of age, the AIs for potassium are: 400 mg of potassium for 0-6-month-old infants, 860 mg of potassium for 7-12-month-old infants, 2,000 mg of potassium for 1-3-year-old children, and 2,300 mg of potassium for 4-8-year-old children.
For males 9 years of age and older, the AIs for potassium are: 2,500 mg of potassium for 9-13-year-old males, 3,000 mg of potassium for 14-18-year-old males, and 3,400 mg for males that are 19 years of age and older.
For females 9 years of age and older, the AIs for potassium are: 2,300 mg of potassium for 9-18-year-old females, and 2,600 mg of potassium for females that are 19 years of age and older.
For pregnant and lactating females, the AIs for potassium are: 2,600 mg of potassium for 14-18-year-old pregnant females, 2,900 mg for pregnant females that are 19 years of age and older; furthermore, 2,500 mg of potassium for 14-18-year-old lactating females, and 2,800 mg for lactating females that are 19 years of age and older. As for safety, the NAM also sets tolerable upper intake levels (ULs) for vitamins and minerals, but for potassium the evidence was insufficient, so no UL was established.[83][84]
As of 2004, most Americans adults consume less than 3,000 mg.[85]
Likewise, in the European Union, in particular in Germany, and Italy, insufficient potassium intake is somewhat common.[86] The British National Health Service recommends a similar intake, saying that adults need 3,500 mg per day and that excess amounts may cause health problems such as stomach pain and diarrhea.[87]
In 2019, the National Academies of Sciences, Engineering, and Medicine revised the Adequate Intake for potassium to 2,600 mg/day for females 19 years of age and older who are not pregnant or lactating, and 3,400 mg/day for males 19 years of age and older.[88][89]
Food sources
Potassium is present in all fruits, vegetables, meat and fish. Foods with high potassium concentrations include yam, parsley, dried apricots, milk, chocolate, all nuts (especially almonds and pistachios), potatoes, bamboo shoots, bananas, avocados, coconut water, soybeans, and bran.[90]
The USDA lists tomato paste, orange juice, beet greens, white beans, potatoes, plantains, bananas, apricots, and many other dietary sources of potassium, ranked in descending order according to potassium content. A day’s worth of potassium is in 5 plantains or 11 bananas.[91]
Deficient intake
Diets low in potassium can lead to hypertension[92] and hypokalemia.
Supplementation
Supplements of potassium are most widely used in conjunction with diuretics that block reabsorption of sodium and water upstream from the distal tubule (thiazides and loop diuretics), because this promotes increased distal tubular potassium secretion, with resultant increased potassium excretion.[medical citation needed] A variety of prescription and over-the counter supplements are available.[citation needed] Potassium chloride may be dissolved in water, but the salty/bitter taste makes liquid supplements unpalatable.[93] Typical doses range from 10 mmol (400 mg), to 20 mmol (800 mg).[medical citation needed] Potassium is also available in tablets or capsules, which are formulated to allow potassium to leach slowly out of a matrix, since very high concentrations of potassium ion that occur adjacent to a solid tablet can injure the gastric or intestinal mucosa.[medical citation needed] For this reason, non-prescription potassium pills are limited by law in the US to a maximum of 99 mg of potassium.[citation needed]
A meta-analysis concluded that a 1640 mg increase in the daily intake of potassium was associated with a 21% lower risk of stroke.[94] Potassium chloride and potassium bicarbonate may be useful to control mild hypertension.[95] In 2020, potassium was the 33rd most commonly prescribed medication in the United States, with more than 17 million prescriptions.[96][97]
Detection by taste buds
Potassium can be detected by taste because it triggers three of the five types of taste sensations, according to concentration. Dilute solutions of potassium ions taste sweet, allowing moderate concentrations in milk and juices, while higher concentrations become increasingly bitter/alkaline, and finally also salty to the taste. The combined bitterness and saltiness of high-potassium solutions makes high-dose potassium supplementation by liquid drinks a palatability challenge.[93][98]
Commercial production
Mining
Potassium salts such as carnallite, langbeinite, polyhalite, and sylvite form extensive evaporite deposits in ancient lake bottoms and seabeds,[35] making extraction of potassium salts in these environments commercially viable. The principal source of potassium – potash – is mined in Canada, Russia, Belarus, Kazakhstan, Germany, Israel, United States, Jordan, and other places around the world.[99][100][101] The first mined deposits were located near Staßfurt, Germany, but the deposits span from Great Britain over Germany into Poland. They are located in the Zechstein and were deposited in the Middle to Late Permian. The largest deposits ever found lie 1,000 meters (3,300 feet) below the surface of the Canadian province of Saskatchewan. The deposits are located in the Elk Point Group produced in the Middle Devonian. Saskatchewan, where several large mines have operated since the 1960s pioneered the technique of freezing of wet sands (the Blairmore formation) to drive mine shafts through them. The main potash mining company in Saskatchewan until its merge was the Potash Corporation of Saskatchewan, now Nutrien.[102] The water of the Dead Sea is used by Israel and Jordan as a source of potash, while the concentration in normal oceans is too low for commercial production at current prices.[100][101]
Several methods are used to separate potassium salts from sodium and magnesium compounds. The most-used method is fractional precipitation using the solubility differences of the salts. Electrostatic separation of the ground salt mixture is also used in some mines. The resulting sodium and magnesium waste is either stored underground or piled up in slag heaps. Most of the mined potassium mineral ends up as potassium chloride after processing. The mineral industry refers to potassium chloride either as potash, muriate of potash, or simply MOP.[36]
Pure potassium metal can be isolated by electrolysis of its hydroxide in a process that has changed little since it was first used by Humphry Davy in 1807. Although the electrolysis process was developed and used in industrial scale in the 1920s, the thermal method by reacting sodium with potassium chloride in a chemical equilibrium reaction became the dominant method in the 1950s.
- Na + KCl → NaCl + K
The production of sodium potassium alloys is accomplished by changing the reaction time and the amount of sodium used in the reaction. The Griesheimer process employing the reaction of potassium fluoride with calcium carbide was also used to produce potassium.[36][103]
- 2 KF + CaC2 → 2 K + CaF2 + 2 C
Reagent-grade potassium metal costs about $10.00/pound ($22/kg) in 2010 when purchased by the tonne. Lower purity metal is considerably cheaper. The market is volatile because long-term storage of the metal is difficult. It must be stored in a dry inert gas atmosphere or anhydrous mineral oil to prevent the formation of a surface layer of potassium superoxide, a pressure-sensitive explosive that detonates when scratched. The resulting explosion often starts a fire difficult to extinguish.[104][105]
Cation identification
Potassium is now quantified by ionization techniques, but at one time it was quantitated by gravimetric analysis.
Reagents used to precipitate potassium salts include sodium tetraphenylborate, hexachloroplatinic acid, and sodium cobaltinitrite into respectively potassium tetraphenylborate, potassium hexachloroplatinate, and potassium cobaltinitrite.[50]
The reaction with sodium cobaltinitrite is illustrative:
- 3 K+ + Na3[Co(NO2)6] → K3[Co(NO2)6] + 3 Na+
The potassium cobaltinitrite is obtained as a yellow solid.
Commercial uses
Fertilizer
Potassium sulfate/magnesium sulfate fertilizer
Potassium ions are an essential component of plant nutrition and are found in most soil types.[11] They are used as a fertilizer in agriculture, horticulture, and hydroponic culture in the form of chloride (KCl), sulfate (K2SO4), or nitrate (KNO3), representing the ‘K’ in ‘NPK’. Agricultural fertilizers consume 95% of global potassium chemical production, and about 90% of this potassium is supplied as KCl.[11] The potassium content of most plants ranges from 0.5% to 2% of the harvested weight of crops, conventionally expressed as amount of K2O. Modern high-yield agriculture depends upon fertilizers to replace the potassium lost at harvest. Most agricultural fertilizers contain potassium chloride, while potassium sulfate is used for chloride-sensitive crops or crops needing higher sulfur content. The sulfate is produced mostly by decomposition of the complex minerals kainite (MgSO4·KCl·3H2O) and langbeinite (MgSO4·K2SO4). Only a very few fertilizers contain potassium nitrate.[106] In 2005, about 93% of world potassium production was consumed by the fertilizer industry.[101] Furthermore, potassium can play a key role in nutrient cycling by controlling litter composition.[107]
Medical use
Potassium citrate
Potassium citrate is used to treat a kidney stone condition called renal tubular acidosis.[108]
Potassium chloride
Potassium, in the form of potassium chloride is used as a medication to treat and prevent low blood potassium.[109] Low blood potassium may occur due to vomiting, diarrhea, or certain medications.[110] It is given by slow injection into a vein or by mouth.[111]
Food additives
Potassium sodium tartrate (KNaC4H4O6, Rochelle salt) is a main constituent of some varieties of baking powder; it is also used in the silvering of mirrors. Potassium bromate (KBrO3) is a strong oxidizer (E924), used to improve dough strength and rise height. Potassium bisulfite (KHSO3) is used as a food preservative, for example in wine and beer-making (but not in meats). It is also used to bleach textiles and straw, and in the tanning of leathers.[112][113]
Industrial
Major potassium chemicals are potassium hydroxide, potassium carbonate, potassium sulfate, and potassium chloride. Megatons of these compounds are produced annually.[114]
Potassium hydroxide KOH is a strong base, which is used in industry to neutralize strong and weak acids, to control pH and to manufacture potassium salts. It is also used to saponify fats and oils, in industrial cleaners, and in hydrolysis reactions, for example of esters.[115][116]
Potassium nitrate (KNO3) or saltpeter is obtained from natural sources such as guano and evaporites or manufactured via the Haber process; it is the oxidant in gunpowder (black powder) and an important agricultural fertilizer. Potassium cyanide (KCN) is used industrially to dissolve copper and precious metals, in particular silver and gold, by forming complexes. Its applications include gold mining, electroplating, and electroforming of these metals; it is also used in organic synthesis to make nitriles. Potassium carbonate (K2CO3 or potash) is used in the manufacture of glass, soap, color TV tubes, fluorescent lamps, textile dyes and pigments.[117] Potassium permanganate (KMnO4) is an oxidizing, bleaching and purification substance and is used for production of saccharin. Potassium chlorate (KClO3) is added to matches and explosives. Potassium bromide (KBr) was formerly used as a sedative and in photography.[11]
While potassium chromate (K2CrO4) is used in the manufacture of a host of different commercial products such as inks, dyes, wood stains (by reacting with the tannic acid in wood), explosives, fireworks, fly paper, and safety matches,[118] as well as in the tanning of leather, all of these uses are due to the chemistry of the chromate ion rather than to that of the potassium ion.[119]
Niche uses
There are thousands of uses of various potassium compounds. One example is potassium superoxide, KO2, an orange solid that acts as a portable source of oxygen and a carbon dioxide absorber. It is widely used in respiration systems in mines, submarines and spacecraft as it takes less volume than the gaseous oxygen.[120][121]
- 4 KO2 + 2 CO2 → 2 K2CO3 + 3 O2
Another example is potassium cobaltinitrite, K3[Co(NO2)6], which is used as artist’s pigment under the name of Aureolin or Cobalt Yellow.[122]
The stable isotopes of potassium can be laser cooled and used to probe fundamental and technological problems in quantum physics. The two bosonic isotopes possess convenient Feshbach resonances to enable studies requiring tunable interactions, while 40
K is one of only two stable fermions amongst the alkali metals.[123]
Laboratory uses
An alloy of sodium and potassium, NaK is a liquid used as a heat-transfer medium and a desiccant for producing dry and air-free solvents. It can also be used in reactive distillation.[124] The ternary alloy of 12% Na, 47% K and 41% Cs has the lowest melting point of −78 °C of any metallic compound.[18]
Metallic potassium is used in several types of magnetometers.[125]
Precautions
| Hazards | |
|---|---|
| GHS labelling: | |
|
Pictograms |
 
|
|
Signal word |
Danger |
|
Hazard statements |
H260, H314 |
|
Precautionary statements |
P223, P231+P232, P280, P305+P351+P338, P370+P378, P422[126] |
| NFPA 704 (fire diamond) |
3 3 2
|
Potassium metal can react violently with water producing potassium hydroxide (KOH) and hydrogen gas.
- 2 K(s) + 2 H2O(l) → 2 KOH(aq) + H2(g)↑
A reaction of potassium metal with water. Hydrogen is produced, and with potassium vapor, burns with a pink or lilac flame. Strongly alkaline potassium hydroxide is formed in solution.
This reaction is exothermic and releases sufficient heat to ignite the resulting hydrogen in the presence of oxygen. Finely powdered potassium ignites in air at room temperature. The bulk metal ignites in air if heated. Because its density is 0.89 g/cm3, burning potassium floats in water that exposes it to atmospheric oxygen. Many common fire extinguishing agents, including water, either are ineffective or make a potassium fire worse. Nitrogen, argon, sodium chloride (table salt), sodium carbonate (soda ash), and silicon dioxide (sand) are effective if they are dry. Some Class D dry powder extinguishers designed for metal fires are also effective. These agents deprive the fire of oxygen and cool the potassium metal.[127]
During storage, potassium forms peroxides and superoxides. These peroxides may react violently with organic compounds such as oils. Both peroxides and superoxides may react explosively with metallic potassium.[128]
Because potassium reacts with water vapor in the air, it is usually stored under anhydrous mineral oil or kerosene. Unlike lithium and sodium, however, potassium should not be stored under oil for longer than six months, unless in an inert (oxygen free) atmosphere, or under vacuum. After prolonged storage in air dangerous shock-sensitive peroxides can form on the metal and under the lid of the container, and can detonate upon opening.[129]
Ingestion of large amounts of potassium compounds can lead to hyperkalemia, strongly influencing the cardiovascular system.[130][131] Potassium chloride is used in the United States for lethal injection executions.[130]
See also
References
- ^ «Standard Atomic Weights: Potassium». CIAAW. 1979.
- ^ a b Aitken, F.; Volino, F. (January 2022). «New equations of state describing both the dynamic viscosity and self-diffusion coefficient for potassium and thallium in their fluid phases». Physics of Fluids. 34 (1): 017112. doi:10.1063/5.0079944.
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.122. ISBN 1-4398-5511-0.
- ^ Magnetic susceptibility of the elements and inorganic compounds, in Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Augustyn, Adam. «Potassium/ Chemical element». Encyclopedia Britannica. Retrieved 2019-04-17.
Potassium Physical properties
- ^ a b Webb, D. A. (April 1939). «The Sodium and Potassium Content of Sea Water» (PDF). The Journal of Experimental Biology (2): 183.
- ^ Anthoni, J. (2006). «Detailed composition of seawater at 3.5% salinity». seafriends.org.nz. Retrieved 2011-09-23.
- ^ Halperin, Mitchell L.; Kamel, Kamel S. (1998-07-11). «Potassium». The Lancet. 352 (9122): 135–140. doi:10.1016/S0140-6736(98)85044-7. ISSN 0140-6736. PMID 9672294. S2CID 208790031.
- ^ a b Marggraf, Andreas Siegmund (1761). Chymische Schriften. p. 167.
- ^ a b c d e Greenwood, p. 73
- ^ Davy, Humphry (1808). «On some new phenomena of chemical changes produced by electricity, in particular the decomposition of the fixed alkalies, and the exhibition of the new substances that constitute their bases; and on the general nature of alkaline bodies». Philosophical Transactions of the Royal Society. 98: 32. doi:10.1098/rstl.1808.0001.
- ^ Klaproth, M. (1797) «Nouvelles données relatives à l’histoire naturelle de l’alcali végétal» (New data regarding the natural history of the vegetable alkali), Mémoires de l’Académie royale des sciences et belles-lettres (Berlin), pp. 9–13 ; see p. 13. From p. 13: «Cet alcali ne pouvant donc plus être envisagé comme un produit de la végétation dans les plantes, occupe une place propre dans la série des substances primitivement simples du règne minéral, &I il devient nécessaire de lui assigner un nom, qui convienne mieux à sa nature.
La dénomination de Potasche (potasse) que la nouvelle nomenclature françoise a consacrée comme nom de tout le genre, ne sauroit faire fortune auprès des chimistes allemands, qui sentent à quel point la dérivation étymologique en est vicieuse. Elle est prise en effet de ce qu’anciennement on se servoit pour la calcination des lessives concentrées des cendres, de pots de fer (pott en dialecte de la Basse-Saxe) auxquels on a substitué depuis des fours à calciner.
Je propose donc ici, de substituer aux mots usités jusqu’ici d’alcali des plantes, alcali végétal, potasse, &c. celui de kali, & de revenir à l’ancienne dénomination de natron, au lieu de dire alcali minéral, soude &c.»
(This alkali [i.e., potash] — [which] therefore can no longer be viewed as a product of growth in plants — occupies a proper place in the originally simple series of the mineral realm, and it becomes necessary to assign it a name that is better suited to its nature.
The name of «potash» (potasse), which the new French nomenclature has bestowed as the name of the entire species [i.e., substance], would not find acceptance among German chemists, who feel to some extent [that] the etymological derivation of it is faulty. Indeed, it is taken from [the vessels] that one formerly used for the roasting of washing powder concentrated from cinders: iron pots (pott in the dialect of Lower Saxony), for which roasting ovens have been substituted since then.
Thus I now propose to substitute for the until now common words of «plant alkali», «vegetable alkali», «potash», etc., that of kali ; and to return to the old name of natron instead of saying «mineral alkali», «soda», etc.) - ^ Davy, Humphry (1809). «Ueber einige neue Erscheinungen chemischer Veränderungen, welche durch die Electricität bewirkt werden; insbesondere über die Zersetzung der feuerbeständigen Alkalien, die Darstellung der neuen Körper, welche ihre Basen ausmachen, und die Natur der Alkalien überhaupt» [On some new phenomena of chemical changes that are achieved by electricity; particularly the decomposition of flame-resistant alkalis [i.e., alkalies that cannot be reduced to their base metals by flames], the preparation of new substances that constitute their [metallic] bases, and the nature of alkalies generally]. Annalen der Physik. 31 (2): 113–175. Bibcode:1809AnP….31..113D. doi:10.1002/andp.18090310202.
p. 157: In unserer deutschen Nomenclatur würde ich die Namen Kalium und Natronium vorschlagen, wenn man nicht lieber bei den von Herrn Erman gebrauchten und von mehreren angenommenen Benennungen Kali-Metalloid and Natron-Metalloid, bis zur völligen Aufklärung der chemischen Natur dieser räthzelhaften Körper bleiben will. Oder vielleicht findet man es noch zweckmässiger fürs Erste zwei Klassen zu machen, Metalle und Metalloide, und in die letztere Kalium und Natronium zu setzen. — Gilbert. (In our German nomenclature, I would suggest the names Kalium and Natronium, if one would not rather continue with the appellations Kali-metalloid and Natron-metalloid which are used by Mr. Erman [i.e., German physics professor Paul Erman (1764–1851)] and accepted by several [people], until the complete clarification of the chemical nature of these puzzling substances. Or perhaps one finds it yet more advisable for the present to create two classes, metals and metalloids, and to place Kalium and Natronium in the latter — Gilbert.)
- ^ Berzelius, J. Jacob (1814) Försök, att, genom användandet af den electrokemiska theorien och de kemiska proportionerna, grundlägga ett rent vettenskapligt system för mineralogien [Attempt, by the use of electrochemical theory and chemical proportions, to found a pure scientific system for mineralogy]. Stockholm, Sweden: A. Gadelius., p. 87.
- ^ 19. Kalium (Potassium) – Elementymology & Elements Multidict. vanderkrogt.net
- ^ McNaught, A. D. and Wilkinson,A. eds. (1997). Compendium of Chemical Terminology, 2nd ed. (the «Gold Book»). IUPAC. Blackwell Scientific Publications, Oxford.
- ^ a b Greenwood, p. 76
- ^ Greenwood, p. 75
- ^ Dye, J. L. (1979). «Compounds of Alkali Metal Anions». Angewandte Chemie International Edition. 18 (8): 587–598. doi:10.1002/anie.197905871.
- ^ Williams, D. Bradley G.; Lawton, Michelle (2010). «Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants». The Journal of Organic Chemistry. 75 (24): 8351–8354. doi:10.1021/jo101589h. PMID 20945830. S2CID 17801540.
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida, United States: CRC Press. pp. 477, 520. ISBN 978-0-8493-0594-8.
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 4–80. ISBN 0-8493-0486-5.
- ^ Schultz, p. 94
- ^ Lincoln, S. F.; Richens, D. T. and Sykes, A. G. «Metal Aqua Ions» in J. A. McCleverty and T. J. Meyer (eds.) Comprehensive Coordination Chemistry II, Vol. 1, pp. 515–555, ISBN 978-0-08-043748-4.
- ^ Anthony Agulyanski (2004). «Fluorine chemistry in the processing of tantalum and niobium». In Anatoly Agulyanski (ed.). Chemistry of Tantalum and Niobium Fluoride Compounds (1st ed.). Burlington: Elsevier. ISBN 9780080529028.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), «The NUBASE evaluation of nuclear and decay properties», Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729….3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ Bowen, Robert; Attendorn, H. G. (1988). «Theory and Assumptions in Potassium–Argon Dating». Isotopes in the Earth Sciences. Springer. pp. 203–8. ISBN 978-0-412-53710-3.
- ^ Anaç, D. & Martin-Prével, P. (1999). Improved crop quality by nutrient management. Springer. pp. 290–. ISBN 978-0-7923-5850-3.
- ^ «Radiation and Radioactive Decay. Radioactive Human Body». Harvard Natural Sciences Lecture Demonstrations. Retrieved July 2, 2016.
- ^ Winteringham, F. P. W; Effects, F.A.O. Standing Committee on Radiation, Land And Water Development Division, Food and Agriculture Organization of the United Nations (1989). Radioactive fallout in soils, crops and food: a background review. Food & Agriculture Org. p. 32. ISBN 978-92-5-102877-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Shimansky, V.; Bikmaev, I. F.; Galeev, A. I.; Shimanskaya, N. N.; et al. (September 2003). «Observational constraints on potassium synthesis during the formation of stars of the Galactic disk». Astronomy Reports. 47 (9): 750–762. Bibcode:2003ARep…47..750S. doi:10.1134/1.1611216. S2CID 120396773.
- ^ The, L.-S.; Eid, M. F. El; Meyer, B. S. (2000). «A New Study of s-Process Nucleosynthesis in Massive Stars». The Astrophysical Journal. 533 (2): 998. arXiv:astro-ph/9812238. Bibcode:2000ApJ…533..998T. doi:10.1086/308677. ISSN 0004-637X. S2CID 7698683.
- ^ Greenwood, p. 69
- ^ a b Micale, Giorgio; Cipollina, Andrea; Rizzuti, Lucio (2009). Seawater Desalination: Conventional and Renewable Energy Processes. Springer. p. 3. ISBN 978-3-642-01149-8.
- ^ a b c d Prud’homme, Michel; Krukowski, Stanley T. (2006). «Potash». Industrial minerals & rocks: commodities, markets, and uses. Society for Mining, Metallurgy, and Exploration. pp. 723–740. ISBN 978-0-87335-233-8.
- ^ du Monceau, H. L. D. (1702–1797). «Sur la Base de Sel Marin». Mémoires de l’Académie Royale des Sciences (in French): 65–68.
- ^ a b Weeks, Mary Elvira (1932). «The discovery of the elements. IX. Three alkali metals: Potassium, sodium, and lithium». Journal of Chemical Education. 9 (6): 1035. Bibcode:1932JChEd…9.1035W. doi:10.1021/ed009p1035.
- ^ a b c Siegfried, R. (1963). «The Discovery of Potassium and Sodium, and the Problem of the Chemical Elements». Isis. 54 (2): 247–258. doi:10.1086/349704. JSTOR 228541. PMID 14147904. S2CID 38152048.
- ^ Browne, C. A. (1926). «Historical notes upon the domestic potash industry in early colonial and later times». Journal of Chemical Education. 3 (7): 749–756. Bibcode:1926JChEd…3..749B. doi:10.1021/ed003p749.
- ^ Liebig, Justus von (1840). Die organische Chemie in ihrer Anwendung auf Agricultur und Physiologie (in German). F. Vieweg und Sohn.
- ^ Cordel, Oskar (1868). Die Stassfurter Kalisalze in der Landwirthschalt: Eine Besprechung … (in German). L. Schnock.
- ^ Birnbaum, Karl (1869). Die Kalidüngung in ihren Vortheilen und Gefahren (in German).
- ^ United Nations Industrial Development Organization and Int’l Fertilizer Development Center (1998). Fertilizer Manual. pp. 46, 417. ISBN 978-0-7923-5032-3.
- ^ Miller, H. (1980). «Potash from Wood Ashes: Frontier Technology in Canada and the United States». Technology and Culture. 21 (2): 187–208. doi:10.2307/3103338. JSTOR 3103338.
- ^ Rittenhouse, P. A. (1979). «Potash and politics». Economic Geology. 74 (2): 353–7. doi:10.2113/gsecongeo.74.2.353.
- ^ Enghag, P. (2004). «11. Sodium and Potassium». Encyclopedia of the elements. Wiley-VCH Weinheim. ISBN 978-3-527-30666-4.
- ^ Davy, Humphry (1808). «On some new phenomena of chemical changes produced by electricity, in particular the decomposition of the fixed alkalies, and the exhibition of the new substances that constitute their bases; and on the general nature of alkaline bodies». Philosophical Transactions of the Royal Society. 98: 1–44. doi:10.1098/rstl.1808.0001.
- ^ Shaposhnik, V. A. (2007). «History of the discovery of potassium and sodium (on the 200th anniversary of the discovery of potassium and sodium)». Journal of Analytical Chemistry. 62 (11): 1100–2. doi:10.1134/S1061934807110160. S2CID 96141217.
- ^ a b c Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). «Potassium». Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. ISBN 978-3-11-007511-3.
- ^ Burkhardt, p. 32
- ^ Rieke, R. D. (1989). «Preparation of Organometallic Compounds from Highly Reactive Metal Powders». Science. 246 (4935): 1260–4. Bibcode:1989Sci…246.1260R. doi:10.1126/science.246.4935.1260. PMID 17832221. S2CID 92794.
- ^ Ross, William H. (1914). «The Origin of Nitrate Deposits». Popular Science. Bonnier Corporation. pp. 134–145.
- ^ Abdel-Wahab, M.; Youssef, S.; Aly, A.; el-Fiki, S.; et al. (1992). «A simple calibration of a whole-body counter for the measurement of total body potassium in humans». International Journal of Radiation Applications and Instrumentation A. 43 (10): 1285–9. doi:10.1016/0883-2889(92)90208-V. PMID 1330980.
- ^ Chang, Raymond (2007). Chemistry. McGraw-Hill Higher Education. p. 52. ISBN 978-0-07-110595-8.
- ^ Vašák, Milan; Schnabl, Joachim (2016). «Chapter 8. Sodium and Potassium Ions in Proteins and Enzyme Catalysis». In Astrid, Sigel; Helmut, Sigel; Roland K.O., Sigel (eds.). The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. Vol. 16. Springer. pp. 259–290. doi:10.1007/978-3-319-21756-7_8. PMID 26860304.
- ^ Weiner ID, Linus S, Wingo CS (2014). «Disorders of potassium metabolism». In Freehally J, Johnson RJ, Floege J (eds.). Comprehensive clinical nephrology (5th ed.). St. Louis: Saunders. p. 118. ISBN 9780323242875.
- ^ Malnic G, Giebisch G, Muto S, Wang W, Bailey MA, Satlin LM (2013). «Regulation of K+ excretion». In Alpern RJ, Caplan MJ, Moe OW (eds.). Seldin and Giebisch’s the kidney: physiology and pathophysiology (5th ed.). London: Academic Press. pp. 1659–1716. ISBN 9780123814630.
- ^ Mount DB, Zandi-Nejad K (2011). «Disorders of potassium balance». In Taal MW, Chertow GM, Marsden PA, Skorecki KL, Yu AS, Brenner BM (eds.). The kidney (9th ed.). Philadelphia: Elsevier. pp. 640–688. ISBN 9781455723041.
- ^ Lockless, S. W.; Zhou, M.; MacKinnon, R. (2007). «Structural and thermodynamic properties of selective ion binding in a K+ channel». PLOS Biol. 5 (5): e121. doi:10.1371/journal.pbio.0050121. PMC 1858713. PMID 17472437.
- ^ Wei, Kuang-Yu; Gritter, Martin; Vogt, Liffert; de Borst, Martin H; Rotmans, Joris I; Hoorn, Ewout J (2020-09-02). «Dietary potassium and the kidney: lifesaving physiology». Clinical Kidney Journal. Oxford University Press (OUP). 13 (6): 952–968. doi:10.1093/ckj/sfaa157. ISSN 2048-8513. PMC 7769543. PMID 33391739.
- ^ Goyal, Abhinav; Spertus, John A.; Gosch, Kensey; Venkitachalam, Lakshmi; Jones, Philip G.; Van den Berghe, Greet; Kosiborod, Mikhail (2012). «Serum Potassium Levels and Mortality in Acute Myocardial Infarction». JAMA. 307 (2): 157–164. doi:10.1001/jama.2011.1967. PMID 22235086.
- ^ Smyth, A.; Dunkler, D.; Gao, P.; et al. (2014). «The relationship between estimated sodium and potassium excretion and subsequent renal outcomes». Kidney Int. 86 (6): 1205–1212. doi:10.1038/ki.2014.214. PMID 24918156.
- ^ Moore-Ede, M. C. (1986). «Physiology of the circadian timing system: predictive versus reactive homeostasis». Am J Physiol. 250 (5 Pt 2): R737–R752. doi:10.1152/ajpregu.1986.250.5.R737. PMID 3706563.
- ^ Slonim, Anthony D.; Pollack, Murray M. (2006). «Potassium». Pediatric critical care medicine. Lippincott Williams & Wilkins. p. 812. ISBN 978-0-7817-9469-5.
- ^ Visveswaran, Kasi (2009). «hypokalemia». Essentials of Nephrology (2nd ed.). BI Publications. p. 257. ISBN 978-81-7225-323-3.
- ^ Gumz, Michelle L.; Rabinowitz, Lawrence; Wingo, Charles S. (2015-07-02). «An Integrated View of Potassium Homeostasis». The New England Journal of Medicine. 373 (1): 60–72. doi:10.1056/NEJMra1313341. ISSN 0028-4793. PMC 5675534. PMID 26132942.
- ^ Campbell, Neil (1987). Biology. Menlo Park, California: Benjamin/Cummings Pub. Co. p. 795. ISBN 978-0-8053-1840-1.
- ^ Hellgren, Mikko; Sandberg, Lars; Edholm, Olle (2006). «A comparison between two prokaryotic potassium channels (KirBac1.1 and KcsA) in a molecular dynamics (MD) simulation study». Biophysical Chemistry. 120 (1): 1–9. doi:10.1016/j.bpc.2005.10.002. PMID 16253415.
- ^ Potts, W. T. W.; Parry, G. (1964). Osmotic and ionic regulation in animals. Pergamon Press.
- ^ Lans, H. S.; Stein, I. F.; Meyer, K. A. (1952). «The relation of serum potassium to erythrocyte potassium in normal subjects and patients with potassium deficiency». American Journal of the Medical Sciences. 223 (1): 65–74. doi:10.1097/00000441-195201000-00011. PMID 14902792.
- ^ Bennett, C. M.; Brenner, B. M.; Berliner, R. W. (1968). «Micropuncture study of nephron function in the rhesus monkey». Journal of Clinical Investigation. 47 (1): 203–216. doi:10.1172/JCI105710. PMC 297160. PMID 16695942.
- ^ Solomon, A. K. (1962). «Pumps in the living cell». Scientific American. 207 (2): 100–8. Bibcode:1962SciAm.207b.100S. doi:10.1038/scientificamerican0862-100. PMID 13914986.
- ^ Kernan, Roderick P. (1980). Cell potassium (Transport in the life sciences). New York: Wiley. pp. 40, 48. ISBN 978-0-471-04806-0.
- ^ Wright, F. S. (1977). «Sites and mechanisms of potassium transport along the renal tubule». Kidney International. 11 (6): 415–432. doi:10.1038/ki.1977.60. PMID 875263.
- ^ Squires, R. D.; Huth, E. J. (1959). «Experimental potassium depletion in normal human subjects. I. Relation of ionic intakes to the renal conservation of potassium». Journal of Clinical Investigation. 38 (7): 1134–48. doi:10.1172/JCI103890. PMC 293261. PMID 13664789.
- ^ Fiebach, Nicholas H.; Barker, Lee Randol; Burton, John Russell & Zieve, Philip D. (2007). Principles of ambulatory medicine. Lippincott Williams & Wilkins. pp. 748–750. ISBN 978-0-7817-6227-4.
- ^ Gadsby, D. C. (2004). «Ion transport: spot the difference». Nature. 427 (6977): 795–7. Bibcode:2004Natur.427..795G. doi:10.1038/427795a. PMID 14985745. S2CID 5923529.; for a diagram of the potassium pores are viewed, see Miller, C (2001). «See potassium run». Nature. 414 (6859): 23–24. Bibcode:2001Natur.414…23M. doi:10.1038/35102126. PMID 11689922. S2CID 4423041.
- ^ Jiang, Y.; Lee, A.; Chen, J.; Cadene, M.; et al. (2002). «Crystal structure and mechanism of a calcium-gated potassium channel» (PDF). Nature. 417 (6888): 515–22. Bibcode:2002Natur.417..515J. doi:10.1038/417515a. PMID 12037559. S2CID 205029269. Archived from the original on 2009-04-24.
{{cite journal}}: CS1 maint: bot: original URL status unknown (link) - ^ Shi, N.; Ye, S.; Alam, A.; Chen, L.; et al. (2006). «Atomic structure of a Na+— and K+-conducting channel». Nature. 440 (7083): 570–4. Bibcode:2006Natur.440..570S. doi:10.1038/nature04508. PMID 16467789. S2CID 4355500.; includes a detailed picture of atoms in the pump.
- ^ Zhou, Y.; Morais-Cabral, J. H.; Kaufman, A.; MacKinnon, R. (2001). «Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution». Nature. 414 (6859): 43–48. Bibcode:2001Natur.414…43Z. doi:10.1038/35102009. PMID 11689936. S2CID 205022645.
- ^ Noskov, S. Y.; Bernèche, S.; Roux, B. (2004). «Control of ion selectivity in potassium channels by electrostatic and dynamic properties of carbonyl ligands». Nature. 431 (7010): 830–4. Bibcode:2004Natur.431..830N. doi:10.1038/nature02943. PMID 15483608. S2CID 4414885.
- ^ National Academies of Sciences, Engineering and Medicine (2019). «Potassium: Dietary Reference Intakes for Adequacy». In Stallings, Virginia A; Harrison, Meghan; Oria, Maria (eds.). Dietary Reference Intakes for Sodium and Potassium. Washington, DC: The National Academies Press. doi:10.17226/25353. ISBN 978-0-309-48834-1. PMID 30844154.
- ^ Stallings, Virginia A; Harrison, Meghan; Oria, Maria, eds. (March 5, 2019). Dietary Reference Intakes for Sodium and Potassium – Publication. Health and Medicine Division. National Academies of Sciences, Engineering and Medicine. doi:10.17226/25353. ISBN 978-0-309-48834-1. PMID 30844154. S2CID 104464967. Retrieved May 13, 2019.
- ^ Panel on Dietary Reference Intakes for Electrolytes and Water, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition (2004). DRI, dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, D.C.: National Academies Press. ISBN 978-0-309-53049-1. Archived from the original on 2011-10-06.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Karger, S. (2004). «Energy and nutrient intake in the European Union». Annals of Nutrition and Metabolism. 48 (2 (suppl)): 1–16. doi:10.1159/000083041.
- ^ «Vitamins and minerals». National Health Service (NHS). 18 November 2021. Retrieved 13 November 2022.
- ^ «Sodium and Potassium Dietary Reference Intake Values Updated in New Report; Introduces New Category for Sodium Based on Chronic Disease Risk Reduction» (Press release). National Academies of Sciences, Engineering, and Medicine. 5 March 2019. Retrieved 29 January 2022.
- ^ National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium (March 2019). Oria M, Harrison M, Stallings VA (eds.). Dietary Reference Intakes for Sodium and Potassium. National Academies Press. doi:10.17226/25353. ISBN 978-0-309-48834-1. PMID 30844154. S2CID 104464967. Bookshelf ID: NBK538102. Retrieved 13 November 2022.
- ^ «Potassium Food Charts». Asia Pacific Journal of Clinical Nutrition. Archived from the original on 2021-04-29. Retrieved 2011-05-18.
- ^ «Potassium Content of Selected Foods per Common Measure, sorted by nutrient content» (PDF). USDA National Nutrient Database for Standard Reference, Release 20. Archived from the original (PDF) on December 17, 2008.
- ^ Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ (1997). «Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials». JAMA. 277 (20): 1624–32. doi:10.1001/jama.1997.03540440058033. PMID 9168293. S2CID 25937399.
- ^ a b Institute of Medicine (U.S.). Committee on Optimization of Nutrient Composition of Military Rations for Short-Term, High-Stress Situations; Institute of Medicine (U.S.). Committee on Military Nutrition Research (2006). Nutrient composition of rations for short-term, high-intensity combat operations. National Academies Press. pp. 287–. ISBN 978-0-309-09641-6.
- ^ D’Elia, L.; Barba, G.; Cappuccio, F.; Strazzullo (2011). «Potassium Intake, Stroke, and Cardiovascular Disease: A Meta-Analysis of Prospective Studies». J Am Coll Cardiol. 57 (10): 1210–9. doi:10.1016/j.jacc.2010.09.070. PMID 21371638.
- ^ He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, Dalton RN, Kaski JC, MacGregor GA (2010). «Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives». Hypertension. 55 (3): 681–8. doi:10.1161/HYPERTENSIONAHA.109.147488. PMID 20083724.
- ^ «The Top 300 of 2020». ClinCalc. Retrieved 7 October 2022.
- ^ «Potassium Chloride — Drug Usage Statistics». ClinCalc. Retrieved 7 October 2022.
- ^ Shallenberger, R. S. (1993). Taste chemistry. Springer. pp. 120–. ISBN 978-0-7514-0150-9.
- ^ Garrett, Donald E. (1995-12-31). Potash: deposits, processing, properties and uses. Springer. ISBN 978-0-412-99071-7.
- ^ a b Ober, Joyce A. «Mineral Commodity Summaries 2008:Potash» (PDF). United States Geological Survey. Retrieved 2008-11-20.
- ^ a b c Ober, Joyce A. «Mineral Yearbook 2006:Potash» (PDF). United States Geological Survey. Retrieved 2008-11-20.
- ^ Wishart, David J. (2004). Encyclopedia of the Great Plains. U of Nebraska Press. p. 433. ISBN 978-0-8032-4787-1.
- ^ Chiu, Kuen-Wai (2000). «Potassium». Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. doi:10.1002/0471238961.1615200103080921.a01.pub2. ISBN 9780471238966.
- ^ Burkhardt, p. 34
- ^ Delahunt, J.; Lindeman, T. (2007). «Review of the safety of potassium and potassium oxides, including deactivation by introduction into water». Journal of Chemical Health and Safety. 14 (2): 21–32. doi:10.1016/j.jchas.2006.09.010.
- ^ Roy, Amit H. (2007). Kent and Riegel’s handbook of industrial chemistry and biotechnology. Springer. pp. 1135–57. Bibcode:2007karh.book……. ISBN 978-0-387-27843-8.
- ^ Ochoa-Hueso, R; Delgado-Baquerizo, M; King, PTA; Benham, M; Arca, V; Power, SA (2019). «Ecosystem type and resource quality are more important than global change drivers in regulating early stages of litter decomposition». Soil Biology and Biochemistry. 129: 144–152. doi:10.1016/j.soilbio.2018.11.009. S2CID 92606851.
- ^ «Potassium Uses, Side Effects & Interactions». Drugs.com.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 491. hdl:10665/44053. ISBN 9789241547659.
- ^ «Potassium chloride medical facts from Drugs.com». www.drugs.com. Archived from the original on 18 January 2017. Retrieved 14 January 2017.
- ^ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 680, 684. ISBN 9780857111562.
- ^ Figoni, Paula I (2010). «Bleaching and Maturing Agents». How Baking Works: Exploring the Fundamentals of Baking Science. John Wiley and Sons. p. 86. ISBN 978-0-470-39267-6.
- ^ Chichester, C. O. (July 1986). «Uses and Exposure to Sulfites in Food». Advances in food research. Academic Press. pp. 4–6. ISBN 978-0-12-016430-1.
- ^ Schultz
- ^ Toedt, John; Koza, Darrell & Cleef-Toedt, Kathleen Van (2005). «Personal Cleansing Products: Bar Soap». Chemical composition of everyday products. Greenwood Publishing Group. ISBN 978-0-313-32579-3.
- ^ Schultz, p. 95
- ^ Schultz, p. 99
- ^ Siegel, Richard S. (1940). «Ignition of the safety match». Journal of Chemical Education. 17 (11): 515. Bibcode:1940JChEd..17..515S. doi:10.1021/ed017p515.
- ^ Anger, Gerd; Halstenberg, Jost; Hochgeschwender, Klaus; Scherhag, Christoph; Korallus, Ulrich; Knopf, Herbert; Schmidt, Peter; Ohlinger, Manfred. «Chromium Compounds». Ullmann’s Encyclopedia of Industrial Chemistry. Vol. 9. Weinheim: Wiley-VCH. p. 178. doi:10.1002/14356007.a07_067.
- ^ Greenwood, p. 74
- ^ Marx, Robert F. (1990). The history of underwater exploration. Courier Dover Publications. p. 93. ISBN 978-0-486-26487-5.
- ^ Gettens, Rutherford John & Stout, George Leslie (1966). Painting materials: A short encyclopaedia. Courier Dover Publications. pp. 109–110. ISBN 978-0-486-21597-6.
- ^ Modugno, G.; Benkő, C.; Hannaford, P.; Roati, G.; Inguscio, M. (1999-11-01). «Sub-Doppler laser cooling of fermionic ${}^{40}mathrm{K}$ atoms». Physical Review A. 60 (5): R3373–R3376. arXiv:cond-mat/9908102. Bibcode:1999PhRvA..60.3373M. doi:10.1103/PhysRevA.60.R3373. S2CID 119001675.
- ^ Jackson, C. B.; Werner, R. C. (1957). «Ch. 18: The Manufacture of Potassium and NaK». Handling and uses of the alkali metals. Advances in Chemistry. Vol. 19. pp. 169–173. doi:10.1021/ba-1957-0019.ch018. ISBN 978-0-8412-0020-3.
- ^ Kearey, Philip; Brooks, M & Hill, Ian (2002). «Optical Pumped Magnetometer». An introduction to geophysical exploration. Wiley-Blackwell. p. 164. ISBN 978-0-632-04929-5.
- ^ «Potassium 244856». Sigma Aldrich.
- ^ Solomon, Robert E. (2002). Fire and Life Safety Inspection Manual. Jones & Bartlett Learning. p. 459. ISBN 978-0-87765-472-8.
- ^ «DOE Handbook-Alkali Metals Sodium, Potassium, NaK, and Lithium». Hss.doe.gov. Archived from the original on 2010-09-28. Retrieved 2010-10-16.
- ^ Wray, Thomas K. «Danger: peroxidazable chemicals» (PDF). Environmental Health & Public Safety, North Carolina State University. Archived from the original on 2016-07-29.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ a b Schonwald, Seth (2004). «Potassium Chloride and Potassium Permanganate». Medical toxicology. Lippincott Williams & Wilkins. pp. 903–5. ISBN 978-0-7817-2845-4.
- ^ Markovchick, Vincent J. & Pons, Peter T. (2003). Emergency medicine secrets. Elsevier Health Sciences. p. 223. ISBN 978-1-56053-503-4.
Bibliography
- Burkhardt, Elizabeth R. (2006). «Potassium and Potassium Alloys». Ullmann’s Encyclopedia of Industrial Chemistry. Vol. A22. pp. 31–38. doi:10.1002/14356007.a22_031.pub2. ISBN 978-3-527-30673-2.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (2007). «Potassium». Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. ISBN 978-3110177701.
- Schultz, H.; et al. (2006). «Potassium compounds». Ullmann’s Encyclopedia of Industrial Chemistry. Vol. A22. pp. 39–103. doi:10.1002/14356007.a22_031.pub2. ISBN 978-3-527-30673-2.
- National Nutrient Database Archived 2014-08-10 at the Wayback Machine at USDA Website
External links
- «Potassium». Drug Information Portal. U.S. National Library of Medicine.

Potassium pearls (in paraffin oil, ~5 mm each) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Potassium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | (pə-TASS-ee-əm) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white, faint bluish-purple hue when exposed to air | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(K) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Potassium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 1: hydrogen and alkali metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | s-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 4s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 8, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 336.7 K (63.5 °C, 146.3 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1030.793 K (757.643 °C, 1395.757 °F)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 0.89 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 0.82948 g/cm3[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 2223 K, 16 MPa[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.33 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 76.9 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 29.6 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −1, +1 (a strongly basic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 227 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 203±12 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 275 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of potassium |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 2000 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 83.3 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 102.5 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 72 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +20.8×10−6 cm3/mol (298 K)[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 3.53 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 1.3 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 3.1 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 0.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 0.363 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-09-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Humphry Davy (1807) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | «K»: from New Latin kalium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of potassium
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Potassium is the chemical element with the symbol K (from Neo-Latin kalium) and atomic number 19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force.[6] Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight[7][8]), and occurs in many minerals such as orthoclase, a common constituent of granites and other igneous rocks.[9]
Potassium is chemically very similar to sodium, the previous element in group 1 of the periodic table. They have a similar first ionization energy, which allows for each atom to give up its sole outer electron. It was suspected in 1702 that they were distinct elements that combine with the same anions to make similar salts,[10] and this was proven in 1807 through using electrolysis. Naturally occurring potassium is composed of three isotopes, of which 40
K is radioactive. Traces of 40
K are found in all potassium, and it is the most common radioisotope in the human body.
Potassium ions are vital for the functioning of all living cells. The transfer of potassium ions across nerve cell membranes is necessary for normal nerve transmission; potassium deficiency and excess can each result in numerous signs and symptoms, including an abnormal heart rhythm and various electrocardiographic abnormalities. Fresh fruits and vegetables are good dietary sources of potassium. The body responds to the influx of dietary potassium, which raises serum potassium levels, with a shift of potassium from outside to inside cells and an increase in potassium excretion by the kidneys.
Most industrial applications of potassium exploit the high solubility in water of potassium compounds, such as potassium soaps. Heavy crop production rapidly depletes the soil of potassium, and this can be remedied with agricultural fertilizers containing potassium, accounting for 95% of global potassium chemical production.[11]
Etymology
The English name for the element potassium comes from the word potash,[12] which refers to an early method of extracting various potassium salts: placing in a pot the ash of burnt wood or tree leaves, adding water, heating, and evaporating the solution. When Humphry Davy first isolated the pure element using electrolysis in 1807, he named it potassium, which he derived from the word potash.
The symbol K stems from kali, itself from the root word alkali, which in turn comes from Arabic: القَلْيَه al-qalyah ‘plant ashes’. In 1797, the German chemist Martin Klaproth discovered «potash» in the minerals leucite and lepidolite, and realized that «potash» was not a product of plant growth but actually contained a new element, which he proposed calling kali.[13] In 1807, Humphry Davy produced the element via electrolysis: in 1809, Ludwig Wilhelm Gilbert proposed the name Kalium for Davy’s «potassium».[14] In 1814, the Swedish chemist Berzelius advocated the name kalium for potassium, with the chemical symbol K.[15]
The English and French-speaking countries adopted Davy and Gay-Lussac/Thénard’s name Potassium, whereas the Germanic countries adopted Gilbert/Klaproth’s name Kalium.[16] The «Gold Book» of the International Union of Pure and Applied Chemistry has designated the official chemical symbol as K.[17]
Properties
Physical
Potassium is the second least dense metal after lithium. It is a soft solid with a low melting point, and can be easily cut with a knife. Freshly cut potassium is silvery in appearance, but it begins to tarnish toward gray immediately on exposure to air.[18] In a flame test, potassium and its compounds emit a lilac color with a peak emission wavelength of 766.5 nanometers.[19]
Neutral potassium atoms have 19 electrons, one more than the configuration of the noble gas argon. Because of its low first ionization energy of 418.8 kJ/mol, the potassium atom is much more likely to lose the last electron and acquire a positive charge, although negatively charged alkalide K− ions are not impossible.[20] In contrast, the second ionization energy is very high (3052 kJ/mol).
Chemical
Potassium reacts with oxygen, water, and carbon dioxide components in air. With oxygen it forms potassium peroxide. With water potassium forms potassium hydroxide. The reaction of potassium with water can be violently exothermic, especially since the coproduced hydrogen gas can ignite. Because of this, potassium and the liquid sodium-potassium (NaK) alloy are potent desiccants, although they are no longer used as such.[21]
Compounds
Structure of solid potassium superoxide (
KO2).
Four oxides of potassium are well studied: potassium oxide (K2O), potassium peroxide (K2O2), potassium superoxide (KO2)[22] and potassium ozonide (KO3). The binary potassium-oxygen compounds react with water forming potassium hydroxide KOH.
Potassium hydroxide is a strong base. Illustrating its hydrophilic character, as much as 1.21 kg of KOH can dissolve in a single liter of water.[23][24] Anhydrous KOH is rarely encountered. KOH reacts readily with carbon dioxide CO2 to produce potassium carbonate K2CO3, and in principle could be used to remove traces of the gas from air. Like the closely related sodium hydroxide, potassium hydroxide reacts with fats to produce soaps.
In general, potassium compounds are ionic and, owing to the high hydration energy of the K+ ion, have excellent water solubility. The main species in water solution are the aquo complexes [K(H2O)n]+ where n = 6 and 7.[25]
Potassium heptafluorotantalate K2[TaF7] is an intermediate in the purification of tantalum from the otherwise persistent contaminant of niobium.[26]
Organopotassium compounds illustrate nonionic compounds of potassium. They feature highly polar covalent K–C bonds. Examples include benzyl potassium KCH2C6H5. Potassium intercalates into graphite to give a variety of graphite intercalation compounds, including KC8.
Isotopes
There are 25 known isotopes of potassium, three of which occur naturally: 39
K (93.3%), 40
K (0.0117%), and 41
K (6.7%) (by mole fraction). Naturally occurring 40
K has a half-life of 1.250×109 years. It decays to stable 40
Ar by electron capture or positron emission (11.2%) or to stable 40
Ca by beta decay (88.8%).[27] The decay of 40
K to 40
Ar is the basis of a common method for dating rocks. The conventional K-Ar dating method depends on the assumption that the rocks contained no argon at the time of formation and that all the subsequent radiogenic argon (40
Ar) was quantitatively retained. Minerals are dated by measurement of the concentration of potassium and the amount of radiogenic 40
Ar that has accumulated. The minerals best suited for dating include biotite, muscovite, metamorphic hornblende, and volcanic feldspar; whole rock samples from volcanic flows and shallow instrusives can also be dated if they are unaltered.[27][28] Apart from dating, potassium isotopes have been used as tracers in studies of weathering and for nutrient cycling studies because potassium is a macronutrient required for life[29] on Earth.
40
K occurs in natural potassium (and thus in some commercial salt substitutes) in sufficient quantity that large bags of those substitutes can be used as a radioactive source for classroom demonstrations. 40
K is the radioisotope with the largest abundance in the body. In healthy animals and people, 40
K represents the largest source of radioactivity, greater even than 14
C. In a human body of 70 kg, about 4,400 nuclei of 40
K decay per second.[30] The activity of natural potassium is 31 Bq/g.[31]
Cosmic formation and distribution
Potassium is formed in supernovae by nucleosynthesis from lighter atoms. Potassium is principally created in Type II supernovae via an explosive oxygen-burning process.[32] (These are fusion reactions; do not confuse with chemical burning between potassium and oxygen.) 40
K is also formed in s-process nucleosynthesis and the neon burning process.[33]
Potassium is the 20th most abundant element in the solar system and the 17th most abundant element by weight in the Earth. It makes up about 2.6% of the weight of the Earth’s crust and is the seventh most abundant element in the crust.[34] The potassium concentration in seawater is 0.39 g/L[7] (0.039 wt/v%), about one twenty-seventh the concentration of sodium.[35][36]
Potash
Potash is primarily a mixture of potassium salts because plants have little or no sodium content, and the rest of a plant’s major mineral content consists of calcium salts of relatively low solubility in water. While potash has been used since ancient times, its composition was not understood. Georg Ernst Stahl obtained experimental evidence that led him to suggest the fundamental difference of sodium and potassium salts in 1702,[10] and Henri Louis Duhamel du Monceau was able to prove this difference in 1736.[37] The exact chemical composition of potassium and sodium compounds, and the status as chemical element of potassium and sodium, was not known then, and thus Antoine Lavoisier did not include the alkali in his list of chemical elements in 1789.[38][39] For a long time the only significant applications for potash were the production of glass, bleach, soap and gunpowder as potassium nitrate.[40] Potassium soaps from animal fats and vegetable oils were especially prized because they tend to be more water-soluble and of softer texture, and are therefore known as soft soaps.[11] The discovery by Justus Liebig in 1840 that potassium is a necessary element for plants and that most types of soil lack potassium[41] caused a steep rise in demand for potassium salts. Wood-ash from fir trees was initially used as a potassium salt source for fertilizer, but, with the discovery in 1868 of mineral deposits containing potassium chloride near Staßfurt, Germany, the production of potassium-containing fertilizers began at an industrial scale.[42][43][44] Other potash deposits were discovered, and by the 1960s Canada became the dominant producer.[45][46]
Metal
Pieces of potassium metal
Potassium metal was first isolated in 1807 by Humphry Davy, who derived it by electrolysis of molten KOH with the newly discovered voltaic pile. Potassium was the first metal that was isolated by electrolysis.[47] Later in the same year, Davy reported extraction of the metal sodium from a mineral derivative (caustic soda, NaOH, or lye) rather than a plant salt, by a similar technique, demonstrating that the elements, and thus the salts, are different.[38][39][48][49] Although the production of potassium and sodium metal should have shown that both are elements, it took some time before this view was universally accepted.[39]
Because of the sensitivity of potassium to water and air, air-free techniques are normally employed for handling the element. It is unreactive toward nitrogen and saturated hydrocarbons such as mineral oil or kerosene.[50] It readily dissolves in liquid ammonia, up to 480 g per 1000 g of ammonia at 0 °C. Depending on the concentration, the ammonia solutions are blue to yellow, and their electrical conductivity is similar to that of liquid metals. Potassium slowly reacts with ammonia to form KNH
2, but this reaction is accelerated by minute amounts of transition metal salts.[51] Because it can reduce the salts to the metal, potassium is often used as the reductant in the preparation of finely divided metals from their salts by the Rieke method.[52] Illustrative is the preparation of magnesium:
- MgCl2 + 2 K → Mg + 2 KCl
Geology
Elemental potassium does not occur in nature because of its high reactivity. It reacts violently with water (see section Precautions below)[50] and also reacts with oxygen. Orthoclase (potassium feldspar) is a common rock-forming mineral. Granite for example contains 5% potassium, which is well above the average in the Earth’s crust. Sylvite (KCl), carnallite (KCl·MgCl2·6H2O), kainite (MgSO4·KCl·3H2O) and langbeinite (MgSO4·K2SO4) are the minerals found in large evaporite deposits worldwide. The deposits often show layers starting with the least soluble at the bottom and the most soluble on top.[36] Deposits of niter (potassium nitrate) are formed by decomposition of organic material in contact with atmosphere, mostly in caves; because of the good water solubility of niter the formation of larger deposits requires special environmental conditions.[53]
Biological role
Potassium is the eighth or ninth most common element by mass (0.2%) in the human body, so that a 60 kg adult contains a total of about 120 g of potassium.[54] The body has about as much potassium as sulfur and chlorine, and only calcium and phosphorus are more abundant (with the exception of the ubiquitous CHON elements).[55] Potassium ions are present in a wide variety of proteins and enzymes.[56]
Biochemical function
Potassium levels influence multiple physiological processes, including[57][58][59]
- resting cellular-membrane potential and the propagation of action potentials in neuronal, muscular, and cardiac tissue. Due to the electrostatic and chemical properties, K+ ions are larger than Na+ ions, and ion channels and pumps in cell membranes can differentiate between the two ions, actively pumping or passively passing one of the two ions while blocking the other.[60]
- hormone secretion and action
- vascular tone
- systemic blood pressure control
- gastrointestinal motility
- acid–base homeostasis
- glucose and insulin metabolism
- mineralocorticoid action
- renal concentrating ability
- fluid and electrolyte balance
Homeostasis
Potassium homeostasis denotes the maintenance of the total body potassium content, plasma potassium level, and the ratio of the intracellular to extracellular potassium concentrations within narrow limits, in the face of pulsatile intake (meals), obligatory renal excretion, and shifts between intracellular and extracellular compartments.
Plasma levels
Plasma potassium is normally kept at 3.5 to 5.5 millimoles (mmol) [or milliequivalents (mEq)] per liter by multiple mechanisms.[61] Levels outside this range are associated with an increasing rate of death from multiple causes,[62] and some cardiac, kidney,[63] and lung diseases progress more rapidly if serum potassium levels are not maintained within the normal range.
An average meal of 40–50 mmol presents the body with more potassium than is present in all plasma (20–25 mmol). However, this surge causes the plasma potassium to rise only 10% at most as a result of prompt and efficient clearance by both renal and extra-renal mechanisms.[64]
Hypokalemia, a deficiency of potassium in the plasma, can be fatal if severe. Common causes are increased gastrointestinal loss (vomiting, diarrhea), and increased renal loss (diuresis).[65] Deficiency symptoms include muscle weakness, paralytic ileus, ECG abnormalities, decreased reflex response; and in severe cases, respiratory paralysis, alkalosis, and cardiac arrhythmia.[66]
Control mechanisms
Potassium content in the plasma is tightly controlled by four basic mechanisms, which have various names and classifications. The four are 1) a reactive negative-feedback system, 2) a reactive feed-forward system, 3) a predictive or circadian system, and 4) an internal or cell membrane transport system. Collectively, the first three are sometimes termed the «external potassium homeostasis system»;[67] and the first two, the «reactive potassium homeostasis system».
- The reactive negative-feedback system refers to the system that induces renal secretion of potassium in response to a rise in the plasma potassium (potassium ingestion, shift out of cells, or intravenous infusion.)
- The reactive feed-forward system refers to an incompletely understood system that induces renal potassium secretion in response to potassium ingestion prior to any rise in the plasma potassium. This is probably initiated by gut cell potassium receptors that detect ingested potassium and trigger vagal afferent signals to the pituitary gland.
- The predictive or circadian system increases renal secretion of potassium during mealtime hours (e.g. daytime for humans, nighttime for rodents) independent of the presence, amount, or absence of potassium ingestion. It is mediated by a circadian oscillator in the suprachiasmatic nucleus of the brain (central clock), which causes the kidney (peripheral clock) to secrete potassium in this rhythmic circadian fashion.
The action of the sodium-potassium pump is an example of primary active transport. The two carrier proteins embedded in the cell membrane on the left are using ATP to move sodium out of the cell against the concentration gradient; The two proteins on the right are using secondary active transport to move potassium into the cell. This process results in reconstitution of ATP.
- The ion transport system moves potassium across the cell membrane using two mechanisms. One is active and pumps sodium out of, and potassium into, the cell. The other is passive and allows potassium to leak out of the cell. Potassium and sodium cations influence fluid distribution between intracellular and extracellular compartments by osmotic forces. The movement of potassium and sodium through the cell membrane is mediated by the Na⁺/K⁺-ATPase pump.[68] This ion pump uses ATP to pump three sodium ions out of the cell and two potassium ions into the cell, creating an electrochemical gradient and electromotive force across the cell membrane. The highly selective potassium ion channels (which are tetramers) are crucial for hyperpolarization inside neurons after an action potential is triggered, to cite one example. The most recently discovered potassium ion channel is KirBac3.1, which makes a total of five potassium ion channels (KcsA, KirBac1.1, KirBac3.1, KvAP, and MthK) with a determined structure. All five are from prokaryotic species.[69]
Renal filtration, reabsorption, and excretion
Renal handling of potassium is closely connected to sodium handling. Potassium is the major cation (positive ion) inside animal cells [150 mmol/L, (4.8 g)], while sodium is the major cation of extracellular fluid [150 mmol/L, (3.345 g)]. In the kidneys, about 180 liters of plasma is filtered through the glomeruli and into the renal tubules per day.[70] This filtering involves about 600 g of sodium and 33 g of potassium. Since only 1–10 g of sodium and 1–4 g of potassium are likely to be replaced by diet, renal filtering must efficiently reabsorb the remainder from the plasma.
Sodium is reabsorbed to maintain extracellular volume, osmotic pressure, and serum sodium concentration within narrow limits. Potassium is reabsorbed to maintain serum potassium concentration within narrow limits.[71] Sodium pumps in the renal tubules operate to reabsorb sodium. Potassium must be conserved, but because the amount of potassium in the blood plasma is very small and the pool of potassium in the cells is about 30 times as large, the situation is not so critical for potassium. Since potassium is moved passively[72][73] in counter flow to sodium in response to an apparent (but not actual) Donnan equilibrium,[74] the urine can never sink below the concentration of potassium in serum except sometimes by actively excreting water at the end of the processing. Potassium is excreted twice and reabsorbed three times before the urine reaches the collecting tubules.[75] At that point, urine usually has about the same potassium concentration as plasma. At the end of the processing, potassium is secreted one more time if the serum levels are too high.[citation needed]
With no potassium intake, it is excreted at about 200 mg per day until, in about a week, potassium in the serum declines to a mildly deficient level of 3.0–3.5 mmol/L.[76] If potassium is still withheld, the concentration continues to fall until a severe deficiency causes eventual death.[77]
The potassium moves passively through pores in the cell membrane. When ions move through Ion transporters (pumps) there is a gate in the pumps on both sides of the cell membrane and only one gate can be open at once. As a result, approximately 100 ions are forced through per second. Ion channel have only one gate, and there only one kind of ion can stream through, at 10 million to 100 million ions per second.[78] Calcium is required to open the pores,[79] although calcium may work in reverse by blocking at least one of the pores.[80] Carbonyl groups inside the pore on the amino acids mimic the water hydration that takes place in water solution[81] by the nature of the electrostatic charges on four carbonyl groups inside the pore.[82]
Nutrition
Dietary recommendations
The U.S. National Academy of Medicine (NAM), on behalf of both the U.S. and Canada, sets Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs), or Adequate Intakes (AIs) for when there is not sufficient information to set EARs and RDAs. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes.
For both males and females under 9 years of age, the AIs for potassium are: 400 mg of potassium for 0-6-month-old infants, 860 mg of potassium for 7-12-month-old infants, 2,000 mg of potassium for 1-3-year-old children, and 2,300 mg of potassium for 4-8-year-old children.
For males 9 years of age and older, the AIs for potassium are: 2,500 mg of potassium for 9-13-year-old males, 3,000 mg of potassium for 14-18-year-old males, and 3,400 mg for males that are 19 years of age and older.
For females 9 years of age and older, the AIs for potassium are: 2,300 mg of potassium for 9-18-year-old females, and 2,600 mg of potassium for females that are 19 years of age and older.
For pregnant and lactating females, the AIs for potassium are: 2,600 mg of potassium for 14-18-year-old pregnant females, 2,900 mg for pregnant females that are 19 years of age and older; furthermore, 2,500 mg of potassium for 14-18-year-old lactating females, and 2,800 mg for lactating females that are 19 years of age and older. As for safety, the NAM also sets tolerable upper intake levels (ULs) for vitamins and minerals, but for potassium the evidence was insufficient, so no UL was established.[83][84]
As of 2004, most Americans adults consume less than 3,000 mg.[85]
Likewise, in the European Union, in particular in Germany, and Italy, insufficient potassium intake is somewhat common.[86] The British National Health Service recommends a similar intake, saying that adults need 3,500 mg per day and that excess amounts may cause health problems such as stomach pain and diarrhea.[87]
In 2019, the National Academies of Sciences, Engineering, and Medicine revised the Adequate Intake for potassium to 2,600 mg/day for females 19 years of age and older who are not pregnant or lactating, and 3,400 mg/day for males 19 years of age and older.[88][89]
Food sources
Potassium is present in all fruits, vegetables, meat and fish. Foods with high potassium concentrations include yam, parsley, dried apricots, milk, chocolate, all nuts (especially almonds and pistachios), potatoes, bamboo shoots, bananas, avocados, coconut water, soybeans, and bran.[90]
The USDA lists tomato paste, orange juice, beet greens, white beans, potatoes, plantains, bananas, apricots, and many other dietary sources of potassium, ranked in descending order according to potassium content. A day’s worth of potassium is in 5 plantains or 11 bananas.[91]
Deficient intake
Diets low in potassium can lead to hypertension[92] and hypokalemia.
Supplementation
Supplements of potassium are most widely used in conjunction with diuretics that block reabsorption of sodium and water upstream from the distal tubule (thiazides and loop diuretics), because this promotes increased distal tubular potassium secretion, with resultant increased potassium excretion.[medical citation needed] A variety of prescription and over-the counter supplements are available.[citation needed] Potassium chloride may be dissolved in water, but the salty/bitter taste makes liquid supplements unpalatable.[93] Typical doses range from 10 mmol (400 mg), to 20 mmol (800 mg).[medical citation needed] Potassium is also available in tablets or capsules, which are formulated to allow potassium to leach slowly out of a matrix, since very high concentrations of potassium ion that occur adjacent to a solid tablet can injure the gastric or intestinal mucosa.[medical citation needed] For this reason, non-prescription potassium pills are limited by law in the US to a maximum of 99 mg of potassium.[citation needed]
A meta-analysis concluded that a 1640 mg increase in the daily intake of potassium was associated with a 21% lower risk of stroke.[94] Potassium chloride and potassium bicarbonate may be useful to control mild hypertension.[95] In 2020, potassium was the 33rd most commonly prescribed medication in the United States, with more than 17 million prescriptions.[96][97]
Detection by taste buds
Potassium can be detected by taste because it triggers three of the five types of taste sensations, according to concentration. Dilute solutions of potassium ions taste sweet, allowing moderate concentrations in milk and juices, while higher concentrations become increasingly bitter/alkaline, and finally also salty to the taste. The combined bitterness and saltiness of high-potassium solutions makes high-dose potassium supplementation by liquid drinks a palatability challenge.[93][98]
Commercial production
Mining
Potassium salts such as carnallite, langbeinite, polyhalite, and sylvite form extensive evaporite deposits in ancient lake bottoms and seabeds,[35] making extraction of potassium salts in these environments commercially viable. The principal source of potassium – potash – is mined in Canada, Russia, Belarus, Kazakhstan, Germany, Israel, United States, Jordan, and other places around the world.[99][100][101] The first mined deposits were located near Staßfurt, Germany, but the deposits span from Great Britain over Germany into Poland. They are located in the Zechstein and were deposited in the Middle to Late Permian. The largest deposits ever found lie 1,000 meters (3,300 feet) below the surface of the Canadian province of Saskatchewan. The deposits are located in the Elk Point Group produced in the Middle Devonian. Saskatchewan, where several large mines have operated since the 1960s pioneered the technique of freezing of wet sands (the Blairmore formation) to drive mine shafts through them. The main potash mining company in Saskatchewan until its merge was the Potash Corporation of Saskatchewan, now Nutrien.[102] The water of the Dead Sea is used by Israel and Jordan as a source of potash, while the concentration in normal oceans is too low for commercial production at current prices.[100][101]
Several methods are used to separate potassium salts from sodium and magnesium compounds. The most-used method is fractional precipitation using the solubility differences of the salts. Electrostatic separation of the ground salt mixture is also used in some mines. The resulting sodium and magnesium waste is either stored underground or piled up in slag heaps. Most of the mined potassium mineral ends up as potassium chloride after processing. The mineral industry refers to potassium chloride either as potash, muriate of potash, or simply MOP.[36]
Pure potassium metal can be isolated by electrolysis of its hydroxide in a process that has changed little since it was first used by Humphry Davy in 1807. Although the electrolysis process was developed and used in industrial scale in the 1920s, the thermal method by reacting sodium with potassium chloride in a chemical equilibrium reaction became the dominant method in the 1950s.
- Na + KCl → NaCl + K
The production of sodium potassium alloys is accomplished by changing the reaction time and the amount of sodium used in the reaction. The Griesheimer process employing the reaction of potassium fluoride with calcium carbide was also used to produce potassium.[36][103]
- 2 KF + CaC2 → 2 K + CaF2 + 2 C
Reagent-grade potassium metal costs about $10.00/pound ($22/kg) in 2010 when purchased by the tonne. Lower purity metal is considerably cheaper. The market is volatile because long-term storage of the metal is difficult. It must be stored in a dry inert gas atmosphere or anhydrous mineral oil to prevent the formation of a surface layer of potassium superoxide, a pressure-sensitive explosive that detonates when scratched. The resulting explosion often starts a fire difficult to extinguish.[104][105]
Cation identification
Potassium is now quantified by ionization techniques, but at one time it was quantitated by gravimetric analysis.
Reagents used to precipitate potassium salts include sodium tetraphenylborate, hexachloroplatinic acid, and sodium cobaltinitrite into respectively potassium tetraphenylborate, potassium hexachloroplatinate, and potassium cobaltinitrite.[50]
The reaction with sodium cobaltinitrite is illustrative:
- 3 K+ + Na3[Co(NO2)6] → K3[Co(NO2)6] + 3 Na+
The potassium cobaltinitrite is obtained as a yellow solid.
Commercial uses
Fertilizer
Potassium sulfate/magnesium sulfate fertilizer
Potassium ions are an essential component of plant nutrition and are found in most soil types.[11] They are used as a fertilizer in agriculture, horticulture, and hydroponic culture in the form of chloride (KCl), sulfate (K2SO4), or nitrate (KNO3), representing the ‘K’ in ‘NPK’. Agricultural fertilizers consume 95% of global potassium chemical production, and about 90% of this potassium is supplied as KCl.[11] The potassium content of most plants ranges from 0.5% to 2% of the harvested weight of crops, conventionally expressed as amount of K2O. Modern high-yield agriculture depends upon fertilizers to replace the potassium lost at harvest. Most agricultural fertilizers contain potassium chloride, while potassium sulfate is used for chloride-sensitive crops or crops needing higher sulfur content. The sulfate is produced mostly by decomposition of the complex minerals kainite (MgSO4·KCl·3H2O) and langbeinite (MgSO4·K2SO4). Only a very few fertilizers contain potassium nitrate.[106] In 2005, about 93% of world potassium production was consumed by the fertilizer industry.[101] Furthermore, potassium can play a key role in nutrient cycling by controlling litter composition.[107]
Medical use
Potassium citrate
Potassium citrate is used to treat a kidney stone condition called renal tubular acidosis.[108]
Potassium chloride
Potassium, in the form of potassium chloride is used as a medication to treat and prevent low blood potassium.[109] Low blood potassium may occur due to vomiting, diarrhea, or certain medications.[110] It is given by slow injection into a vein or by mouth.[111]
Food additives
Potassium sodium tartrate (KNaC4H4O6, Rochelle salt) is a main constituent of some varieties of baking powder; it is also used in the silvering of mirrors. Potassium bromate (KBrO3) is a strong oxidizer (E924), used to improve dough strength and rise height. Potassium bisulfite (KHSO3) is used as a food preservative, for example in wine and beer-making (but not in meats). It is also used to bleach textiles and straw, and in the tanning of leathers.[112][113]
Industrial
Major potassium chemicals are potassium hydroxide, potassium carbonate, potassium sulfate, and potassium chloride. Megatons of these compounds are produced annually.[114]
Potassium hydroxide KOH is a strong base, which is used in industry to neutralize strong and weak acids, to control pH and to manufacture potassium salts. It is also used to saponify fats and oils, in industrial cleaners, and in hydrolysis reactions, for example of esters.[115][116]
Potassium nitrate (KNO3) or saltpeter is obtained from natural sources such as guano and evaporites or manufactured via the Haber process; it is the oxidant in gunpowder (black powder) and an important agricultural fertilizer. Potassium cyanide (KCN) is used industrially to dissolve copper and precious metals, in particular silver and gold, by forming complexes. Its applications include gold mining, electroplating, and electroforming of these metals; it is also used in organic synthesis to make nitriles. Potassium carbonate (K2CO3 or potash) is used in the manufacture of glass, soap, color TV tubes, fluorescent lamps, textile dyes and pigments.[117] Potassium permanganate (KMnO4) is an oxidizing, bleaching and purification substance and is used for production of saccharin. Potassium chlorate (KClO3) is added to matches and explosives. Potassium bromide (KBr) was formerly used as a sedative and in photography.[11]
While potassium chromate (K2CrO4) is used in the manufacture of a host of different commercial products such as inks, dyes, wood stains (by reacting with the tannic acid in wood), explosives, fireworks, fly paper, and safety matches,[118] as well as in the tanning of leather, all of these uses are due to the chemistry of the chromate ion rather than to that of the potassium ion.[119]
Niche uses
There are thousands of uses of various potassium compounds. One example is potassium superoxide, KO2, an orange solid that acts as a portable source of oxygen and a carbon dioxide absorber. It is widely used in respiration systems in mines, submarines and spacecraft as it takes less volume than the gaseous oxygen.[120][121]
- 4 KO2 + 2 CO2 → 2 K2CO3 + 3 O2
Another example is potassium cobaltinitrite, K3[Co(NO2)6], which is used as artist’s pigment under the name of Aureolin or Cobalt Yellow.[122]
The stable isotopes of potassium can be laser cooled and used to probe fundamental and technological problems in quantum physics. The two bosonic isotopes possess convenient Feshbach resonances to enable studies requiring tunable interactions, while 40
K is one of only two stable fermions amongst the alkali metals.[123]
Laboratory uses
An alloy of sodium and potassium, NaK is a liquid used as a heat-transfer medium and a desiccant for producing dry and air-free solvents. It can also be used in reactive distillation.[124] The ternary alloy of 12% Na, 47% K and 41% Cs has the lowest melting point of −78 °C of any metallic compound.[18]
Metallic potassium is used in several types of magnetometers.[125]
Precautions
| Hazards | |
|---|---|
| GHS labelling: | |
|
Pictograms |
 
|
|
Signal word |
Danger |
|
Hazard statements |
H260, H314 |
|
Precautionary statements |
P223, P231+P232, P280, P305+P351+P338, P370+P378, P422[126] |
| NFPA 704 (fire diamond) |
3 3 2
|
Potassium metal can react violently with water producing potassium hydroxide (KOH) and hydrogen gas.
- 2 K(s) + 2 H2O(l) → 2 KOH(aq) + H2(g)↑
A reaction of potassium metal with water. Hydrogen is produced, and with potassium vapor, burns with a pink or lilac flame. Strongly alkaline potassium hydroxide is formed in solution.
This reaction is exothermic and releases sufficient heat to ignite the resulting hydrogen in the presence of oxygen. Finely powdered potassium ignites in air at room temperature. The bulk metal ignites in air if heated. Because its density is 0.89 g/cm3, burning potassium floats in water that exposes it to atmospheric oxygen. Many common fire extinguishing agents, including water, either are ineffective or make a potassium fire worse. Nitrogen, argon, sodium chloride (table salt), sodium carbonate (soda ash), and silicon dioxide (sand) are effective if they are dry. Some Class D dry powder extinguishers designed for metal fires are also effective. These agents deprive the fire of oxygen and cool the potassium metal.[127]
During storage, potassium forms peroxides and superoxides. These peroxides may react violently with organic compounds such as oils. Both peroxides and superoxides may react explosively with metallic potassium.[128]
Because potassium reacts with water vapor in the air, it is usually stored under anhydrous mineral oil or kerosene. Unlike lithium and sodium, however, potassium should not be stored under oil for longer than six months, unless in an inert (oxygen free) atmosphere, or under vacuum. After prolonged storage in air dangerous shock-sensitive peroxides can form on the metal and under the lid of the container, and can detonate upon opening.[129]
Ingestion of large amounts of potassium compounds can lead to hyperkalemia, strongly influencing the cardiovascular system.[130][131] Potassium chloride is used in the United States for lethal injection executions.[130]
See also
References
- ^ «Standard Atomic Weights: Potassium». CIAAW. 1979.
- ^ a b Aitken, F.; Volino, F. (January 2022). «New equations of state describing both the dynamic viscosity and self-diffusion coefficient for potassium and thallium in their fluid phases». Physics of Fluids. 34 (1): 017112. doi:10.1063/5.0079944.
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.122. ISBN 1-4398-5511-0.
- ^ Magnetic susceptibility of the elements and inorganic compounds, in Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Augustyn, Adam. «Potassium/ Chemical element». Encyclopedia Britannica. Retrieved 2019-04-17.
Potassium Physical properties
- ^ a b Webb, D. A. (April 1939). «The Sodium and Potassium Content of Sea Water» (PDF). The Journal of Experimental Biology (2): 183.
- ^ Anthoni, J. (2006). «Detailed composition of seawater at 3.5% salinity». seafriends.org.nz. Retrieved 2011-09-23.
- ^ Halperin, Mitchell L.; Kamel, Kamel S. (1998-07-11). «Potassium». The Lancet. 352 (9122): 135–140. doi:10.1016/S0140-6736(98)85044-7. ISSN 0140-6736. PMID 9672294. S2CID 208790031.
- ^ a b Marggraf, Andreas Siegmund (1761). Chymische Schriften. p. 167.
- ^ a b c d e Greenwood, p. 73
- ^ Davy, Humphry (1808). «On some new phenomena of chemical changes produced by electricity, in particular the decomposition of the fixed alkalies, and the exhibition of the new substances that constitute their bases; and on the general nature of alkaline bodies». Philosophical Transactions of the Royal Society. 98: 32. doi:10.1098/rstl.1808.0001.
- ^ Klaproth, M. (1797) «Nouvelles données relatives à l’histoire naturelle de l’alcali végétal» (New data regarding the natural history of the vegetable alkali), Mémoires de l’Académie royale des sciences et belles-lettres (Berlin), pp. 9–13 ; see p. 13. From p. 13: «Cet alcali ne pouvant donc plus être envisagé comme un produit de la végétation dans les plantes, occupe une place propre dans la série des substances primitivement simples du règne minéral, &I il devient nécessaire de lui assigner un nom, qui convienne mieux à sa nature.
La dénomination de Potasche (potasse) que la nouvelle nomenclature françoise a consacrée comme nom de tout le genre, ne sauroit faire fortune auprès des chimistes allemands, qui sentent à quel point la dérivation étymologique en est vicieuse. Elle est prise en effet de ce qu’anciennement on se servoit pour la calcination des lessives concentrées des cendres, de pots de fer (pott en dialecte de la Basse-Saxe) auxquels on a substitué depuis des fours à calciner.
Je propose donc ici, de substituer aux mots usités jusqu’ici d’alcali des plantes, alcali végétal, potasse, &c. celui de kali, & de revenir à l’ancienne dénomination de natron, au lieu de dire alcali minéral, soude &c.»
(This alkali [i.e., potash] — [which] therefore can no longer be viewed as a product of growth in plants — occupies a proper place in the originally simple series of the mineral realm, and it becomes necessary to assign it a name that is better suited to its nature.
The name of «potash» (potasse), which the new French nomenclature has bestowed as the name of the entire species [i.e., substance], would not find acceptance among German chemists, who feel to some extent [that] the etymological derivation of it is faulty. Indeed, it is taken from [the vessels] that one formerly used for the roasting of washing powder concentrated from cinders: iron pots (pott in the dialect of Lower Saxony), for which roasting ovens have been substituted since then.
Thus I now propose to substitute for the until now common words of «plant alkali», «vegetable alkali», «potash», etc., that of kali ; and to return to the old name of natron instead of saying «mineral alkali», «soda», etc.) - ^ Davy, Humphry (1809). «Ueber einige neue Erscheinungen chemischer Veränderungen, welche durch die Electricität bewirkt werden; insbesondere über die Zersetzung der feuerbeständigen Alkalien, die Darstellung der neuen Körper, welche ihre Basen ausmachen, und die Natur der Alkalien überhaupt» [On some new phenomena of chemical changes that are achieved by electricity; particularly the decomposition of flame-resistant alkalis [i.e., alkalies that cannot be reduced to their base metals by flames], the preparation of new substances that constitute their [metallic] bases, and the nature of alkalies generally]. Annalen der Physik. 31 (2): 113–175. Bibcode:1809AnP….31..113D. doi:10.1002/andp.18090310202.
p. 157: In unserer deutschen Nomenclatur würde ich die Namen Kalium und Natronium vorschlagen, wenn man nicht lieber bei den von Herrn Erman gebrauchten und von mehreren angenommenen Benennungen Kali-Metalloid and Natron-Metalloid, bis zur völligen Aufklärung der chemischen Natur dieser räthzelhaften Körper bleiben will. Oder vielleicht findet man es noch zweckmässiger fürs Erste zwei Klassen zu machen, Metalle und Metalloide, und in die letztere Kalium und Natronium zu setzen. — Gilbert. (In our German nomenclature, I would suggest the names Kalium and Natronium, if one would not rather continue with the appellations Kali-metalloid and Natron-metalloid which are used by Mr. Erman [i.e., German physics professor Paul Erman (1764–1851)] and accepted by several [people], until the complete clarification of the chemical nature of these puzzling substances. Or perhaps one finds it yet more advisable for the present to create two classes, metals and metalloids, and to place Kalium and Natronium in the latter — Gilbert.)
- ^ Berzelius, J. Jacob (1814) Försök, att, genom användandet af den electrokemiska theorien och de kemiska proportionerna, grundlägga ett rent vettenskapligt system för mineralogien [Attempt, by the use of electrochemical theory and chemical proportions, to found a pure scientific system for mineralogy]. Stockholm, Sweden: A. Gadelius., p. 87.
- ^ 19. Kalium (Potassium) – Elementymology & Elements Multidict. vanderkrogt.net
- ^ McNaught, A. D. and Wilkinson,A. eds. (1997). Compendium of Chemical Terminology, 2nd ed. (the «Gold Book»). IUPAC. Blackwell Scientific Publications, Oxford.
- ^ a b Greenwood, p. 76
- ^ Greenwood, p. 75
- ^ Dye, J. L. (1979). «Compounds of Alkali Metal Anions». Angewandte Chemie International Edition. 18 (8): 587–598. doi:10.1002/anie.197905871.
- ^ Williams, D. Bradley G.; Lawton, Michelle (2010). «Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants». The Journal of Organic Chemistry. 75 (24): 8351–8354. doi:10.1021/jo101589h. PMID 20945830. S2CID 17801540.
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida, United States: CRC Press. pp. 477, 520. ISBN 978-0-8493-0594-8.
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 4–80. ISBN 0-8493-0486-5.
- ^ Schultz, p. 94
- ^ Lincoln, S. F.; Richens, D. T. and Sykes, A. G. «Metal Aqua Ions» in J. A. McCleverty and T. J. Meyer (eds.) Comprehensive Coordination Chemistry II, Vol. 1, pp. 515–555, ISBN 978-0-08-043748-4.
- ^ Anthony Agulyanski (2004). «Fluorine chemistry in the processing of tantalum and niobium». In Anatoly Agulyanski (ed.). Chemistry of Tantalum and Niobium Fluoride Compounds (1st ed.). Burlington: Elsevier. ISBN 9780080529028.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), «The NUBASE evaluation of nuclear and decay properties», Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729….3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ Bowen, Robert; Attendorn, H. G. (1988). «Theory and Assumptions in Potassium–Argon Dating». Isotopes in the Earth Sciences. Springer. pp. 203–8. ISBN 978-0-412-53710-3.
- ^ Anaç, D. & Martin-Prével, P. (1999). Improved crop quality by nutrient management. Springer. pp. 290–. ISBN 978-0-7923-5850-3.
- ^ «Radiation and Radioactive Decay. Radioactive Human Body». Harvard Natural Sciences Lecture Demonstrations. Retrieved July 2, 2016.
- ^ Winteringham, F. P. W; Effects, F.A.O. Standing Committee on Radiation, Land And Water Development Division, Food and Agriculture Organization of the United Nations (1989). Radioactive fallout in soils, crops and food: a background review. Food & Agriculture Org. p. 32. ISBN 978-92-5-102877-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Shimansky, V.; Bikmaev, I. F.; Galeev, A. I.; Shimanskaya, N. N.; et al. (September 2003). «Observational constraints on potassium synthesis during the formation of stars of the Galactic disk». Astronomy Reports. 47 (9): 750–762. Bibcode:2003ARep…47..750S. doi:10.1134/1.1611216. S2CID 120396773.
- ^ The, L.-S.; Eid, M. F. El; Meyer, B. S. (2000). «A New Study of s-Process Nucleosynthesis in Massive Stars». The Astrophysical Journal. 533 (2): 998. arXiv:astro-ph/9812238. Bibcode:2000ApJ…533..998T. doi:10.1086/308677. ISSN 0004-637X. S2CID 7698683.
- ^ Greenwood, p. 69
- ^ a b Micale, Giorgio; Cipollina, Andrea; Rizzuti, Lucio (2009). Seawater Desalination: Conventional and Renewable Energy Processes. Springer. p. 3. ISBN 978-3-642-01149-8.
- ^ a b c d Prud’homme, Michel; Krukowski, Stanley T. (2006). «Potash». Industrial minerals & rocks: commodities, markets, and uses. Society for Mining, Metallurgy, and Exploration. pp. 723–740. ISBN 978-0-87335-233-8.
- ^ du Monceau, H. L. D. (1702–1797). «Sur la Base de Sel Marin». Mémoires de l’Académie Royale des Sciences (in French): 65–68.
- ^ a b Weeks, Mary Elvira (1932). «The discovery of the elements. IX. Three alkali metals: Potassium, sodium, and lithium». Journal of Chemical Education. 9 (6): 1035. Bibcode:1932JChEd…9.1035W. doi:10.1021/ed009p1035.
- ^ a b c Siegfried, R. (1963). «The Discovery of Potassium and Sodium, and the Problem of the Chemical Elements». Isis. 54 (2): 247–258. doi:10.1086/349704. JSTOR 228541. PMID 14147904. S2CID 38152048.
- ^ Browne, C. A. (1926). «Historical notes upon the domestic potash industry in early colonial and later times». Journal of Chemical Education. 3 (7): 749–756. Bibcode:1926JChEd…3..749B. doi:10.1021/ed003p749.
- ^ Liebig, Justus von (1840). Die organische Chemie in ihrer Anwendung auf Agricultur und Physiologie (in German). F. Vieweg und Sohn.
- ^ Cordel, Oskar (1868). Die Stassfurter Kalisalze in der Landwirthschalt: Eine Besprechung … (in German). L. Schnock.
- ^ Birnbaum, Karl (1869). Die Kalidüngung in ihren Vortheilen und Gefahren (in German).
- ^ United Nations Industrial Development Organization and Int’l Fertilizer Development Center (1998). Fertilizer Manual. pp. 46, 417. ISBN 978-0-7923-5032-3.
- ^ Miller, H. (1980). «Potash from Wood Ashes: Frontier Technology in Canada and the United States». Technology and Culture. 21 (2): 187–208. doi:10.2307/3103338. JSTOR 3103338.
- ^ Rittenhouse, P. A. (1979). «Potash and politics». Economic Geology. 74 (2): 353–7. doi:10.2113/gsecongeo.74.2.353.
- ^ Enghag, P. (2004). «11. Sodium and Potassium». Encyclopedia of the elements. Wiley-VCH Weinheim. ISBN 978-3-527-30666-4.
- ^ Davy, Humphry (1808). «On some new phenomena of chemical changes produced by electricity, in particular the decomposition of the fixed alkalies, and the exhibition of the new substances that constitute their bases; and on the general nature of alkaline bodies». Philosophical Transactions of the Royal Society. 98: 1–44. doi:10.1098/rstl.1808.0001.
- ^ Shaposhnik, V. A. (2007). «History of the discovery of potassium and sodium (on the 200th anniversary of the discovery of potassium and sodium)». Journal of Analytical Chemistry. 62 (11): 1100–2. doi:10.1134/S1061934807110160. S2CID 96141217.
- ^ a b c Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). «Potassium». Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. ISBN 978-3-11-007511-3.
- ^ Burkhardt, p. 32
- ^ Rieke, R. D. (1989). «Preparation of Organometallic Compounds from Highly Reactive Metal Powders». Science. 246 (4935): 1260–4. Bibcode:1989Sci…246.1260R. doi:10.1126/science.246.4935.1260. PMID 17832221. S2CID 92794.
- ^ Ross, William H. (1914). «The Origin of Nitrate Deposits». Popular Science. Bonnier Corporation. pp. 134–145.
- ^ Abdel-Wahab, M.; Youssef, S.; Aly, A.; el-Fiki, S.; et al. (1992). «A simple calibration of a whole-body counter for the measurement of total body potassium in humans». International Journal of Radiation Applications and Instrumentation A. 43 (10): 1285–9. doi:10.1016/0883-2889(92)90208-V. PMID 1330980.
- ^ Chang, Raymond (2007). Chemistry. McGraw-Hill Higher Education. p. 52. ISBN 978-0-07-110595-8.
- ^ Vašák, Milan; Schnabl, Joachim (2016). «Chapter 8. Sodium and Potassium Ions in Proteins and Enzyme Catalysis». In Astrid, Sigel; Helmut, Sigel; Roland K.O., Sigel (eds.). The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. Vol. 16. Springer. pp. 259–290. doi:10.1007/978-3-319-21756-7_8. PMID 26860304.
- ^ Weiner ID, Linus S, Wingo CS (2014). «Disorders of potassium metabolism». In Freehally J, Johnson RJ, Floege J (eds.). Comprehensive clinical nephrology (5th ed.). St. Louis: Saunders. p. 118. ISBN 9780323242875.
- ^ Malnic G, Giebisch G, Muto S, Wang W, Bailey MA, Satlin LM (2013). «Regulation of K+ excretion». In Alpern RJ, Caplan MJ, Moe OW (eds.). Seldin and Giebisch’s the kidney: physiology and pathophysiology (5th ed.). London: Academic Press. pp. 1659–1716. ISBN 9780123814630.
- ^ Mount DB, Zandi-Nejad K (2011). «Disorders of potassium balance». In Taal MW, Chertow GM, Marsden PA, Skorecki KL, Yu AS, Brenner BM (eds.). The kidney (9th ed.). Philadelphia: Elsevier. pp. 640–688. ISBN 9781455723041.
- ^ Lockless, S. W.; Zhou, M.; MacKinnon, R. (2007). «Structural and thermodynamic properties of selective ion binding in a K+ channel». PLOS Biol. 5 (5): e121. doi:10.1371/journal.pbio.0050121. PMC 1858713. PMID 17472437.
- ^ Wei, Kuang-Yu; Gritter, Martin; Vogt, Liffert; de Borst, Martin H; Rotmans, Joris I; Hoorn, Ewout J (2020-09-02). «Dietary potassium and the kidney: lifesaving physiology». Clinical Kidney Journal. Oxford University Press (OUP). 13 (6): 952–968. doi:10.1093/ckj/sfaa157. ISSN 2048-8513. PMC 7769543. PMID 33391739.
- ^ Goyal, Abhinav; Spertus, John A.; Gosch, Kensey; Venkitachalam, Lakshmi; Jones, Philip G.; Van den Berghe, Greet; Kosiborod, Mikhail (2012). «Serum Potassium Levels and Mortality in Acute Myocardial Infarction». JAMA. 307 (2): 157–164. doi:10.1001/jama.2011.1967. PMID 22235086.
- ^ Smyth, A.; Dunkler, D.; Gao, P.; et al. (2014). «The relationship between estimated sodium and potassium excretion and subsequent renal outcomes». Kidney Int. 86 (6): 1205–1212. doi:10.1038/ki.2014.214. PMID 24918156.
- ^ Moore-Ede, M. C. (1986). «Physiology of the circadian timing system: predictive versus reactive homeostasis». Am J Physiol. 250 (5 Pt 2): R737–R752. doi:10.1152/ajpregu.1986.250.5.R737. PMID 3706563.
- ^ Slonim, Anthony D.; Pollack, Murray M. (2006). «Potassium». Pediatric critical care medicine. Lippincott Williams & Wilkins. p. 812. ISBN 978-0-7817-9469-5.
- ^ Visveswaran, Kasi (2009). «hypokalemia». Essentials of Nephrology (2nd ed.). BI Publications. p. 257. ISBN 978-81-7225-323-3.
- ^ Gumz, Michelle L.; Rabinowitz, Lawrence; Wingo, Charles S. (2015-07-02). «An Integrated View of Potassium Homeostasis». The New England Journal of Medicine. 373 (1): 60–72. doi:10.1056/NEJMra1313341. ISSN 0028-4793. PMC 5675534. PMID 26132942.
- ^ Campbell, Neil (1987). Biology. Menlo Park, California: Benjamin/Cummings Pub. Co. p. 795. ISBN 978-0-8053-1840-1.
- ^ Hellgren, Mikko; Sandberg, Lars; Edholm, Olle (2006). «A comparison between two prokaryotic potassium channels (KirBac1.1 and KcsA) in a molecular dynamics (MD) simulation study». Biophysical Chemistry. 120 (1): 1–9. doi:10.1016/j.bpc.2005.10.002. PMID 16253415.
- ^ Potts, W. T. W.; Parry, G. (1964). Osmotic and ionic regulation in animals. Pergamon Press.
- ^ Lans, H. S.; Stein, I. F.; Meyer, K. A. (1952). «The relation of serum potassium to erythrocyte potassium in normal subjects and patients with potassium deficiency». American Journal of the Medical Sciences. 223 (1): 65–74. doi:10.1097/00000441-195201000-00011. PMID 14902792.
- ^ Bennett, C. M.; Brenner, B. M.; Berliner, R. W. (1968). «Micropuncture study of nephron function in the rhesus monkey». Journal of Clinical Investigation. 47 (1): 203–216. doi:10.1172/JCI105710. PMC 297160. PMID 16695942.
- ^ Solomon, A. K. (1962). «Pumps in the living cell». Scientific American. 207 (2): 100–8. Bibcode:1962SciAm.207b.100S. doi:10.1038/scientificamerican0862-100. PMID 13914986.
- ^ Kernan, Roderick P. (1980). Cell potassium (Transport in the life sciences). New York: Wiley. pp. 40, 48. ISBN 978-0-471-04806-0.
- ^ Wright, F. S. (1977). «Sites and mechanisms of potassium transport along the renal tubule». Kidney International. 11 (6): 415–432. doi:10.1038/ki.1977.60. PMID 875263.
- ^ Squires, R. D.; Huth, E. J. (1959). «Experimental potassium depletion in normal human subjects. I. Relation of ionic intakes to the renal conservation of potassium». Journal of Clinical Investigation. 38 (7): 1134–48. doi:10.1172/JCI103890. PMC 293261. PMID 13664789.
- ^ Fiebach, Nicholas H.; Barker, Lee Randol; Burton, John Russell & Zieve, Philip D. (2007). Principles of ambulatory medicine. Lippincott Williams & Wilkins. pp. 748–750. ISBN 978-0-7817-6227-4.
- ^ Gadsby, D. C. (2004). «Ion transport: spot the difference». Nature. 427 (6977): 795–7. Bibcode:2004Natur.427..795G. doi:10.1038/427795a. PMID 14985745. S2CID 5923529.; for a diagram of the potassium pores are viewed, see Miller, C (2001). «See potassium run». Nature. 414 (6859): 23–24. Bibcode:2001Natur.414…23M. doi:10.1038/35102126. PMID 11689922. S2CID 4423041.
- ^ Jiang, Y.; Lee, A.; Chen, J.; Cadene, M.; et al. (2002). «Crystal structure and mechanism of a calcium-gated potassium channel» (PDF). Nature. 417 (6888): 515–22. Bibcode:2002Natur.417..515J. doi:10.1038/417515a. PMID 12037559. S2CID 205029269. Archived from the original on 2009-04-24.
{{cite journal}}: CS1 maint: bot: original URL status unknown (link) - ^ Shi, N.; Ye, S.; Alam, A.; Chen, L.; et al. (2006). «Atomic structure of a Na+— and K+-conducting channel». Nature. 440 (7083): 570–4. Bibcode:2006Natur.440..570S. doi:10.1038/nature04508. PMID 16467789. S2CID 4355500.; includes a detailed picture of atoms in the pump.
- ^ Zhou, Y.; Morais-Cabral, J. H.; Kaufman, A.; MacKinnon, R. (2001). «Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution». Nature. 414 (6859): 43–48. Bibcode:2001Natur.414…43Z. doi:10.1038/35102009. PMID 11689936. S2CID 205022645.
- ^ Noskov, S. Y.; Bernèche, S.; Roux, B. (2004). «Control of ion selectivity in potassium channels by electrostatic and dynamic properties of carbonyl ligands». Nature. 431 (7010): 830–4. Bibcode:2004Natur.431..830N. doi:10.1038/nature02943. PMID 15483608. S2CID 4414885.
- ^ National Academies of Sciences, Engineering and Medicine (2019). «Potassium: Dietary Reference Intakes for Adequacy». In Stallings, Virginia A; Harrison, Meghan; Oria, Maria (eds.). Dietary Reference Intakes for Sodium and Potassium. Washington, DC: The National Academies Press. doi:10.17226/25353. ISBN 978-0-309-48834-1. PMID 30844154.
- ^ Stallings, Virginia A; Harrison, Meghan; Oria, Maria, eds. (March 5, 2019). Dietary Reference Intakes for Sodium and Potassium – Publication. Health and Medicine Division. National Academies of Sciences, Engineering and Medicine. doi:10.17226/25353. ISBN 978-0-309-48834-1. PMID 30844154. S2CID 104464967. Retrieved May 13, 2019.
- ^ Panel on Dietary Reference Intakes for Electrolytes and Water, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition (2004). DRI, dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, D.C.: National Academies Press. ISBN 978-0-309-53049-1. Archived from the original on 2011-10-06.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Karger, S. (2004). «Energy and nutrient intake in the European Union». Annals of Nutrition and Metabolism. 48 (2 (suppl)): 1–16. doi:10.1159/000083041.
- ^ «Vitamins and minerals». National Health Service (NHS). 18 November 2021. Retrieved 13 November 2022.
- ^ «Sodium and Potassium Dietary Reference Intake Values Updated in New Report; Introduces New Category for Sodium Based on Chronic Disease Risk Reduction» (Press release). National Academies of Sciences, Engineering, and Medicine. 5 March 2019. Retrieved 29 January 2022.
- ^ National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium (March 2019). Oria M, Harrison M, Stallings VA (eds.). Dietary Reference Intakes for Sodium and Potassium. National Academies Press. doi:10.17226/25353. ISBN 978-0-309-48834-1. PMID 30844154. S2CID 104464967. Bookshelf ID: NBK538102. Retrieved 13 November 2022.
- ^ «Potassium Food Charts». Asia Pacific Journal of Clinical Nutrition. Archived from the original on 2021-04-29. Retrieved 2011-05-18.
- ^ «Potassium Content of Selected Foods per Common Measure, sorted by nutrient content» (PDF). USDA National Nutrient Database for Standard Reference, Release 20. Archived from the original (PDF) on December 17, 2008.
- ^ Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ (1997). «Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials». JAMA. 277 (20): 1624–32. doi:10.1001/jama.1997.03540440058033. PMID 9168293. S2CID 25937399.
- ^ a b Institute of Medicine (U.S.). Committee on Optimization of Nutrient Composition of Military Rations for Short-Term, High-Stress Situations; Institute of Medicine (U.S.). Committee on Military Nutrition Research (2006). Nutrient composition of rations for short-term, high-intensity combat operations. National Academies Press. pp. 287–. ISBN 978-0-309-09641-6.
- ^ D’Elia, L.; Barba, G.; Cappuccio, F.; Strazzullo (2011). «Potassium Intake, Stroke, and Cardiovascular Disease: A Meta-Analysis of Prospective Studies». J Am Coll Cardiol. 57 (10): 1210–9. doi:10.1016/j.jacc.2010.09.070. PMID 21371638.
- ^ He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, Dalton RN, Kaski JC, MacGregor GA (2010). «Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives». Hypertension. 55 (3): 681–8. doi:10.1161/HYPERTENSIONAHA.109.147488. PMID 20083724.
- ^ «The Top 300 of 2020». ClinCalc. Retrieved 7 October 2022.
- ^ «Potassium Chloride — Drug Usage Statistics». ClinCalc. Retrieved 7 October 2022.
- ^ Shallenberger, R. S. (1993). Taste chemistry. Springer. pp. 120–. ISBN 978-0-7514-0150-9.
- ^ Garrett, Donald E. (1995-12-31). Potash: deposits, processing, properties and uses. Springer. ISBN 978-0-412-99071-7.
- ^ a b Ober, Joyce A. «Mineral Commodity Summaries 2008:Potash» (PDF). United States Geological Survey. Retrieved 2008-11-20.
- ^ a b c Ober, Joyce A. «Mineral Yearbook 2006:Potash» (PDF). United States Geological Survey. Retrieved 2008-11-20.
- ^ Wishart, David J. (2004). Encyclopedia of the Great Plains. U of Nebraska Press. p. 433. ISBN 978-0-8032-4787-1.
- ^ Chiu, Kuen-Wai (2000). «Potassium». Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. doi:10.1002/0471238961.1615200103080921.a01.pub2. ISBN 9780471238966.
- ^ Burkhardt, p. 34
- ^ Delahunt, J.; Lindeman, T. (2007). «Review of the safety of potassium and potassium oxides, including deactivation by introduction into water». Journal of Chemical Health and Safety. 14 (2): 21–32. doi:10.1016/j.jchas.2006.09.010.
- ^ Roy, Amit H. (2007). Kent and Riegel’s handbook of industrial chemistry and biotechnology. Springer. pp. 1135–57. Bibcode:2007karh.book……. ISBN 978-0-387-27843-8.
- ^ Ochoa-Hueso, R; Delgado-Baquerizo, M; King, PTA; Benham, M; Arca, V; Power, SA (2019). «Ecosystem type and resource quality are more important than global change drivers in regulating early stages of litter decomposition». Soil Biology and Biochemistry. 129: 144–152. doi:10.1016/j.soilbio.2018.11.009. S2CID 92606851.
- ^ «Potassium Uses, Side Effects & Interactions». Drugs.com.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 491. hdl:10665/44053. ISBN 9789241547659.
- ^ «Potassium chloride medical facts from Drugs.com». www.drugs.com. Archived from the original on 18 January 2017. Retrieved 14 January 2017.
- ^ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 680, 684. ISBN 9780857111562.
- ^ Figoni, Paula I (2010). «Bleaching and Maturing Agents». How Baking Works: Exploring the Fundamentals of Baking Science. John Wiley and Sons. p. 86. ISBN 978-0-470-39267-6.
- ^ Chichester, C. O. (July 1986). «Uses and Exposure to Sulfites in Food». Advances in food research. Academic Press. pp. 4–6. ISBN 978-0-12-016430-1.
- ^ Schultz
- ^ Toedt, John; Koza, Darrell & Cleef-Toedt, Kathleen Van (2005). «Personal Cleansing Products: Bar Soap». Chemical composition of everyday products. Greenwood Publishing Group. ISBN 978-0-313-32579-3.
- ^ Schultz, p. 95
- ^ Schultz, p. 99
- ^ Siegel, Richard S. (1940). «Ignition of the safety match». Journal of Chemical Education. 17 (11): 515. Bibcode:1940JChEd..17..515S. doi:10.1021/ed017p515.
- ^ Anger, Gerd; Halstenberg, Jost; Hochgeschwender, Klaus; Scherhag, Christoph; Korallus, Ulrich; Knopf, Herbert; Schmidt, Peter; Ohlinger, Manfred. «Chromium Compounds». Ullmann’s Encyclopedia of Industrial Chemistry. Vol. 9. Weinheim: Wiley-VCH. p. 178. doi:10.1002/14356007.a07_067.
- ^ Greenwood, p. 74
- ^ Marx, Robert F. (1990). The history of underwater exploration. Courier Dover Publications. p. 93. ISBN 978-0-486-26487-5.
- ^ Gettens, Rutherford John & Stout, George Leslie (1966). Painting materials: A short encyclopaedia. Courier Dover Publications. pp. 109–110. ISBN 978-0-486-21597-6.
- ^ Modugno, G.; Benkő, C.; Hannaford, P.; Roati, G.; Inguscio, M. (1999-11-01). «Sub-Doppler laser cooling of fermionic ${}^{40}mathrm{K}$ atoms». Physical Review A. 60 (5): R3373–R3376. arXiv:cond-mat/9908102. Bibcode:1999PhRvA..60.3373M. doi:10.1103/PhysRevA.60.R3373. S2CID 119001675.
- ^ Jackson, C. B.; Werner, R. C. (1957). «Ch. 18: The Manufacture of Potassium and NaK». Handling and uses of the alkali metals. Advances in Chemistry. Vol. 19. pp. 169–173. doi:10.1021/ba-1957-0019.ch018. ISBN 978-0-8412-0020-3.
- ^ Kearey, Philip; Brooks, M & Hill, Ian (2002). «Optical Pumped Magnetometer». An introduction to geophysical exploration. Wiley-Blackwell. p. 164. ISBN 978-0-632-04929-5.
- ^ «Potassium 244856». Sigma Aldrich.
- ^ Solomon, Robert E. (2002). Fire and Life Safety Inspection Manual. Jones & Bartlett Learning. p. 459. ISBN 978-0-87765-472-8.
- ^ «DOE Handbook-Alkali Metals Sodium, Potassium, NaK, and Lithium». Hss.doe.gov. Archived from the original on 2010-09-28. Retrieved 2010-10-16.
- ^ Wray, Thomas K. «Danger: peroxidazable chemicals» (PDF). Environmental Health & Public Safety, North Carolina State University. Archived from the original on 2016-07-29.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ a b Schonwald, Seth (2004). «Potassium Chloride and Potassium Permanganate». Medical toxicology. Lippincott Williams & Wilkins. pp. 903–5. ISBN 978-0-7817-2845-4.
- ^ Markovchick, Vincent J. & Pons, Peter T. (2003). Emergency medicine secrets. Elsevier Health Sciences. p. 223. ISBN 978-1-56053-503-4.
Bibliography
- Burkhardt, Elizabeth R. (2006). «Potassium and Potassium Alloys». Ullmann’s Encyclopedia of Industrial Chemistry. Vol. A22. pp. 31–38. doi:10.1002/14356007.a22_031.pub2. ISBN 978-3-527-30673-2.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (2007). «Potassium». Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. ISBN 978-3110177701.
- Schultz, H.; et al. (2006). «Potassium compounds». Ullmann’s Encyclopedia of Industrial Chemistry. Vol. A22. pp. 39–103. doi:10.1002/14356007.a22_031.pub2. ISBN 978-3-527-30673-2.
- National Nutrient Database Archived 2014-08-10 at the Wayback Machine at USDA Website
External links
- «Potassium». Drug Information Portal. U.S. National Library of Medicine.
| Калий | |
|---|---|
| Серебристо-белый мягкий металл | |

Элементарный калий |
|
| Название, символ, номер | Калий / Kalium (K), 19 |
| Атомная масса (молярная масса) |
39,0983(1) а. е. м. (г/моль) |
| Электронная конфигурация | [Ar] 4s1 |
| Радиус атома | 235 пм |
| Ковалентный радиус | 203 пм |
| Радиус иона | 133 пм |
| Электроотрицательность | 0,82 (шкала Полинга) |
| Электродный потенциал | −2,92 В |
| Степени окисления | 0; +1 |
| Энергия ионизации (первый электрон) |
418,5 (4,34) кДж/моль (эВ) |
| Плотность (при н. у.) | 0,856 г/см³ |
| Температура плавления | 336,8 К; +63,65 °C |
| Температура кипения | 1047 К; 773,85 °C |
| Уд. теплота плавления | 2,33 кДж/моль |
| Уд. теплота испарения | 76,9 кДж/моль |
| Молярная теплоёмкость | 29,6 Дж/(K·моль) |
| Молярный объём | 45,3 см³/моль |
| Структура решётки | кубическая объёмно-центрированная |
| Параметры решётки | 5,332 Å |
| Температура Дебая | 100 K |
| Теплопроводность | (300 K) 79,0 Вт/(м·К) |
| Номер CAS | 7440-09-7 |
Калий — элемент первой группы (по старой классификации — главной подгруппы первой группы), четвёртого периода периодической системы химических элементов Д. И. Менделеева, с атомным номером 19. Обозначается символом K (лат. Kalium). Простое вещество калий — мягкий щелочной металл серебристо-белого цвета.
В природе калий встречается только в соединениях с другими элементами, например, в морской воде, а также во многих минералах.
Очень быстро окисляется на воздухе и очень легко вступает в химические реакции, особенно с водой, образуя щёлочь.
Во многих свойствах калий очень близок натрию, но с точки зрения биологической функции и использования клетками живых организмов они антагонистичны.
Содержание
- 1 История и происхождение названия
- 2 Нахождение в природе
- 2.1 Месторождения
- 3 Получение
- 4 Физические свойства
- 5 Химические свойства
- 5.1 Взаимодействие с простыми веществами
- 5.2 Взаимодействие со сложными веществами
- 5.3 Соединения с кислородом
- 5.4 Гидроксид
- 6 Применение
- 6.1 Важные соединения
- 7 Биологическая роль
- 7.1 Калий в организме человека
- 8 Изотопы
История и происхождение названия
Соединения калия используются с древнейших времён. Так, производство поташа (который применялся как моющее средство) существовало уже в XI веке. Золу, образующуюся при сжигании соломы или древесины, обрабатывали водой, а полученный раствор (щёлок) после фильтрования выпаривали. Сухой остаток, помимо карбоната калия K2CO3, содержал сульфат калия K2SO4, соду и хлорид калия KCl.
19 ноября 1807 года в Бейкеровской лекции английский химик Дэви сообщил о выделении калия электролизом расплава едкого кали (KOH)(в рукописи лекции Дэви указал, что он открыл калий 6 октября 1807 года). Дэви назвал его «потасий» (лат. potasium; это название (правда, в некоторых языках с двумя буквами s) до сих пор употребительно в английском, французском, испанском, португальском и польском языках. При электролизе влажного едкого кали KOH на ртутном катоде он получил амальгаму калия, а после отгонки ртути — чистый металл. Дэви определил его плотность, изучил химические свойства, в том числе разложение воды и поглощение водорода.
В 1808 году французские химики Гей-Люссак и Л. Тенар выделили калий химическим путём — прокаливанием KOH с углём.
В 1809 году немецкий физик Л. В. Гильберт предложил название «калий» (лат. kalium, от араб. аль-кали — поташ). Это название вошло в немецкий язык, оттуда в большинство языков Северной и Восточной Европы (в том числе русский) и «победило» при выборе символа для этого элемента — K.
Нахождение в природе
Ввиду высокой химической активности калий в свободном состоянии в природе не встречается. Породообразующий элемент, входит в состав слюд, полевых шпатов и т. д. Также калий входит в состав минералов сильвина KCl, сильвинита KCl·NaCl, карналлита KCl·MgCl2·6H2O, каинита KCl·MgSO4·6H2O, а также присутствует в золе некоторых растений в виде карбоната K2CO3 (поташ). Калий входит в состав всех клеток (см. ниже раздел Биологическая роль). Кларк калия в земной коре составляет 2,4 % (5-й по распространённости металл, 7-й по содержанию в коре элемент). Средняя концентрация в морской воде — 380 мг/л.
Месторождения
Крупнейшие месторождения калия находятся на территории Канады (производитель PotashCorp), России (ПАО «Уралкалий», г. Березники, г. Соликамск, Пермский край, Верхнекамское месторождение калийных руд), Белоруссии (ПО «Беларуськалий», г. Солигорск, Старобинское месторождение калийных руд).
Получение
Калий, как и другие щелочные металлы, получают электролизом расплавленных хлоридов или щелочей. Так как хлориды имеют более высокую температуру плавления (600—650 °C), то чаще проводят электролиз расплавленных щелочей с добавкой к ним соды или поташа (до 12 %). При электролизе расплавленных хлоридов на катоде выделяется расплавленный калий, а на аноде — хлор:
-
- K+ + e− → K
- 2Cl− → Cl2
При электролизе гидроксида калия на катоде также выделяется расплавленный калий, а на аноде — кислород:
-
- 4OH− → 2H2O + O2
Вода из расплава быстро испаряется. Чтобы калий не взаимодействовал с хлором или кислородом, катод изготовляют из меди и над ним помещают медный цилиндр. Образовавшийся калий в расплавленном виде собирается в цилиндре. Анод изготовляют также в виде цилиндра из никеля (при электролизе щелочей) либо из графита (при электролизе хлоридов).
Важное промышленное значение имеют и методы термохимического восстановления:
-
- Na + KOH →N2,380−450oC NaOH + K
и восстановление из расплава хлорида калия карбидом кальция, алюминием или кремнием.
Физические свойства
Калий — серебристый металл с характерным блеском на свежеобразованной поверхности. Очень лёгок и легкоплавок. Относительно хорошо растворяется в ртути, образуя амальгамы. Будучи внесённым в пламя горелки, калий (а также его соединения) окрашивает пламя в характерный розово-фиолетовый цвет.
Калий активно взаимодействует с водой. Выделяющийся водород воспламеняется, а ионы калия придают пламени фиолетовый цвет. Раствор фенолфталеина в воде становится малиновым, демонстрируя щелочную реакцию образующегося KOH
Калий образует кристаллы кубической сингонии, пространственная группа I m3m, параметры ячейки a = 0,5247 нм, Z = 2.
Химические свойства
Элементарный калий, как и другие щелочные металлы, проявляет типичные металлические свойства и очень химически активен, является сильным восстановителем. На воздухе свежий срез быстро тускнеет из-за образования плёнок соединений (оксиды и карбонат). При длительном контакте с атмосферой способен полностью разрушиться. С водой реагирует со взрывом. Хранить его необходимо под слоем бензина, керосина или силикона, дабы исключить контакт воздуха и воды с его поверхностью. С Na, Tl, Sn, Pb, Bi калий образует интерметаллиды.
Взаимодействие с простыми веществами
Калий при комнатной температуре реагирует с кислородом воздуха, галогенами; практически не реагирует с азотом (в отличие от лития и натрия). При умеренном нагревании реагирует с водородом с образованием гидрида (200—350 °C):
-
- 2K + H2 ⟶ 2KH
с халькогенами (100—200 °C, E = S, Se, Te):
-
- 2K + E ⟶ K2E
При сгорании калия на воздухе образуется надпероксид калия KO2 (с примесью K2O2):
-
- K + O2 ⟶ KO2
В реакции с фосфором в инертной атмосфере образуется фосфид калия зелёного цвета (200 °C):
-
- 3K + P ⟶ K3P
Взаимодействие со сложными веществами
Калий при комнатной температуре (+20 °C) активно реагирует с водой, кислотами, растворяется в жидком аммиаке (−50 °C) с образованием тёмно-синего раствора аммиаката калия.
-
- 2K + 2H2O ⟶ 2KOH + H2↑
-
- 2K + 2HCl ⟶ 2KCl + H2↑
-
- K + 6NH3 ⟶ [K(NH3)]6
Калий глубоко восстанавливает разбавленные серную и азотную кислоты:
-
- 8K + 6H2SO4 ⟶ 4K2SO4 + SO2↑ + S↓ + 6H2O
-
- 21K + 26HNO3 ⟶ 21KNO3 + NO↑ + N2O↑ + N2↑ + 13H2O
При сплавлении металлического калия со щелочами он восстанавливает водород гидроксогруппы:
-
- 2K + 2KOH ⟶ 2K2O + H2↑ (450∘C)
При умеренном нагревании реагирует с газообразным аммиаком с образованием амида (+65…+105 °C):
-
- 2K + 2NH3 ⟶ 2KNH2 + H2
Металлический калий реагирует со спиртами с образованием алкоголятов:
-
- 2K + 2C2H5OH ⟶ 2C2H5OK + H2↑
Алкоголяты щелочных металлов (в данном случае — этилат калия) широко используются в органическом синтезе.
Соединения с кислородом
При взаимодействии калия с кислородом воздуха образуется не оксид, а пероксид и супероксид:
-
- 2K + O2 ⟶ K2O2
- K + O2 ⟶ KO2
Оксид калия может быть получен при нагревании металла до температуры не выше 180 °C в среде, содержащей очень мало кислорода, или при нагревании смеси супероксида калия с металлическим калием:
-
- 4K + O2 ⟶ 2K2O
- KO2 + 3K ⟶ 2K2O
Оксиды калия обладают ярко выраженными осно́вными свойствами, бурно реагируют с водой, кислотами и кислотными оксидами. Практического значения они не имеют. Пероксиды представляют собой желтовато-белые порошки, которые, хорошо растворяясь в воде, образуют щёлочи и пероксид водорода:
-
- K2O2 + 2H2O ⟶ 2KOH + H2O2
-
- 4KO2 + 2H2O ⟶ 4KOH + 3O2↑
-
- 4KO2 + 2CO2 ⟶ 2K2CO3 + 3O2↑
Советский изолирующий противогаз ИП-5
Свойство обменивать углекислый газ на кислород используется в изолирующих противогазах и на подводных лодках. В качестве поглотителя используют эквимолярную смесь супероксида калия и пероксида натрия. Если смесь не эквимолярна, то в случае избытка пероксида натрия поглотится больше газа, чем выделится (при поглощении двух объёмов CO2 выделяется один объём O2), и давление в замкнутом пространстве упадёт, а в случае избытка супероксида калия (при поглощении двух объёмов CO2 выделяется три объёма O2) выделяется больше газа, чем поглотится, и давление повысится.
В случае эквимолярной смеси (Na2O2:K2O4 = 1:1) объёмы поглощаемого и выделяемого газов будут равны (при поглощении четырёх объёмов CO2 выделяется четыре объёма O2).
Пероксиды являются сильными окислителями, поэтому их применяют для отбеливания тканей в текстильной промышленности.
Получают пероксиды прокаливанием металлов на воздухе, освобождённом от углекислого газа.
Также известен озонид калия KO3, оранжево-красного цвета. Получить его можно взаимодействием гидроксида калия с озоном при температуре не выше +20 °C:
-
- 4KOH + 4O3 ⟶ 4KO3 + O2 + 2H2O
Озонид калия является очень сильным окислителем, например, окисляет элементарную серу до сульфата и дисульфата уже при +50 °C:
-
- 6KO3 + 5S ⟶ K2SO4 + 2K2S2O7
Гидроксид
Основная статья: Гидроксид калия
Гидроксид калия (или едкое кали) представляет собой твёрдые белые непрозрачные, очень гигроскопичные кристаллы, плавящиеся при температуре 360 °C. Гидроксид калия относится к щелочам. Он хорошо растворяется в воде с выделением большого количества тепла. Растворимость едкого кали при +20 °C в 100 г воды составляет 112 г.
Применение
- Жидкий при комнатной температуре сплав калия и натрия используется в качестве теплоносителя в замкнутых системах, например, в атомных силовых установках на быстрых нейтронах. Кроме того, широко применяются его жидкие сплавы с рубидием и цезием. Сплав с составом 12 % натрия, 47 % калия, 41 % цезия обладает рекордно низкой температурой плавления −78 °C.
- Соединения калия — важнейший биогенный элемент и потому применяются в качестве удобрений. Калий является одним из трёх базовых элементов, которые необходимы для роста растений наряду с азотом и фосфором. В отличие от азота и фосфора, калий является основным клеточным катионом. При его недостатке у растения прежде всего нарушается структура мембран хлоропластов — клеточных органелл, в которых проходит фотосинтез. Внешне это проявляется в пожелтении и последующем отмирании листьев. При внесении калийных удобрений у растений увеличивается вегетативная масса, урожайность и устойчивость к вредителям.
- Соли калия широко используются в гальванотехнике, так как, несмотря на относительно высокую стоимость, они часто более растворимы, чем соответствующие соли натрия, и потому обеспечивают интенсивную работу электролитов при повышенной плотности тока.
Важные соединения
- Бромид калия применяется в медицине и как успокаивающее средство для нервной системы.
- Гидроксид калия (едкое кали) применяется в щелочных аккумуляторах и при сушке газов.
- Карбонат калия (поташ) используется как удобрение, при варке стекла, как кормовая добавка для птицы.
- Хлорид калия (сильвин, «калийная соль») используется как удобрение.
- Нитрат калия (калийная селитра) — удобрение, компонент чёрного пороха.
- Перхлорат и хлорат калия (бертолетова соль) используются в производстве спичек, ракетных порохов, осветительных зарядов, взрывчатых веществ, в гальванотехнике.
- Дихромат калия (хромпик) — сильный окислитель, используется для приготовления «хромовой смеси» для мытья химической посуды и при обработке кожи (дубление). Также используется для очистки ацетилена на ацетиленовых заводах от аммиака, сероводорода и фосфина.
Кристаллы перманганата калия
- Перманганат калия — сильный окислитель, используется как антисептическое средство в медицине и для лабораторного получения кислорода.
- Тартрат натрия-калия (сегнетова соль) в качестве пьезоэлектрика.
- Дигидрофосфат и дидейтерофосфат калия в виде монокристаллов в лазерной технике.
- Пероксид калия и супероксид калия используются для регенерации воздуха на подводных лодках и в изолирующих противогазах (поглощает углекислый газ с выделением кислорода).
- Фтороборат калия — важный флюс для пайки сталей и цветных металлов.
- Цианид калия применяется в гальванотехнике (серебрение, золочение), при добыче золота и при нитроцементации стали. Чрезвычайно ядовит, один из сильнейших ядов.
- Калий совместно с перекисью калия применяется при термохимическом разложении воды на водород и кислород (калиевый цикл «Газ де Франс», Франция).
- Сульфат калия применяется как удобрение.
Биологическая роль
Калий — важнейший биогенный элемент, особенно в растительном мире. При недостатке калия в почве растения развиваются очень плохо, уменьшается урожай, поэтому около 90 % добываемых солей калия используют в качестве удобрений.
Калий в качестве катиона наряду с катионами натрия является базовым элементом так называемого калиево-натриевого насоса клеточной мембраны, который играет важную роль в проведении нервных импульсов.
Калий в организме человека
Калий содержится большей частью в клетках, до 40 раз больше, чем в межклеточном пространстве. В процессе функционирования клеток избыточный калий покидает цитоплазму, поэтому для сохранения концентрации он должен нагнетаться обратно при помощи натрий-калиевого насоса. Калий и натрий между собой функционально связаны и выполняют следующие функции:
- Создание условий для возникновения мембранного потенциала и мышечных сокращений.
- Поддержание осмотической концентрации крови.
- Поддержание кислотно-щелочного баланса.
- Нормализация водного баланса.
Рекомендуемая суточная доля калия составляет для детей от 600 до 1700 миллиграммов, для взрослых — от 1800 до 5000 миллиграммов. Потребность в калии зависит от массы тела, физической активности, физиологического состояния и климата места проживания. Рвота, продолжительные поносы, обильное потение, использование мочегонных повышают потребность организма в калии.
Основными пищевыми источниками являются бобы (в первую очередь белая фасоль), шпинат и капуста кормовая, финики, картофель, батат, сушёные абрикосы, дыня, киви, авокадо, помело, бананы, брокколи, печень, молоко, ореховое масло, цитрусовые, виноград. Калия достаточно много в рыбе и молочных продуктах.
Практически все сорта рыбы содержат более 200 мг калия на 100 г. Количество калия в разных видах рыбы различается.
Овощи, грибы и травы также содержат много калия, однако в консервированных продуктах его уровень может быть гораздо меньше. Много калия содержится в шоколаде.
Всасывание происходит в тонком кишечнике. Усвоение калия облегчает витамин B6, затрудняет — алкоголь.
При недостатке калия развивается гипокалиемия. Возникают нарушения работы сердечной и скелетной мускулатуры. Продолжительный дефицит калия может быть причиной острой невралгии.
При избытке калия развивается гиперкалиемия, для которой основным симптомом является язва тонкого кишечника. Настоящая гиперкалиемия может вызвать остановку сердца.
Изотопы
Основная статья: Изотопы калия
Природный калий состоит из трёх изотопов. Два из них стабильны: 39K (изотопная распространённость 93,258 %) и 41K (6,730 %). Третий изотоп 40K (0,0117 %) является бета-активным с периодом полураспада 1,251 миллиарда лет. Сравнительно малый период полураспада и большая распространённость калия по сравнению с ураном и торием означает, что на Земле ещё 2 млрд лет назад и ранее калий-40 вносил главный вклад в естественный радиационный фон. В каждом грамме природного калия в секунду распадается в среднем 31,0±0,3 ядра 40K, благодаря чему, например, в организме человека массой 70 кг ежесекундно происходит около 4000 радиоактивных распадов. Поэтому легкодоступные в быту соединения калия (поташ, хлорид калия, калийная селитра и т. д.) можно использовать как пробные радиоактивные источники для проверки бытовых дозиметров. 40K наряду с ураном и торием считается одним из основных источников геотермальной энергии, выделяемой в недрах Земли (полная скорость энерговыделения оценивается в 40—44 ТВт). В минералах, содержащих калий, постепенно накапливается 40Ar, один из продуктов распада калия-40, что позволяет измерять возраст горных пород; калий-аргоновый метод является одним из основных методов ядерной геохронологии.
Один из искусственных изотопов — 37K, — с временем полураспада 1,23651 секунды, применяется в экспериментах по изучению Стандартной модели слабого взаимодействия.
|
Периодическая система химических элементов Д. И. Менделеева |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Электрохимический ряд активности металлов |
|---|
|
Eu, Sm, Li, Cs, Rb, K, Ra, Ba, Sr, Ca, Na, Ac, La, Ce, Pr, Nd, Pm, Gd, Tb, Mg, Y, Dy, Am, Ho, Er, Tm, Lu, Sc, Pu, |
|
Щелочные металлы |
||||||
|---|---|---|---|---|---|---|
|
Калий в таблице менделеева занимает 19 место, в 4 периоде.
| Символ | K |
| Номер | 19 |
| Атомный вес | 39.0983000 |
| Латинское название | Kalium, Calium |
| Русское название | Калий |
Как самостоятельно построить электронную конфигурацию? Ответ здесь
Электронная схема калия
K: 1s2 2s2 2p6 3s2 3p6 4s1
Короткая запись:
K: [Ar]4s1
Одинаковую электронную конфигурацию имеют
атом калия и
Cl-2, Sc+2, Ti+3, V+4, Mn+6
Порядок заполнения оболочек атома калия (K) электронами:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d →
5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
На подуровне ‘s’ может находиться до 2 электронов, на ‘s’ — до 6, на
‘d’ — до 10 и на ‘f’ до 14
Калий имеет 19 электронов,
заполним электронные оболочки в описанном выше порядке:
2 электрона на 1s-подуровне
2 электрона на 2s-подуровне
6 электронов на 2p-подуровне
2 электрона на 3s-подуровне
6 электронов на 3p-подуровне
1 электрон на 4s-подуровне
Степень окисления калия
Атомы калия в соединениях имеют степени окисления 1, -1.
Степень окисления — это условный заряд атома в соединении: связь в молекуле
между атомами основана на разделении электронов, таким образом, если у атома виртуально увеличивается
заряд, то степень окисления отрицательная (электроны несут отрицательный заряд), если заряд уменьшается,
то степень окисления положительная.
Ионы калия
Валентность K
Атомы калия в соединениях проявляют валентность I.
Валентность калия характеризует способность атома K к образованию хмических связей.
Валентность следует из строения электронной оболочки атома, электроны, участвующие в образовании
химических соединений называются валентными электронами. Более обширное определение валентности это:
Число химических связей, которыми данный атом соединён с другими атомами
Валентность не имеет знака.
Квантовые числа K
Квантовые числа определяются последним электроном в конфигурации,
для атома K эти числа имеют значение N = 4, L = 0, Ml = 0, Ms = +½
Видео заполнения электронной конфигурации (gif):
Результат:
Энергия ионизации
Чем ближе электрон к центру атома — тем больше энергии необходимо, что бы его оторвать.
Энергия, затрачиваемая на отрыв электрона от атома называется энергией ионизации и обозначается Eo.
Если не указано иное, то энергия ионизации — это энергия отрыва первого электрона, также существуют энергии
ионизации для каждого последующего электрона.
Энергия ионизации K:
Eo = 419 кДж/моль
— Что такое ион читайте в статье.
Перейти к другим элементам таблицы менделеева
Где K в таблице менделеева?
Таблица Менделеева
Скачать таблицу менделеева в хорошем качестве
Калий имеет серебристый, с проблесками белого, цвет. Относится к металлам. В окружающей среде встретить его в чистом виде невозможно. В природе существует только в виде соединений. По свойствам схож с натрием, но эти элементы – соперники.
Оглавление
- История открытия
- Калий в таблице Менделеева
- Строение атома
- Физические свойства
- Химические свойства
- Калий в природе
- Применение
История открытия
Ученые, которые принесли вклад в открытие элемента:
- Хэмфри Дэви;
- Жозеф Луи Гей-Люссак;
- Луи Жак Тенар;
- Людвиг Вильгельм Гильберт.
Хэмфри Дэви – британский химик, первооткрыватель. Первое публичное упоминание и краткое описание элемента принадлежит ему и датируется 1807 годом. 12-го ноября ученый рассказал о выделении калия из процесса распада гидроксида.
Согласно его рукописи открытие было сделано 6 октября 1807 года. Название калий получил позже. Сам Хэмфри Дэви присвоил ему термин «потассий». Он все еще употребляется в польском, испанском языках.
Ученый проводил химические эксперименты на протяжении многих лет, в итоге получил результат – чистый металл. Он изучил и описал свойства как физические, так и химические, задокументировал особенности взаимодействия элемента с другими веществами, определил его плотность.
Жозеф Луи Гей-Люссак, Луи Жак Тенар – французские химики, в 1808 году также выделили чистый калий. Они получили его, нагревая до высокой температуры смесь гидроксида и железного наполнителя.
Людвиг Вильгельм Гильберт – немецкий физик. Он считается первым, кто предложил известное всем слово – «калий». В 1809 году это название начало употребляться в Германии, а позже перешло в Северо-Восточную часть Европы.
Термин приняли в обществе, после того, как вышла книга Гесса в Германии.
Калий в таблице Менделеева
Калий в таблице Менделеева кратко обозначается латинской заглавной буквой «K». Находится под номером 14, в четвертом периоде, относится к первой группе периодической таблицы. Имеет следующее описание и отличительные черты.
| Название | Kalium (K) |
| Молярная масса | 39,0983 г/моль |
| Температура плавления | 63,2°С |
| Температура кипения | 760°C |
| Теплота парообразования | 231,5 кДж/моль |
| Молярный объем | 45,3 см³/моль |
| Удельная теплота плавления | 2,33 кДж/моль |
| Удельная теплота испарения | 76,9 кДж/моль |
Химические формулы соединений калия:
- KOH – гидроксид;
- KCl – хлорид калия;
- KNO₃ – нитрат;
- K₂SO₄ – сульфат;
- K₃PO₄ – фосфат;
- K₂CO₃ – карбонат калия.
Строение атома
Атом имеет четыре орбиты, на которых движутся 19 электронов, у ядра положительный заряд (+19). Степень окисления равна +1. Порядковый номер в таблице – 19, семейство – s. Валентность – 1.
Существует 2 стабильных изотопа.
Физические свойства
Элемент характеризуется следующими физическими свойствами:
- серебристый цвет;
- мягкая текстура;
- легкое плавление;
- активность.
Калий меняет свою окраску при горении. Из серебристо-белого становится фиолетового цвета. О наличии калия в реакции свидетельствует ярко-фиолетовый оттенок пламени.
Химические свойства
Получение калия идет химическим путем, за счет электролиза расплавленных оснований, хлоридов. Чаще применяют щелочи из-за более низкой температуры, требующейся для плавления.
Калий сам по себе – активное вещество, которое легко взаимодействует, отдает электроны. Чтобы предотвратить нежелательное окисление, хранить его требуется под слоем керосина.
Имеет типичные для металлов свойства:
- действует как восстановитель;
- вступает в реакцию с кислородом, образуя прочный оксид;
- образует гидрид при температуре 200-350°C;
- взаимодействует с хлором, серой, азотом, серой и т. д. (они считаются простыми элементами, эти реакции носят название «присоединение»);
- реагирует со сложными компонентами – соль, кислоты, вода, оксиды (происходит реакция замещения, калий оказывается на месте водорода).
При соприкосновении с водой, при первом же контакте воспламеняется, выделяет тепло. В результате реакции получается щелочь (гидроксид калия).
Реакция с кислородом называется окислением. Уравнение реакции калия с кислородом имеет вид:
- 2K+O₂=K₂О₂ (при протекании в условиях пониженных температур);
- K+O₂=KО₂ (возможна только при горении).
Калий в природе
Калий – щелочной металл. В природе в чистом виде его практически нет, так как он химически активен.
Использовали калий еще с 1807 года. Получали элемент путем выделения из продуктов горения.
Несколько фактов о содержании калия в природе:
- концентрация в земной коре достигает 2,5%;
- элемент присутствует во многих горных породах, полезных ископаемых;
- концентрация в почве 0,5-3%;
- калийные соли содержатся на остатках высушенных морей;
- высокая концентрация в морских, океанических водах способствует выгодной добыче этого вещества.
Калий – важный элемент для живых организмов. При его участии протекает множество биологических процессов. Его количество в человеческом теле достигает 0,25% массы.
Применение
Область применения обширна. Вещество и его соединения используются:
- При производстве спичек.
- В медицине и биологии как радиоактивный индикатор.
- В качестве подкормки для растений, почвы.
- В машиностроении для изготовления деталей.
- В пищевой промышленности. Результатом взаимодействия с азотом является пищевая добавка – нитрат.
- В прикладной электрохимии.
- В химической промышленности.
Большая часть калия, порядка 95%, тратится на удобрения.
Была ли полезна Вам наша статья? Сохраняйте ее в закладки, чтобы не потерять полезную информацию. Делитесь ею в социальных сетях.
Также рекомендуем посмотреть подобранные видео по нашей теме.
Калий – самый горючий металл на земле.
Химия 10 класс. Натрий, калий и их важнейшие соединения — свойства.
Источники:
- https://melscience.com/RU-ru/articles/svojstva-kaliya-i-ego-vzaimodejstvie-s-vodoj
- http://www.chem100.ru/elem.php?n=19
- https://ru.wikipedia.org/wiki/Калий
- http://www.himsnab-spb.ru/article/ps/k
- http://www.mining-enc.ru/k/kalij
Калий (лат. Kalium), K (читается «калий»), химический элемент с атомным номером 19, атомная масса 39,0983.
Калий встречается в природе в виде двух стабильных нуклидов: 39К (93,10% по массе) и 41К (6,88%), а также одного радиоактивного 40К (0,02%). Период полураспада калия-40 Т1/2 примерно в 3 раза меньше, чем Т1/2 урана-238 и составляет 1,28 миллиарда лет. При bраспаде калия-40 образуется стабильный кальций-40, а при распаде по типу электронного захвата образуется инертный газ аргон-40.
Калий принадлежит к числу щелочных металлов. В периодической системе Менделеева калий занимает место в четвертом периоде в подгруппе IА. Конфигурация внешнего электронного слоя 4s1, поэтому калий всегда проявляет степень окисления +1 (валентность I).
Атомный радиус калия 0,227 нм, радиус иона K+ 0,133 нм. Энергии последовательной ионизации атома калия 4,34 и 31,8 эВ. Электроотрицательность калия по Полингу 0,82, что говорит о его ярко выраженных металлических свойствах.
В свободном виде — мягкий, легкий, серебристый металл.

Особенности обращения с металлическим калием: металлический калий может вызвать очень сильные ожоги кожи, при попадании мельчайших частичек калия в глаза возникают тяжелые поражения с потерей зрения, поэтому работать с металлическим калием можно только в защитных перчатках и очках. Загоревшийся калий заливают минеральным маслом или засыпают смесью талька и NaCl. Хранят калий в герметично закрытых железных контейнерах под слоем обезвоженного керосина или минерального масла.
История открытия
Атомный номер калия 19, что указывает на его расположение в химической таблице Менделеева.
Примерная молярная масса 39,1 г/моль.
Электронная конфигурация калия 1s22s22p63s23p64s1
Единственная возможная степень окисления +1 (плюс один).
На внешнем энергетическом уровне имеется всего 1 электрон. Это значит, что максимальная валентность элемента 1.
Кристаллическая решётка простого вещества кубическая объёмно-центрированная.
В 1807 году английский химик Х. Дэви опытным путём получил потассий (латинское название — потассиум). Именно так изначально был назван калий. Проводя электролиз каустической воды и расплавов поташа, учёный заметил образование мягкого легкоплавкого металла. Такое достижение подтолкнуло его к новым открытиям и он стал изучать химические и физические свойства нового вещества.
Такая сенсация потрясла весь научный мир и зарубежные коллеги решили не оставаться в стороне. Уже через 2 года британский эксперт Л. В. Гилберт предложил название «Аль-кали», что в переводе с арабского означает «зола растений». И это не удивительно, ведь золу, которая оставалась после сжигания растений, обрабатывали водой, а полученную смесь выпаривали до сухого остатка. В далёкие времена это использовали как моющее средство. В 1831 году немецкий физик Г. И. Гесс, изучавший свойства нового вещества, предложил своё название для элемента, который также называли «Аль-калий».
Калий в природе
После многолетних поисков выяснилось, что в природе калий не находится в чистом виде. Он один из десяти элементов, которые составляют большую часть окружающего мира.
Калий — неотъемлемый элемент в составе клеток живых организмов. Также большое количество содержится в минералах и морской воде. Минералы, формулы которых могут «похвастаться» большим содержанием этого элемента:
- Сильвинита KCl·NaCl.
- Карналлита KCl·MgCl 2 ·6H 2 O.
- Каинита KCl·MgSO 4 ·6H 2 O.
- Зола растений как поташ K 2 CO 3.
Способы получения
Современные условия позволяют учёным получать калий несколькими способами.
Уравнение взаимодействия с жидким натрием расплавленного основания при 380−450°C или хлорида при 760- 890 °C: Na + KOH = NaOH + K
Электролиз расплава хлорида в смеси с карбонатом калия при температуре около 700 °C:
2KCl = 2K + Cl 2.
Физические свойства
Легкоплавкий металл серебристого цвета. При надрезе быстро образует оксидную плёнку после нескольких секунд контакта с кислородом, что объясняет требования особых условий содержания в помещениях. Хранится только в посуде с керосином, силиконами или бензином. Обладает хорошей растворимостью при соединении со ртутью. Образует амальгамы.
На соединение с водой реагирует взрывом. При поднесении горелки окрашивает пламя в розово-фиолетовый цвет.
Химические характеристики
Калий имеет много общего с натрием. Это обусловлено их расположением в периодической таблице химических элементов Д. И. Менделеева. Оба элемента — щелочные металлы, которые ярко выражают свои свойства. Однако у потассия металлические свойства проявляются сильнее, чем у натрия и кальция, но слабее, чем у рубидия.
Калию свойственно проявлять такие характеристики, которые делают его незаменимым для химической промышленности:
- Химически активен.
- Легко отдаёт электроны.
- Сильный восстановитель.
Оксиды или пероксиды
При взаимодействии с кислородом образует не оксид, а пероксид или супероксид, что заметно невооружённым глазом (очень быстро образует оксидную плёнку на поверхности).
Может образовать оксид только лишь при медленном нагревании до температуры меньше 180 °C при низком содержании кислорода в окружающей среде.
Оксиды ярко проявляют основные свойства. Как и сам металл, бурно реагируют с водой, кислотными оксидами и самими кислотами. Практического применения в промышленности они не нашли, используются для обучения в университетах.
Пероксиды — белые порошки с жёлтым тоном. Хорошо растворяются в воде, образуя щёлочи и пероксид водорода.
Сильные окислители, поэтому обрели популярность в текстильной промышленности как отбеливающее средство.
Гидроксиды калия
Гидроксиды калия и натрия имеют особые названия: едкий кали и едкий натри. Белые, твёрдые, непрозрачные вещества. Очень гигроскопичны, это значит, что быстро впитывают влагу и требуют особого внимания при работе с ними. Лаборанту необходимо надевать перчатки и защитные очки, иначе получит сильный ожог и раздражение слизистых оболочек. Кристаллы плавятся при температуре 360 °C. Гидроксиды относят к щелочам, они быстро растворяются в воде, выделяя большое количество тепла.
Сфера применения
Соединения калия используют в качестве удобрений, что свидетельствует о его ценных биологических характеристиках. Один из важнейших компонентов биосистемы вместе с азотом и фосфором. Помимо этого, необходим обмен элемента в натриево-калиевом насосе клетки любого живого организма.
Большую популярность приобрел в гальванотехнике. Соли металла быстро растворяются, по сравнению с солями натрия. Это свойство позволяет устанавливать высокие цены компаниям, которые занимаются обработкой калия.
Жидкий сплав калия и натрия используется в качестве теплоносителей в атомных установках. Необходимое условие: комнатная температура.
Особые соединения калия
Бромид используется в фармацевтике для изготовления успокоительных лекарств.
Карбонат, хлорид и нитрат пользуются популярностью у садоводов, так как представляют собой удобрения, обогащённые большим количеством полезных микроэлементов.
Перманганат применяется в химических лабораториях для получения кислорода, а также ранее широко применялся в быту благодаря своим антисептическим свойствам.
Пероксид и супероксид обеспечивают регенерацию воздуха на подводных лодках и противогазах, благодаря своей способности поглощать углекислый газ и выделять кислород.
Из описания характеристик калия с другими элементами становится понятно, что это крайне важная составляющая организма, которая должны взаимодействовать с другими металлами и неметаллами, чтобы обеспечить гармоничный рост и развитие организма. Норма потребления этого элемента для человека — 2040 мг в сутки.
Роль этого металла и реакций организма, в которых он принимает участие, имеют большое значение для строения клеток, из которых состоит любой живой организм. Благодаря образованию различных химических связей, калий помог человечеству добиться новых вершин в кораблестроении, садоводстве, фармацевтической промышленности.
Повсеместное нахождение в природе позволяет добывать металл и его соединения беспрерывно, а благодаря успехам учёных в области химии возможно регулировать плотность содержания калия в препаратах. При соблюдении правильных пропорций и составлении схем алгоритмов можно предугадать пользу или вред.
Калий может быть полезным, но также он способен обрекать людей на тяжкие мучения, такие как: ожоги рук, раздражение слизистых оболочек. Об этом следует помнить всем, кто стремится узнать больше о свойствах этого металла. Прежде чем начинать работу с опасными веществами, лучше узнать всё о многолетнем опыте предшественников, чтобы избежать печальных и необратимых последствий.