|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Copper | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | red-orange metallic luster | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Cu) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Copper in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
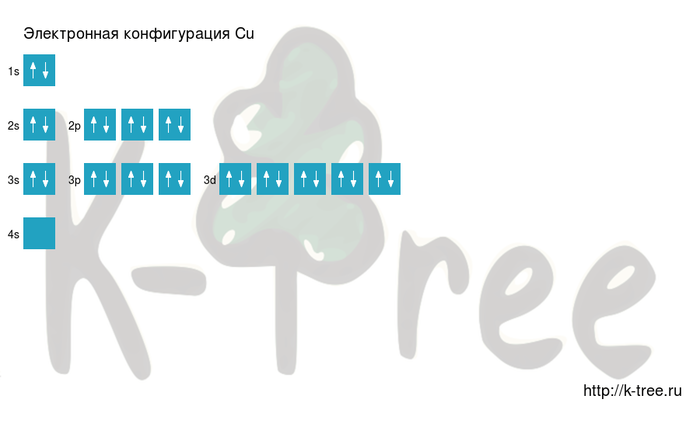
| Electron configuration | [Ar] 3d10 4s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1357.77 K (1084.62 °C, 1984.32 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2835 K (2562 °C, 4643 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.96 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 8.02 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.26 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 300.4 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.440 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −2, 0,[2] +1, +2, +3, +4 (a mildly basic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 128 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 132±4 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 140 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of copper |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | (annealed) 3810 m/s (at r.t.) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 16.5 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 401 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 16.78 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −5.46×10−6 cm3/mol[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 110–128 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 48 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 140 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 3.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 343–369 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 235–878 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-50-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Cyprus, principal mining place in Roman era (Cyprium) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Middle East (9000 BC) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | «Cu»: from Latin cuprum | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of copper
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Copper is a chemical element with the symbol Cu (from Latin: cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement.
Copper is one of the few metals that can occur in nature in a directly usable metallic form (native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create bronze, c. 3500 BC.[5]
In the Roman era, copper was mined principally on Cyprus, the origin of the name of the metal, from aes cyprium (metal of Cyprus), later corrupted to cuprum (Latin). Coper (Old English) and copper were derived from this, the later spelling first used around 1530.[6]
Commonly encountered compounds are copper(II) salts, which often impart blue or green colors to such minerals as azurite, malachite, and turquoise, and have been used widely and historically as pigments.
Copper used in buildings, usually for roofing, oxidizes to form a green verdigris (or patina). Copper is sometimes used in decorative art, both in its elemental metal form and in compounds as pigments. Copper compounds are used as bacteriostatic agents, fungicides, and wood preservatives.
Copper is essential to all living organisms as a trace dietary mineral because it is a key constituent of the respiratory enzyme complex cytochrome c oxidase. In molluscs and crustaceans, copper is a constituent of the blood pigment hemocyanin, replaced by the iron-complexed hemoglobin in fish and other vertebrates. In humans, copper is found mainly in the liver, muscle, and bone.[7] The adult body contains between 1.4 and 2.1 mg of copper per kilogram of body weight.[8]
Characteristics
Physical
Copper just above its melting point keeps its pink luster color when enough light outshines the orange incandescence color
Copper, silver, and gold are in group 11 of the periodic table; these three metals have one s-orbital electron on top of a filled d-electron shell and are characterized by high ductility, and electrical and thermal conductivity. The filled d-shells in these elements contribute little to interatomic interactions, which are dominated by the s-electrons through metallic bonds. Unlike metals with incomplete d-shells, metallic bonds in copper are lacking a covalent character and are relatively weak. This observation explains the low hardness and high ductility of single crystals of copper.[9] At the macroscopic scale, introduction of extended defects to the crystal lattice, such as grain boundaries, hinders flow of the material under applied stress, thereby increasing its hardness. For this reason, copper is usually supplied in a fine-grained polycrystalline form, which has greater strength than monocrystalline forms.[10]
The softness of copper partly explains its high electrical conductivity (59.6×106 S/m) and high thermal conductivity, second highest (second only to silver) among pure metals at room temperature.[11] This is because the resistivity to electron transport in metals at room temperature originates primarily from scattering of electrons on thermal vibrations of the lattice, which are relatively weak in a soft metal.[9] The maximum permissible current density of copper in open air is approximately 3.1×106 A/m2 of cross-sectional area, above which it begins to heat excessively.[12]
Copper is one of a few metallic elements with a natural color other than gray or silver.[13] Pure copper is orange-red and acquires a reddish tarnish when exposed to air. The is due to the low plasma frequency of the metal, which lies in the red part of the visible spectrum, causing it to absorb the higher-frequency green and blue colors.[14]
As with other metals, if copper is put in contact with another metal, galvanic corrosion will occur.[15]
Chemical
Unoxidized copper wire (left) and oxidized copper wire (right)
The East Tower of the Royal Observatory, Edinburgh, showing the contrast between the refurbished copper installed in 2010 and the green color of the original 1894 copper.
Copper does not react with water, but it does slowly react with atmospheric oxygen to form a layer of brown-black copper oxide which, unlike the rust that forms on iron in moist air, protects the underlying metal from further corrosion (passivation). A green layer of verdigris (copper carbonate) can often be seen on old copper structures, such as the roofing of many older buildings[16] and the Statue of Liberty.[17] Copper tarnishes when exposed to some sulfur compounds, with which it reacts to form various copper sulfides.[18]
Isotopes
There are 29 isotopes of copper. 63
Cu
and 65
Cu
are stable, with 63
Cu
comprising approximately 69% of naturally occurring copper; both have a spin of 3⁄2.[19] The other isotopes are radioactive, with the most stable being 67
Cu
with a half-life of 61.83 hours.[19] Seven metastable isotopes have been characterized; 68m
Cu
is the longest-lived with a half-life of 3.8 minutes. Isotopes with a mass number above 64 decay by β−, whereas those with a mass number below 64 decay by β+. 64
Cu
, which has a half-life of 12.7 hours, decays both ways.[20]
62
Cu
and 64
Cu
have significant applications. 62
Cu
is used in 62
Cu
Cu-PTSM as a radioactive tracer for positron emission tomography.[21]
Occurrence
Native copper from the Keweenaw Peninsula, Michigan, about 2.5 inches (6.4 cm) long
Copper is produced in massive stars[22] and is present in the Earth’s crust in a proportion of about 50 parts per million (ppm).[23] In nature, copper occurs in a variety of minerals, including native copper, copper sulfides such as chalcopyrite, bornite, digenite, covellite, and chalcocite, copper sulfosalts such as tetrahedite-tennantite, and enargite, copper carbonates such as azurite and malachite, and as copper(I) or copper(II) oxides such as cuprite and tenorite, respectively.[11] The largest mass of elemental copper discovered weighed 420 tonnes and was found in 1857 on the Keweenaw Peninsula in Michigan, US.[23] Native copper is a polycrystal, with the largest single crystal ever described measuring 4.4 × 3.2 × 3.2 cm.[24] Copper is the 25th most abundant element in Earth’s crust, representing 50 ppm compared with 75 ppm for zinc, and 14 ppm for lead.[25]
Typical background concentrations of copper do not exceed 1 ng/m3 in the atmosphere; 150 mg/kg in soil; 30 mg/kg in vegetation; 2 μg/L in freshwater and 0.5 μg/L in seawater.[26]
Production
Most copper is mined or extracted as copper sulfides from large open pit mines in porphyry copper deposits that contain 0.4 to 1.0% copper. Sites include Chuquicamata, in Chile, Bingham Canyon Mine, in Utah, United States, and El Chino Mine, in New Mexico, United States. According to the British Geological Survey, in 2005, Chile was the top producer of copper with at least one-third of the world share followed by the United States, Indonesia and Peru.[11] Copper can also be recovered through the in-situ leach process. Several sites in the state of Arizona are considered prime candidates for this method.[27] The amount of copper in use is increasing and the quantity available is barely sufficient to allow all countries to reach developed world levels of usage.[28] An alternative source of copper for collection currently being researched are polymetallic nodules, which are located at the depths of the Pacific Ocean approximately 3000–6500 meters below sea level. These nodules contain other valuable metals such as cobalt and nickel.[29]
Reserves and prices
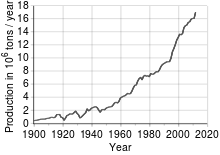
Price of Copper 1959-2022
Copper has been in use at least 10,000 years, but more than 95% of all copper ever mined and smelted has been extracted since 1900.[30] As with many natural resources, the total amount of copper on Earth is vast, with around 1014 tons in the top kilometer of Earth’s crust, which is about 5 million years’ worth at the current rate of extraction. However, only a tiny fraction of these reserves is economically viable with present-day prices and technologies. Estimates of copper reserves available for mining vary from 25 to 60 years, depending on core assumptions such as the growth rate.[31] Recycling is a major source of copper in the modern world.[30] Because of these and other factors, the future of copper production and supply is the subject of much debate, including the concept of peak copper, analogous to peak oil.[citation needed]
The price of copper has historically been unstable,[32] and its price increased from the 60-year low of US$0.60/lb (US$1.32/kg) in June 1999 to $3.75 per pound ($8.27/kg) in May 2006. It dropped to $2.40/lb ($5.29/kg) in February 2007, then rebounded to $3.50/lb ($7.71/kg) in April 2007.[33][better source needed] In February 2009, weakening global demand and a steep fall in commodity prices since the previous year’s highs left copper prices at $1.51/lb ($3.32/kg).[34] Between September 2010 and February 2011, the price of copper rose from £5,000 a metric ton to £6,250 a metric ton.[35]
Methods
Scheme of flash smelting process
The concentration of copper in ores averages only 0.6%, and most commercial ores are sulfides, especially chalcopyrite (CuFeS2), bornite (Cu5FeS4) and, to a lesser extent, covellite (CuS) and chalcocite (Cu2S).[36] Conversely, the average concentration of copper in polymetallic nodules is estimated at 1.3%. The methods of extracting copper as well as other metals found in these nodules include sulphuric leaching, smelting and an application of the Cuprion process.[37][38] For minerals found in land ores, they are concentrated from crushed ores to the level of 10–15% copper by froth flotation or bioleaching.[39] Heating this material with silica in flash smelting removes much of the iron as slag. The process exploits the greater ease of converting iron sulfides into oxides, which in turn react with the silica to form the silicate slag that floats on top of the heated mass. The resulting copper matte, consisting of Cu2S, is roasted to convert the sulfides into oxides:[36]
- 2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
The cuprous oxide reacts with cuprous sulfide to converted to blister copper upon heating:
- 2 Cu2O + Cu2S → 6 Cu + 2 SO2
The Sudbury matte process converted only half the sulfide to oxide and then used this oxide to remove the rest of the sulfur as oxide. It was then electrolytically refined and the anode mud exploited for the platinum and gold it contained. This step exploits the relatively easy reduction of copper oxides to copper metal. Natural gas is blown across the blister to remove most of the remaining oxygen and electrorefining is performed on the resulting material to produce pure copper:[40]
- Cu2+ + 2 e− → Cu

Recycling
Like aluminium,[41] copper is recyclable without any loss of quality, both from raw state and from manufactured products.[42] In volume, copper is the third most recycled metal after iron and aluminium.[43] An estimated 80% of all copper ever mined is still in use today.[44] According to the International Resource Panel’s Metal Stocks in Society report, the global per capita stock of copper in use in society is 35–55 kg. Much of this is in more-developed countries (140–300 kg per capita) rather than less-developed countries (30–40 kg per capita).
The process of recycling copper is roughly the same as is used to extract copper but requires fewer steps. High-purity scrap copper is melted in a furnace and then reduced and cast into billets and ingots; lower-purity scrap is refined by electroplating in a bath of sulfuric acid.[45]
Alloys
Copper alloys are widely used in the production of coinage; seen here are two examples — post-1964 American dimes, which are composed of the alloy cupronickel[46] and a pre-1968 Canadian dime, which is composed of an alloy of 80 percent silver and 20 percent copper.[47]
Numerous copper alloys have been formulated, many with important uses. Brass is an alloy of copper and zinc. Bronze usually refers to copper-tin alloys, but can refer to any alloy of copper such as aluminium bronze. Copper is one of the most important constituents of silver and karat gold solders used in the jewelry industry, modifying the color, hardness and melting point of the resulting alloys.[48] Some lead-free solders consist of tin alloyed with a small proportion of copper and other metals.[49]
The alloy of copper and nickel, called cupronickel, is used in low-denomination coins, often for the outer cladding. The US five-cent coin (currently called a nickel) consists of 75% copper and 25% nickel in homogeneous composition. Prior to the introduction of cupronickel, which was widely adopted by countries in the latter half of the 20th century,[50] alloys of copper and silver were also used, with the United States using an alloy of 90% silver and 10% copper until 1965, when circulating silver was removed from all coins with the exception of the Half dollar — these were debased to an alloy of 40% silver and 60% copper between 1965 and 1970.[51] The alloy of 90% copper and 10% nickel, remarkable for its resistance to corrosion, is used for various objects exposed to seawater, though it is vulnerable to the sulfides sometimes found in polluted harbors and estuaries.[52] Alloys of copper with aluminium (about 7%) have a golden color and are used in decorations.[23] Shakudō is a Japanese decorative alloy of copper containing a low percentage of gold, typically 4–10%, that can be patinated to a dark blue or black color.[53]
Compounds
Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively.[54] Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes.[55]
Binary compounds
As with other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements, the principal examples being oxides, sulfides, and halides. Both cuprous and cupric oxides are known. Among the numerous copper sulfides, important examples include copper(I) sulfide and copper(II) sulfide.[citation needed]
Cuprous halides with fluorine, chlorine, bromine, and iodine are known, as are cupric halides with fluorine, chlorine, and bromine. Attempts to prepare copper(II) iodide yield only copper(I) iodide and iodine.[54]
- 2 Cu2+ + 4 I− → 2 CuI + I2
Coordination chemistry
Copper forms coordination complexes with ligands. In aqueous solution, copper(II) exists as [Cu(H
2O)
6]2+
. This complex exhibits the fastest water exchange rate (speed of water ligands attaching and detaching) for any transition metal aquo complex. Adding aqueous sodium hydroxide causes the precipitation of light blue solid copper(II) hydroxide. A simplified equation is:
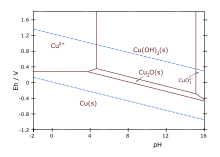
Pourbaix diagram for copper in uncomplexed media (anions other than OH- not considered). Ion concentration 0.001 m (mol/kg water). Temperature 25 °C.
- Cu2+ + 2 OH− → Cu(OH)2
Aqueous ammonia results in the same precipitate. Upon adding excess ammonia, the precipitate dissolves, forming tetraamminecopper(II):
- Cu(H
2O)
4(OH)
2 + 4 NH3 → [Cu(H
2O)
2(NH
3)
4]2+
+ 2 H2O + 2 OH−
Many other oxyanions form complexes; these include copper(II) acetate, copper(II) nitrate, and copper(II) carbonate. Copper(II) sulfate forms a blue crystalline pentahydrate, the most familiar copper compound in the laboratory. It is used in a fungicide called the Bordeaux mixture.[56]
Polyols, compounds containing more than one alcohol functional group, generally interact with cupric salts. For example, copper salts are used to test for reducing sugars. Specifically, using Benedict’s reagent and Fehling’s solution the presence of the sugar is signaled by a color change from blue Cu(II) to reddish copper(I) oxide.[57] Schweizer’s reagent and related complexes with ethylenediamine and other amines dissolve cellulose.[58] Amino acids such as cystine form very stable chelate complexes with copper(II)[59][60][61] including in the form of metal-organic biohybrids (MOBs). Many wet-chemical tests for copper ions exist, one involving potassium ferrocyanide, which gives a brown precipitate with copper(II) salts.[citation needed]
Organocopper chemistry
Compounds that contain a carbon-copper bond are known as organocopper compounds. They are very reactive towards oxygen to form copper(I) oxide and have many uses in chemistry. They are synthesized by treating copper(I) compounds with Grignard reagents, terminal alkynes or organolithium reagents;[62] in particular, the last reaction described produces a Gilman reagent. These can undergo substitution with alkyl halides to form coupling products; as such, they are important in the field of organic synthesis. Copper(I) acetylide is highly shock-sensitive but is an intermediate in reactions such as the Cadiot-Chodkiewicz coupling[63] and the Sonogashira coupling.[64] Conjugate addition to enones[65] and carbocupration of alkynes[66] can also be achieved with organocopper compounds. Copper(I) forms a variety of weak complexes with alkenes and carbon monoxide, especially in the presence of amine ligands.[67]
Copper(III) and copper(IV)
Copper(III) is most often found in oxides. A simple example is potassium cuprate, KCuO2, a blue-black solid.[68] The most extensively studied copper(III) compounds are the cuprate superconductors. Yttrium barium copper oxide (YBa2Cu3O7) consists of both Cu(II) and Cu(III) centres. Like oxide, fluoride is a highly basic anion[69] and is known to stabilize metal ions in high oxidation states. Both copper(III) and even copper(IV) fluorides are known, K3CuF6 and Cs2CuF6, respectively.[54]
Some copper proteins form oxo complexes, which also feature copper(III).[70] With tetrapeptides, purple-colored copper(III) complexes are stabilized by the deprotonated amide ligands.[71]
Complexes of copper(III) are also found as intermediates in reactions of organocopper compounds.[72] For example, in the Kharasch–Sosnovsky reaction.[citation needed]
History
A timeline of copper illustrates how this metal has advanced human civilization for the past 11,000 years.[73]
Prehistoric
Copper Age
A corroded copper ingot from Zakros, Crete, shaped in the form of an animal skin (oxhide) typical in that era.
Many tools during the Chalcolithic Era included copper, such as the blade of this replica of Ötzi’s axe
Copper occurs naturally as native metallic copper and was known to some of the oldest civilizations on record. The history of copper use dates to 9000 BC in the Middle East;[74] a copper pendant was found in northern Iraq that dates to 8700 BC.[75] Evidence suggests that gold and meteoric iron (but not smelted iron) were the only metals used by humans before copper.[76] The history of copper metallurgy is thought to follow this sequence: First, cold working of native copper, then annealing, smelting, and, finally, lost-wax casting. In southeastern Anatolia, all four of these techniques appear more or less simultaneously at the beginning of the Neolithic c. 7500 BC.[77]
Copper smelting was independently invented in different places. It was probably discovered in China before 2800 BC, in Central America around 600 AD, and in West Africa about the 9th or 10th century AD.[78] Investment casting was invented in 4500–4000 BC in Southeast Asia[74] and carbon dating has established mining at Alderley Edge in Cheshire, UK, at 2280 to 1890 BC.[79] Ötzi the Iceman, a male dated from 3300 to 3200 BC, was found with an axe with a copper head 99.7% pure; high levels of arsenic in his hair suggest an involvement in copper smelting.[80] Experience with copper has assisted the development of other metals; in particular, copper smelting led to the discovery of iron smelting.[80] Production in the Old Copper Complex in Michigan and Wisconsin is dated between 6000 and 3000 BC.[81][82] Natural bronze, a type of copper made from ores rich in silicon, arsenic, and (rarely) tin, came into general use in the Balkans around 5500 BC.[83]
Bronze Age
Alloying copper with tin to make bronze was first practiced about 4000 years after the discovery of copper smelting, and about 2000 years after «natural bronze» had come into general use.[84] Bronze artifacts from the Vinča culture date to 4500 BC.[85] Sumerian and Egyptian artifacts of copper and bronze alloys date to 3000 BC.[86] The Bronze Age began in Southeastern Europe around 3700–3300 BC, in Northwestern Europe about 2500 BC. It ended with the beginning of the Iron Age, 2000–1000 BC in the Near East, and 600 BC in Northern Europe. The transition between the Neolithic period and the Bronze Age was formerly termed the Chalcolithic period (copper-stone), when copper tools were used with stone tools. The term has gradually fallen out of favor because in some parts of the world, the Chalcolithic and Neolithic are coterminous at both ends. Brass, an alloy of copper and zinc, is of much more recent origin. It was known to the Greeks, but became a significant supplement to bronze during the Roman Empire.[86]
Ancient and post-classical
In alchemy the symbol for copper was also the symbol for the goddess and planet Venus.
In Greece, copper was known by the name chalkos (χαλκός). It was an important resource for the Romans, Greeks and other ancient peoples. In Roman times, it was known as aes Cyprium, aes being the generic Latin term for copper alloys and Cyprium from Cyprus, where much copper was mined. The phrase was simplified to cuprum, hence the English copper. Aphrodite (Venus in Rome) represented copper in mythology and alchemy because of its lustrous beauty and its ancient use in producing mirrors; Cyprus, the source of copper, was sacred to the goddess. The seven heavenly bodies known to the ancients were associated with the seven metals known in antiquity, and Venus was assigned to copper, both because of the connection to the goddess and because Venus was the brightest heavenly body after the Sun and Moon and so corresponded to the most lustrous and desirable metal after gold and silver.[87]
Copper was first mined in ancient Britain as early as 2100 BC. Mining at the largest of these mines, the Great Orme, continued into the late Bronze Age. Mining seems to have been largely restricted to supergene ores, which were easier to smelt. The rich copper deposits of Cornwall seem to have been largely untouched, in spite of extensive tin mining in the region, for reasons likely social and political rather than technological.[88]
In North America, copper mining began with marginal workings by Native Americans. Native copper is known to have been extracted from sites on Isle Royale with primitive stone tools between 800 and 1600.[89] Copper metallurgy was flourishing in South America, particularly in Peru around 1000 AD. Copper burial ornamentals from the 15th century have been uncovered, but the metal’s commercial production did not start until the early 20th century.[citation needed]
The cultural role of copper has been important, particularly in currency. Romans in the 6th through 3rd centuries BC used copper lumps as money. At first, the copper itself was valued, but gradually the shape and look of the copper became more important. Julius Caesar had his own coins made from brass, while Octavianus Augustus Caesar’s coins were made from Cu-Pb-Sn alloys. With an estimated annual output of around 15,000 t, Roman copper mining and smelting activities reached a scale unsurpassed until the time of the Industrial Revolution; the provinces most intensely mined were those of Hispania, Cyprus and in Central Europe.[90][91]
The gates of the Temple of Jerusalem used Corinthian bronze treated with depletion gilding.[clarification needed][citation needed] The process was most prevalent in Alexandria, where alchemy is thought to have begun.[92] In ancient India, copper was used in the holistic medical science Ayurveda for surgical instruments and other medical equipment. Ancient Egyptians (~2400 BC) used copper for sterilizing wounds and drinking water, and later to treat headaches, burns, and itching.[citation needed]
Modern
18th-century copper kettle from Norway made from Swedish copper
The Great Copper Mountain was a mine in Falun, Sweden, that operated from the 10th century to 1992. It satisfied two-thirds of Europe’s copper consumption in the 17th century and helped fund many of Sweden’s wars during that time.[93] It was referred to as the nation’s treasury; Sweden had a copper backed currency.[94]
Chalcography of the city of Vyborg at the turn of the 17th and 18th centuries. The year 1709 carved on the printing plate.
Copper is used in roofing,[16] currency, and for photographic technology known as the daguerreotype. Copper was used in Renaissance sculpture, and was used to construct the Statue of Liberty; copper continues to be used in construction of various types. Copper plating and copper sheathing were widely used to protect the under-water hulls of ships, a technique pioneered by the British Admiralty in the 18th century.[95] The Norddeutsche Affinerie in Hamburg was the first modern electroplating plant, starting its production in 1876.[96] The German scientist Gottfried Osann invented powder metallurgy in 1830 while determining the metal’s atomic mass; around then it was discovered that the amount and type of alloying element (e.g., tin) to copper would affect bell tones.[citation needed]
During the rise in demand for copper for the Age of Electricity, from the 1880s until the Great Depression of the 1930s, the United States produced one third to half the world’s newly mined copper.[97] Major districts included the Keweenaw district of northern Michigan, primarily native copper deposits, which was eclipsed by the vast sulphide deposits of Butte, Montana in the late 1880s, which itself was eclipsed by porphyry deposits of the Souhwest United States, especially at Bingham Canyon, Utah and Morenci, Arizona. Introduction of open pit steam shovel mining and innovations in smelting, refining, flotation concentration and other processing steps led to mass production. Early in the twentieth century, Arizona ranked first, followed by Montana, then Utah and Michigan.[98]
Flash smelting was developed by Outokumpu in Finland and first applied at Harjavalta in 1949; the energy-efficient process accounts for 50% of the world’s primary copper production.[99]
The Intergovernmental Council of Copper Exporting Countries, formed in 1967 by Chile, Peru, Zaire and Zambia, operated in the copper market as OPEC does in oil, though it never achieved the same influence, particularly because the second-largest producer, the United States, was never a member; it was dissolved in 1988.[100]
Applications
Copper fittings for soldered plumbing joints
The major applications of copper are electrical wire (60%), roofing and plumbing (20%), and industrial machinery (15%). Copper is used mostly as a pure metal, but when greater hardness is required, it is put into such alloys as brass and bronze (5% of total use).[23] For more than two centuries, copper paint has been used on boat hulls to control the growth of plants and shellfish.[101] A small part of the copper supply is used for nutritional supplements and fungicides in agriculture.[56][102] Machining of copper is possible, although alloys are preferred for good machinability in creating intricate parts.
Wire and cable
Despite competition from other materials, copper remains the preferred electrical conductor in nearly all categories of electrical wiring except overhead electric power transmission where aluminium is often preferred.[103][104] Copper wire is used in power generation, power transmission, power distribution, telecommunications, electronics circuitry, and countless types of electrical equipment.[105] Electrical wiring is the most important market for the copper industry.[106] This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used for electrical wire and cable conductors.[107] Many electrical devices rely on copper wiring because of its multitude of inherent beneficial properties, such as its high electrical conductivity, tensile strength, ductility, creep (deformation) resistance, corrosion resistance, low thermal expansion, high thermal conductivity, ease of soldering, malleability, and ease of installation.
For a short period from the late 1960s to the late 1970s, copper wiring was replaced by aluminium wiring in many housing construction projects in America. The new wiring was implicated in a number of house fires and the industry returned to copper.[108]
Electronics and related devices
Copper electrical busbars distributing power to a large building
Integrated circuits and printed circuit boards increasingly feature copper in place of aluminium because of its superior electrical conductivity; heat sinks and heat exchangers use copper because of its superior heat dissipation properties. Electromagnets, vacuum tubes, cathode ray tubes, and magnetrons in microwave ovens use copper, as do waveguides for microwave radiation.[109]
Electric motors
Copper’s superior conductivity enhances the efficiency of electrical motors.[110] This is important because motors and motor-driven systems account for 43%–46% of all global electricity consumption and 69% of all electricity used by industry.[111] Increasing the mass and cross section of copper in a coil increases the efficiency of the motor. Copper motor rotors, a new technology designed for motor applications where energy savings are prime design objectives,[112][113] are enabling general-purpose induction motors to meet and exceed National Electrical Manufacturers Association (NEMA) premium efficiency standards.[114]
Renewable energy production
Renewable energy sources such as solar, wind, tidal, hydro, biomass, and geothermal have become significant sectors of the energy market.[115][116] The rapid growth of these sources in the 21st century has been prompted by increasing costs of fossil fuels as well as their environmental impact issues that significantly lowered their use.
Copper plays an important role in these renewable energy systems.[117][118][119][120][121] Copper usage averages up to five times more in renewable energy systems than in traditional power generation, such as fossil fuel and nuclear power plants.[122] Since copper is an excellent thermal and electrical conductor among engineering metals (second only to silver),[123] electrical systems that utilize copper generate and transmit energy with high efficiency and with minimum environmental impacts.
When choosing electrical conductors, facility planners and engineers factor capital investment costs of materials against operational savings due to their electrical energy efficiencies over their useful lives, plus maintenance costs. Copper often fares well in these calculations. A factor called «copper usage intensity,” is a measure of the quantity of copper necessary to install one megawatt of new power-generating capacity.
Copper wires for recycling
When planning for a new renewable power facility, engineers and product specifiers seek to avoid supply shortages of selected materials. According to the United States Geological Survey, in-ground copper reserves have increased more than 700% since 1950, from almost 100 million tonnes to 720 million tonnes in 2017, despite the fact that world refined usage has more than tripled in the last 50 years.[124] Copper resources are estimated to exceed 5,000 million tonnes.[125][126]
Bolstering the supply from copper extraction is the fact that more than 30 percent of copper installed during the last decade came from recycled sources.[127] Its recycling rate is higher than any other metal.[128]
This article discusses the role of copper in various renewable energy generation systems.
Architecture
Old copper utensils in a Jerusalem restaurant
Copper has been used since ancient times as a durable, corrosion resistant, and weatherproof architectural material.[129][130][131][132] Roofs, flashings, rain gutters, downspouts, domes, spires, vaults, and doors have been made from copper for hundreds or thousands of years. Copper’s architectural use has been expanded in modern times to include interior and exterior wall cladding, building expansion joints, radio frequency shielding, and antimicrobial and decorative indoor products such as attractive handrails, bathroom fixtures, and counter tops. Some of copper’s other important benefits as an architectural material include low thermal movement, light weight, lightning protection, and recyclability
The metal’s distinctive natural green patina has long been coveted by architects and designers. The final patina is a particularly durable layer that is highly resistant to atmospheric corrosion, thereby protecting the underlying metal against further weathering.[133][134][135] It can be a mixture of carbonate and sulfate compounds in various amounts, depending upon environmental conditions such as sulfur-containing acid rain.[136][137][138][139] Architectural copper and its alloys can also be ‘finished’ to take on a particular look, feel, or color. Finishes include mechanical surface treatments, chemical coloring, and coatings.[140]
Copper has excellent brazing and soldering properties and can be welded; the best results are obtained with gas metal arc welding.[141]
Antibiofouling
Copper is biostatic, meaning bacteria and many other forms of life will not grow on it. For this reason it has long been used to line parts of ships to protect against barnacles and mussels. It was originally used pure, but has since been superseded by Muntz metal and copper-based paint. Similarly, as discussed in copper alloys in aquaculture, copper alloys have become important netting materials in the aquaculture industry because they are antimicrobial and prevent biofouling, even in extreme conditions[142] and have strong structural and corrosion-resistant[143] properties in marine environments.
Antimicrobial
Copper-alloy touch surfaces have natural properties that destroy a wide range of microorganisms (e.g., E. coli O157:H7, methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus, Clostridium difficile, influenza A virus, adenovirus, SARS-Cov-2, and fungi).[144][145] Indians have been using copper vessels since ancient times for storing water, even before modern science realized its antimicrobial properties.[146] Some copper alloys were proven to kill more than 99.9% of disease-causing bacteria within just two hours when cleaned regularly.[147] The United States Environmental Protection Agency (EPA) has approved the registrations of these copper alloys as «antimicrobial materials with public health benefits»;[147] that approval allows manufacturers to make legal claims to the public health benefits of products made of registered alloys. In addition, the EPA has approved a long list of antimicrobial copper products made from these alloys, such as bedrails, handrails, over-bed tables, sinks, faucets, door knobs, toilet hardware, computer keyboards, health club equipment, and shopping cart handles (for a comprehensive list, see: Antimicrobial copper-alloy touch surfaces#Approved products). Copper doorknobs are used by hospitals to reduce the transfer of disease, and Legionnaires’ disease is suppressed by copper tubing in plumbing systems.[148] Antimicrobial copper alloy products are now being installed in healthcare facilities in the U.K., Ireland, Japan, Korea, France, Denmark, and Brazil, as well as being called for in the US,[149] and in the subway transit system in Santiago, Chile, where copper-zinc alloy handrails were installed in some 30 stations between 2011 and 2014.[150][151][152]
Textile fibers can be blended with copper to create antimicrobial protective fabrics.[153][unreliable source?]
Speculative investing
Copper may be used as a speculative investment due to the predicted increase in use from worldwide infrastructure growth, and the important role it has in producing wind turbines, solar panels, and other renewable energy sources.[154][155] Another reason predicted demand increases is the fact that electric cars contain an average of 3.6 times as much copper as conventional cars, although the effect of electric cars on copper demand is debated.[156][157] Some people invest in copper through copper mining stocks, ETFs, and futures. Others store physical copper in the form of copper bars or rounds although these tend to carry a higher premium in comparison to precious metals.[158] Those who want to avoid the premiums of copper bullion alternatively store old copper wire, copper tubing or American pennies made before 1982.[159]
Folk medicine
Copper is commonly used in jewelry, and according to some folklore, copper bracelets relieve arthritis symptoms.[160] In one trial for osteoarthritis and one trial for rheumatoid arthritis, no differences is found between copper bracelet and control (non-copper) bracelet.[161][162] No evidence shows that copper can be absorbed through the skin. If it were, it might lead to copper poisoning.[163]
Compression clothing
Recently, some compression clothing with inter-woven copper has been marketed with health claims similar to the folk medicine claims. Because compression clothing is a valid treatment for some ailments, the clothing may have that benefit, but the added copper may have no benefit beyond a placebo effect.[164]
Degradation
Chromobacterium violaceum and Pseudomonas fluorescens can both mobilize solid copper as a cyanide compound.[165] The ericoid mycorrhizal fungi associated with Calluna, Erica and Vaccinium can grow in metalliferous soils containing copper.[165] The ectomycorrhizal fungus Suillus luteus protects young pine trees from copper toxicity. A sample of the fungus Aspergillus niger was found growing from gold mining solution and was found to contain cyano complexes of such metals as gold, silver, copper, iron, and zinc. The fungus also plays a role in the solubilization of heavy metal sulfides.[166]
Biological role
Rich sources of copper include oysters, beef and lamb liver, Brazil nuts, blackstrap molasses, cocoa, and black pepper. Good sources include lobster, nuts and sunflower seeds, green olives, avocados, and wheat bran.
Biochemistry
Copper proteins have diverse roles in biological electron transport and oxygen transportation, processes that exploit the easy interconversion of Cu(I) and Cu(II).[167] Copper is essential in the aerobic respiration of all eukaryotes. In mitochondria, it is found in cytochrome c oxidase, which is the last protein in oxidative phosphorylation. Cytochrome c oxidase is the protein that binds the O2 between a copper and an iron; the protein transfers 8 electrons to the O2 molecule to reduce it to two molecules of water. Copper is also found in many superoxide dismutases, proteins that catalyze the decomposition of superoxides by converting it (by disproportionation) to oxygen and hydrogen peroxide:
- Cu2+-SOD + O2− → Cu+-SOD + O2 (reduction of copper; oxidation of superoxide)
- Cu+-SOD + O2− + 2H+ → Cu2+-SOD + H2O2 (oxidation of copper; reduction of superoxide)
The protein hemocyanin is the oxygen carrier in most mollusks and some arthropods such as the horseshoe crab (Limulus polyphemus).[168] Because hemocyanin is blue, these organisms have blue blood rather than the red blood of iron-based hemoglobin. Structurally related to hemocyanin are the laccases and tyrosinases. Instead of reversibly binding oxygen, these proteins hydroxylate substrates, illustrated by their role in the formation of lacquers.[169] The biological role for copper commenced with the appearance of oxygen in earth’s atmosphere.[170] Several copper proteins, such as the «blue copper proteins», do not interact directly with substrates; hence they are not enzymes. These proteins relay electrons by the process called electron transfer.[169]
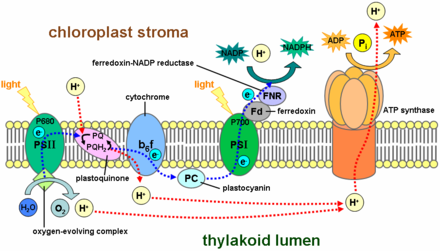
Photosynthesis functions by an elaborate electron transport chain within the thylakoid membrane. A central link in this chain is plastocyanin, a blue copper protein.
A unique tetranuclear copper center has been found in nitrous-oxide reductase.[171]
Chemical compounds which were developed for treatment of Wilson’s disease have been investigated for use in cancer therapy.[172]
Nutrition
Copper is an essential trace element in plants and animals, but not all microorganisms. The human body contains copper at a level of about 1.4 to 2.1 mg per kg of body mass.[173]
Absorption
Copper is absorbed in the gut, then transported to the liver bound to albumin.[174] After processing in the liver, copper is distributed to other tissues in a second phase, which involves the protein ceruloplasmin, carrying the majority of copper in blood. Ceruloplasmin also carries the copper that is excreted in milk, and is particularly well-absorbed as a copper source.[175] Copper in the body normally undergoes enterohepatic circulation (about 5 mg a day, vs. about 1 mg per day absorbed in the diet and excreted from the body), and the body is able to excrete some excess copper, if needed, via bile, which carries some copper out of the liver that is not then reabsorbed by the intestine.[176][177]
Dietary recommendations
The U.S. Institute of Medicine (IOM) updated the estimated average requirements (EARs) and recommended dietary allowances (RDAs) for copper in 2001. If there is not sufficient information to establish EARs and RDAs, an estimate designated Adequate Intake (AI) is used instead. The AIs for copper are: 200 μg of copper for 0–6-month-old males and females, and 220 μg of copper for 7–12-month-old males and females. For both sexes, the RDAs for copper are: 340 μg of copper for 1–3 years old, 440 μg of copper for 4–8 years old, 700 μg of copper for 9–13 years old, 890 μg of copper for 14–18 years old and 900 μg of copper for ages 19 years and older. For pregnancy, 1,000 μg. For lactation, 1,300 μg.[178] As for safety, the IOM also sets tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. In the case of copper the UL is set at 10 mg/day. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes.[179]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women and men ages 18 and older the AIs are set at 1.3 and 1.6 mg/day, respectively. AIs for pregnancy and lactation is 1.5 mg/day. For children ages 1–17 years the AIs increase with age from 0.7 to 1.3 mg/day. These AIs are higher than the U.S. RDAs.[180] The European Food Safety Authority reviewed the same safety question and set its UL at 5 mg/day, which is half the U.S. value.[181]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For copper labeling purposes 100% of the Daily Value was 2.0 mg, but as of May 27, 2016 it was revised to 0.9 mg to bring it into agreement with the RDA.[182][183] A table of the old and new adult daily values is provided at Reference Daily Intake.
Deficiency
Because of its role in facilitating iron uptake, copper deficiency can produce anemia-like symptoms, neutropenia, bone abnormalities, hypopigmentation, impaired growth, increased incidence of infections, osteoporosis, hyperthyroidism, and abnormalities in glucose and cholesterol metabolism. Conversely, Wilson’s disease causes an accumulation of copper in body tissues.
Severe deficiency can be found by testing for low plasma or serum copper levels, low ceruloplasmin, and low red blood cell superoxide dismutase levels; these are not sensitive to marginal copper status. The «cytochrome c oxidase activity of leucocytes and platelets» has been stated as another factor in deficiency, but the results have not been confirmed by replication.[184]
Toxicity
Gram quantities of various copper salts have been taken in suicide attempts and produced acute copper toxicity in humans, possibly due to redox cycling and the generation of reactive oxygen species that damage DNA.[185][186] Corresponding amounts of copper salts (30 mg/kg) are toxic in animals.[187] A minimum dietary value for healthy growth in rabbits has been reported to be at least 3 ppm in the diet.[188] However, higher concentrations of copper (100 ppm, 200 ppm, or 500 ppm) in the diet of rabbits may favorably influence feed conversion efficiency, growth rates, and carcass dressing percentages.[189]
Chronic copper toxicity does not normally occur in humans because of transport systems that regulate absorption and excretion. Autosomal recessive mutations in copper transport proteins can disable these systems, leading to Wilson’s disease with copper accumulation and cirrhosis of the liver in persons who have inherited two defective genes.[173]
Elevated copper levels have also been linked to worsening symptoms of Alzheimer’s disease.[190][191]
Human exposure
In the US, the Occupational Safety and Health Administration (OSHA) has designated a permissible exposure limit (PEL) for copper dust and fumes in the workplace as a time-weighted average (TWA) of 1 mg/m3.[192] The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 1 mg/m3, time-weighted average. The IDLH (immediately dangerous to life and health) value is 100 mg/m3.[193]
Copper is a constituent of tobacco smoke.[194][195] The tobacco plant readily absorbs and accumulates heavy metals, such as copper from the surrounding soil into its leaves. These are readily absorbed into the user’s body following smoke inhalation.[196] The health implications are not clear.[197]
See also
- Copper in renewable energy
- Copper nanoparticle
- Erosion corrosion of copper water tubes
- Cold water pitting of copper tube
- List of countries by copper production
- Metal theft
- Operation Tremor
- Anaconda Copper
- Antofagasta PLC
- Codelco
- El Boleo mine
- Grasberg mine
References
- ^ «Standard Atomic Weights: Copper». CIAAW. 1969.
- ^ Moret, Marc-Etienne; Zhang, Limei; Peters, Jonas C. (2013). «A Polar Copper–Boron One-Electron σ-Bond». J. Am. Chem. Soc. 135 (10): 3792–3795. doi:10.1021/ja4006578. PMID 23418750.
- ^ Lide, D. R., ed. (2005). «Magnetic susceptibility of the elements and inorganic compounds». CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5. Archived from the original (PDF) on 3 March 2011.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Robert McHenry, ed. (1992). «Bronze». The New Encyclopædia Britannica. Vol. 3 (15 ed.). Chicago: Encyclopædia Britannica, Incorporated. p. 612. ISBN 978-0-85229-553-3. OCLC 25228234.
- ^ «Copper». Merriam-Webster Dictionary. 2018. Retrieved 22 August 2018.
- ^ Johnson, MD PhD, Larry E., ed. (2008). «Copper». Merck Manual Home Health Handbook. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Archived from the original on 7 March 2016. Retrieved 7 April 2013.
- ^ «Copper in human health».
- ^ a b Trigg, George L.; Immergut, Edmund H. (1992). Encyclopedia of Applied Physics. Vol. 4: Combustion to Diamagnetism. VCH. pp. 267–272. ISBN 978-3-527-28126-8. Retrieved 2 May 2011.
- ^ Smith, William F. & Hashemi, Javad (2003). Foundations of Materials Science and Engineering. McGraw-Hill Professional. p. 223. ISBN 978-0-07-292194-6.
- ^ a b c Hammond, C. R. (2004). The Elements, in Handbook of Chemistry and Physics (81st ed.). CRC Press. ISBN 978-0-8493-0485-9.
- ^ Resistance Welding Manufacturing Alliance (2003). Resistance Welding Manual (4th ed.). Resistance Welding Manufacturing Alliance. pp. 18–12. ISBN 978-0-9624382-0-2.
- ^ Chambers, William; Chambers, Robert (1884). Chambers’s Information for the People. Vol. L (5th ed.). W. & R. Chambers. p. 312. ISBN 978-0-665-46912-1.
- ^ Ramachandran, Harishankar (14 March 2007). «Why is Copper Red?» (PDF). IIT Madras. Retrieved 27 December 2022.
- ^ «Galvanic Corrosion». Corrosion Doctors. Retrieved 29 April 2011.
- ^ a b Grieken, Rene van; Janssens, Koen (2005). Cultural Heritage Conservation and Environmental Impact Assessment by Non-Destructive Testing and Micro-Analysis. CRC Press. p. 197. ISBN 978-0-203-97078-2.
- ^ «Copper.org: Education: Statue of Liberty: Reclothing the First Lady of Metals – Repair Concerns». Copper.org. Retrieved 11 April 2011.
- ^ Rickett, B. I.; Payer, J. H. (1995). «Composition of Copper Tarnish Products Formed in Moist Air with Trace Levels of Pollutant Gas: Hydrogen Sulfide and Sulfur Dioxide/Hydrogen Sulfide». Journal of the Electrochemical Society. 142 (11): 3723–3728. Bibcode:1995JElS..142.3723R. doi:10.1149/1.2048404.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), «The NUBASE evaluation of nuclear and decay properties», Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729….3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ «Interactive Chart of Nuclides». National Nuclear Data Center. Archived from the original on 25 August 2013. Retrieved 8 April 2011.
- ^ Okazawad, Hidehiko; Yonekura, Yoshiharu; Fujibayashi, Yasuhisa; Nishizawa, Sadahiko; Magata, Yasuhiro; Ishizu, Koichi; Tanaka, Fumiko; Tsuchida, Tatsuro; Tamaki, Nagara; Konishi, Junji (1994). «Clinical Application and Quantitative Evaluation of Generator-Produced Copper-62-PTSM as a Brain Perfusion Tracer for PET» (PDF). Journal of Nuclear Medicine. 35 (12): 1910–1915. PMID 7989968.
- ^ Romano, Donatella; Matteucci, Fransesca (2007). «Contrasting copper evolution in ω Centauri and the Milky Way». Monthly Notices of the Royal Astronomical Society: Letters. 378 (1): L59–L63. arXiv:astro-ph/0703760. Bibcode:2007MNRAS.378L..59R. doi:10.1111/j.1745-3933.2007.00320.x. S2CID 14595800.
- ^ a b c d Emsley, John (2003). Nature’s building blocks: an A–Z guide to the elements. Oxford University Press. pp. 121–125. ISBN 978-0-19-850340-8. Retrieved 2 May 2011.
- ^ Rickwood, P. C. (1981). «The largest crystals» (PDF). American Mineralogist. 66: 885.
- ^ Emsley, John (2003). Nature’s building blocks: an A–Z guide to the elements. Oxford University Press. pp. 124, 231, 449, 503. ISBN 978-0-19-850340-8. Retrieved 2 May 2011.
- ^ Rieuwerts, John (2015). The Elements of Environmental Pollution. London and New York: Earthscan Routledge. p. 207. ISBN 978-0-415-85919-6. OCLC 886492996.
- ^ Randazzo, Ryan (19 June 2011). «A new method to harvest copper». Azcentral.com. Retrieved 25 April 2014.
- ^ Gordon, R.B.; Bertram, M.; Graedel, T.E. (2006). «Metal stocks and sustainability». Proceedings of the National Academy of Sciences. 103 (5): 1209–1214. Bibcode:2006PNAS..103.1209G. doi:10.1073/pnas.0509498103. PMC 1360560. PMID 16432205.
- ^ Beaudoin, Yannick C.; Baker, Elaine (December 2013). Deep Sea Minerals: Manganese Nodules, a physical, biological, environmental and technical review. Secretariat of the Pacific Community. pp. 7–18. ISBN 978-82-7701-119-6. Retrieved 8 February 2021.
- ^ a b Leonard, Andrew (3 March 2006). «Peak copper?». Salon. Retrieved 8 March 2022.
- ^ Brown, Lester (2006). Plan B 2.0: Rescuing a Planet Under Stress and a Civilization in Trouble. New York: W.W. Norton. p. 109. ISBN 978-0-393-32831-8.
- ^ Schmitz, Christopher (1986). «The Rise of Big Business in the World, Copper Industry 1870–1930». Economic History Review. 2. 39 (3): 392–410. doi:10.1111/j.1468-0289.1986.tb00411.x. JSTOR 2596347.
- ^ «Copper Trends: Live Metal Spot Prices». Archived from the original on 1 May 2012.
- ^ Ackerman, R. (2 April 2009). «A Bottom in Sight For Copper». Forbes. Archived from the original on 8 December 2012.
- ^ Employment Appeal Tribunal, AEI Cables Ltd. v GMB and others, 5 April 2013, accessed 5 February 2021
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Su, Kun; Ma, Xiaodong; Parianos, John; Zhao, Baojun (2020). «Thermodynamic and Experimental Study on Efficient Extraction of Valuable Metals from Polymetallic Nodules». Minerals. 10 (4): 360. Bibcode:2020Mine…10..360S. doi:10.3390/min10040360.
- ^ International Seabed Authority. «Polymetallic Nodules» (PDF). International Seabed Authority. Retrieved 8 February 2021.
- ^ Watling, H.R. (2006). «The bioleaching of sulphide minerals with emphasis on copper sulphides – A review» (PDF). Hydrometallurgy. 84 (1): 81–108. doi:10.1016/j.hydromet.2006.05.001. Archived from the original (PDF) on 18 August 2011.
- ^ Samans, Carl (1949). Engineering metals and their alloys. New York: Macmillan. OCLC 716492542.
- ^ Burton, Julie McCulloch (2015). Pen to Paper: Making Fun of Life. iUniverse. ISBN 978-1-4917-5394-1.
- ^ Bahadir, Ali Mufit; Duca, Gheorghe (2009). The Role of Ecological Chemistry in Pollution Research and Sustainable Development. Springer. ISBN 978-90-481-2903-4.
- ^ Green, Dan (2016). The Periodic Table in Minutes. Quercus. ISBN 978-1-68144-329-4.
- ^ «International Copper Association». Archived from the original on 5 March 2012. Retrieved 22 July 2009.
- ^ «Overview of Recycled Copper» Copper.org. (25 August 2010). Retrieved on 8 November 2011.
- ^ «Dime». US Mint. Retrieved 9 July 2019.[permanent dead link]
- ^ «Pride and skill – the 10-cent coin». Royal Canadian Mint. Retrieved 9 July 2019.
- ^ «Gold Jewellery Alloys». World Gold Council. Archived from the original on 14 April 2009. Retrieved 6 June 2009.
- ^ Balver Zinn Solder Sn97Cu3 Archived 7 July 2011 at the Wayback Machine. (PDF) . balverzinn.com. Retrieved on 8 November 2011.
- ^ Deane, D. V. «Modern Coinage Systems» (PDF). British Numismatic Society. Retrieved 1 July 2019.
- ^ «What is 90% Silver?». American Precious Metals Exchange (APMEX). Archived from the original on 28 July 2020. Retrieved 1 July 2019.
- ^ Corrosion Tests and Standards. ASTM International. 2005. p. 368.
- ^ Oguchi, Hachiro (1983). «Japanese Shakudō: its history, properties and production from gold-containing alloys». Gold Bulletin. 16 (4): 125–132. doi:10.1007/BF03214636.
- ^ a b c Holleman, A.F.; Wiberg, N. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- ^ Trammell, Rachel; Rajabimoghadam, Khashayar; Garcia-Bosch, Isaac (30 January 2019). «Copper-Promoted Functionalization of Organic Molecules: from Biologically Relevant Cu/O2 Model Systems to Organometallic Transformations». Chemical Reviews. 119 (4): 2954–3031. doi:10.1021/acs.chemrev.8b00368. PMC 6571019. PMID 30698952.
- ^ a b Wiley-Vch (2 April 2007). «Nonsystematic (Contact) Fungicides». Ullmann’s Agrochemicals. p. 623. ISBN 978-3-527-31604-5.
- ^ Ralph L. Shriner, Christine K.F. Hermann, Terence C. Morrill, David Y. Curtin, Reynold C. Fuson «The Systematic Identification of Organic Compounds» 8th edition, J. Wiley, Hoboken. ISBN 0-471-21503-1
- ^ Saalwächter, Kay; Burchard, Walther; Klüfers, Peter; Kettenbach, G.; Mayer, Peter; Klemm, Dieter; Dugarmaa, Saran (2000). «Cellulose Solutions in Water Containing Metal Complexes». Macromolecules. 33 (11): 4094–4107. Bibcode:2000MaMol..33.4094S. CiteSeerX 10.1.1.951.5219. doi:10.1021/ma991893m.
- ^ Deodhar, S., Huckaby, J., Delahoussaye, M. and DeCoster, M.A., 2014, August. High-aspect ratio bio-metallic nanocomposites for cellular interactions. In IOP Conference Series: Materials Science and Engineering (Vol. 64, No. 1, p. 012014). https://iopscience.iop.org/article/10.1088/1757-899X/64/1/012014/meta.
- ^ Kelly, K.C., Wasserman, J.R., Deodhar, S., Huckaby, J. and DeCoster, M.A., 2015. Generation of scalable, metallic high-aspect ratio nanocomposites in a biological liquid medium. JoVE (Journal of Visualized Experiments), (101), p.e52901. https://www.jove.com/t/52901/generation-scalable-metallic-high-aspect-ratio-nanocomposites.
- ^ Karan, A., Darder, M., Kansakar, U., Norcross, Z. and DeCoster, M.A., 2018. Integration of a Copper-Containing Biohybrid (CuHARS) with Cellulose for Subsequent Degradation and Biomedical Control. International journal of environmental research and public health, 15(5), p.844. https://www.mdpi.com/1660-4601/15/5/844
- ^ «Modern Organocopper Chemistry» Norbert Krause, Ed., Wiley-VCH, Weinheim, 2002. ISBN 978-3-527-29773-3.
- ^ Berná, José; Goldup, Stephen; Lee, Ai-Lan; Leigh, David; Symes, Mark; Teobaldi, Gilberto; Zerbetto, Fransesco (26 May 2008). «Cadiot–Chodkiewicz Active Template Synthesis of Rotaxanes and Switchable Molecular Shuttles with Weak Intercomponent Interactions». Angewandte Chemie. 120 (23): 4464–4468. Bibcode:2008AngCh.120.4464B. doi:10.1002/ange.200800891.
- ^ Rafael Chinchilla & Carmen Nájera (2007). «The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry». Chemical Reviews. 107 (3): 874–922. doi:10.1021/cr050992x. PMID 17305399.
- ^ «An Addition of an Ethylcopper Complex to 1-Octyne: (E)-5-Ethyl-1,4-Undecadiene» (PDF). Organic Syntheses. 64: 1. 1986. doi:10.15227/orgsyn.064.0001. Archived from the original (PDF) on 19 June 2012.
- ^ Kharasch, M.S.; Tawney, P.O. (1941). «Factors Determining the Course and Mechanisms of Grignard Reactions. II. The Effect of Metallic Compounds on the Reaction between Isophorone and Methylmagnesium Bromide». Journal of the American Chemical Society. 63 (9): 2308–2316. doi:10.1021/ja01854a005.
- ^ Imai, Sadako; Fujisawa, Kiyoshi; Kobayashi, Takako; Shirasawa, Nobuhiko; Fujii, Hiroshi; Yoshimura, Tetsuhiko; Kitajima, Nobumasa; Moro-oka, Yoshihiko (1998). «63Cu NMR Study of Copper(I) Carbonyl Complexes with Various Hydrotris(pyrazolyl)borates: Correlation between 63Cu Chemical Shifts and CO Stretching Vibrations». Inorganic Chemistry. 37 (12): 3066–3070. doi:10.1021/ic970138r.
- ^ G. Brauer, ed. (1963). «Potassium Cuprate (III)». Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). NY: Academic Press. p. 1015.
- ^ Schwesinger, Reinhard; Link, Reinhard; Wenzl, Peter; Kossek, Sebastian (2006). «Anhydrous phosphazenium fluorides as sources for extremely reactive fluoride ions in solution». Chemistry: A European Journal. 12 (2): 438–45. doi:10.1002/chem.200500838. PMID 16196062.
- ^ Lewis, E.A.; Tolman, W.B. (2004). «Reactivity of Dioxygen-Copper Systems». Chemical Reviews. 104 (2): 1047–1076. doi:10.1021/cr020633r. PMID 14871149.
- ^ McDonald, M.R.; Fredericks, F.C.; Margerum, D.W. (1997). «Characterization of Copper(III)–Tetrapeptide Complexes with Histidine as the Third Residue». Inorganic Chemistry. 36 (14): 3119–3124. doi:10.1021/ic9608713. PMID 11669966.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1187. ISBN 978-0-08-037941-8.
- ^ A Timeline of Copper Technologies, Copper Development Association, https://www.copper.org/education/history/timeline/
- ^ a b «CSA – Discovery Guides, A Brief History of Copper». Csa.com. Archived from the original on 3 February 2015. Retrieved 12 September 2008.
- ^ Rayner W. Hesse (2007). Jewelrymaking through History: an Encyclopedia. Greenwood Publishing Group. p. 56. ISBN 978-0-313-33507-5.No primary source is given in that book.
- ^ «Copper». Elements.vanderkrogt.net. Retrieved 12 September 2008.
- ^ Renfrew, Colin (1990). Before civilization: the radiocarbon revolution and prehistoric Europe. Penguin. ISBN 978-0-14-013642-5. Retrieved 21 December 2011.
- ^ Cowen, R. «Essays on Geology, History, and People: Chapter 3: Fire and Metals». Retrieved 7 July 2009.
- ^ Timberlake, S. & Prag A.J.N.W. (2005). The Archaeology of Alderley Edge: Survey, excavation and experiment in an ancient mining landscape. Oxford: John and Erica Hedges Ltd. p. 396. doi:10.30861/9781841717159. ISBN 9781841717159.
- ^ a b «CSA – Discovery Guides, A Brief History of Copper». CSA Discovery Guides. Archived from the original on 3 February 2015. Retrieved 29 April 2011.
- ^ Pleger, Thomas C. «A Brief Introduction to the Old Copper Complex of the Western Great Lakes: 4000–1000 BC», Proceedings of the Twenty-Seventh Annual Meeting of the Forest History Association of Wisconsin, Oconto, Wisconsin, 5 October 2002, pp. 10–18.
- ^ Emerson, Thomas E. and McElrath, Dale L. Archaic Societies: Diversity and Complexity Across the Midcontinent, SUNY Press, 2009 ISBN 1-4384-2701-8.
- ^ Dainian, Fan. Chinese Studies in the History and Philosophy of Science and Technology. p. 228.
- ^ Wallach, Joel. Epigenetics: The Death of the Genetic Theory of Disease Transmission.
- ^ Radivojević, Miljana; Rehren, Thilo (December 2013). «Tainted ores and the rise of tin bronzes in Eurasia, c. 6500 years ago». Antiquity Publications Ltd.
- ^ a b McNeil, Ian (2002). Encyclopaedia of the History of Technology. London; New York: Routledge. pp. 13, 48–66. ISBN 978-0-203-19211-5.
- ^ Rickard, T.A. (1932). «The Nomenclature of Copper and its Alloys». Journal of the Royal Anthropological Institute. 62: 281–290. doi:10.2307/2843960. JSTOR 2843960.
- ^ Timberlake, Simon (11 June 2017). «New ideas on the exploitation of copper, tin, gold, and lead ores in Bronze Age Britain: The mining, smelting, and movement of metal». Materials and Manufacturing Processes. 32 (7–8): 709–727. doi:10.1080/10426914.2016.1221113. S2CID 138178474.
- ^ Martin, Susan R. (1995). «The State of Our Knowledge About Ancient Copper Mining in Michigan». The Michigan Archaeologist. 41 (2–3): 119. Archived from the original on 7 February 2016.
- ^ Hong, S.; Candelone, J.-P.; Patterson, C.C.; Boutron, C.F. (1996). «History of Ancient Copper Smelting Pollution During Roman and Medieval Times Recorded in Greenland Ice». Science. 272 (5259): 246–249 (247f.). Bibcode:1996Sci…272..246H. doi:10.1126/science.272.5259.246. S2CID 176767223.
- ^ de Callataÿ, François (2005). «The Graeco-Roman Economy in the Super Long-Run: Lead, Copper, and Shipwrecks». Journal of Roman Archaeology. 18: 361–372 (366–369). doi:10.1017/S104775940000742X. S2CID 232346123.
- ^ Savenije, Tom J.; Warman, John M.; Barentsen, Helma M.; van Dijk, Marinus; Zuilhof, Han; Sudhölter, Ernst J.R. (2000). «Corinthian Bronze and the Gold of the Alchemists» (PDF). Macromolecules. 33 (2): 60–66. Bibcode:2000MaMol..33…60S. doi:10.1021/ma9904870. Archived from the original (PDF) on 29 September 2007.
- ^ Lynch, Martin (2004). Mining in World History. p. 60. ISBN 978-1-86189-173-0.
- ^ «Gold: prices, facts, figures and research: A brief history of money». Retrieved 22 April 2011.
- ^ «Copper and Brass in Ships». Retrieved 6 September 2016.
- ^ Stelter, M.; Bombach, H. (2004). «Process Optimization in Copper Electrorefining». Advanced Engineering Materials. 6 (7): 558–562. doi:10.1002/adem.200400403. S2CID 138550311.
- ^ Gardner, E. D.; et al. (1938). Copper Mining in North America. Washington, D. C.: U. S. Bureau of Mines. Retrieved 19 March 2019.
- ^ Hyde, Charles (1998). Copper for America, the United States Copper Industry from Colonial Times to the 1990s. Tucson, Arizona: University of Arizona Press. p. passim. ISBN 0-8165-1817-3.
- ^ «Outokumpu Flash Smelting» (PDF). Outokumpu. p. 2. Archived from the original (PDF) on 24 July 2011.
- ^ Karen A. Mingst (1976). «Cooperation or illusion: an examination of the intergovernmental council of copper exporting countries». International Organization. 30 (2): 263–287. doi:10.1017/S0020818300018270. S2CID 154183817.
- ^ Ryck Lydecker. «Is Copper Bottom Paint Sinking?». BoatUS Magazine. Retrieved 3 June 2016.
- ^ «Copper». American Elements. 2008. Archived from the original on 8 June 2008. Retrieved 12 July 2008.
- ^ Pops, Horace, 2008, «Processing of wire from antiquity to the future», Wire Journal International, June, pp. 58–66
- ^ The Metallurgy of Copper Wire, http://www.litz-wire.com/pdf%20files/Metallurgy_Copper_Wire.pdf Archived 1 September 2013 at the Wayback Machine
- ^ Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, pp. 141–192 and pp. 331–375.
- ^ «Copper, Chemical Element – Overview, Discovery and naming, Physical properties, Chemical properties, Occurrence in nature, Isotopes». Chemistryexplained.com. Retrieved 16 October 2012.
- ^ Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, p.348
- ^ «Aluminum Wiring Hazards and Pre-Purchase Inspections». www.heimer.com. Archived from the original on 28 May 2016. Retrieved 3 June 2016.
- ^ «Accelerator: Waveguides (SLAC VVC)». SLAC Virtual Visitor Center. Retrieved 29 April 2011.
- ^ IE3 energy-saving motors, Engineer Live, http://www.engineerlive.com/Design-Engineer/Motors_and_Drives/IE3_energy-saving_motors/22687/
- ^ Energy‐efficiency policy opportunities for electric motor‐driven systems, International Energy Agency, 2011 Working Paper in the Energy Efficiency Series, by Paul Waide and Conrad U. Brunner, OECD/IEA 2011
- ^ Fuchsloch, J. and E.F. Brush, (2007), «Systematic Design Approach for a New Series of Ultra‐NEMA Premium Copper Rotor Motors», in EEMODS 2007 Conference Proceedings, 10–15 June, Beijing.
- ^ Copper motor rotor project; Copper Development Association; «Copper.org: Copper Motor Rotor Project». Archived from the original on 13 March 2012. Retrieved 7 November 2012.
- ^ NEMA Premium Motors, The Association of Electrical Equipment and Medical Imaging Manufacturers; «NEMA — NEMA Premium Motors». Archived from the original on 2 April 2010. Retrieved 12 October 2009.
- ^ International Energy Agency, IEA sees renewable energy growth accelerating over next 5 years, http://www.iea.org/newsroomandevents/pressreleases/2012/july/name,28200,en.html
- ^ Global trends in renewable energy investment 2012, by REN21 (Renewable Energy Policy Network for the 21st Century); http://www.ren21.net/gsr
- ^ Will the Transition to Renewable Energy Be Paved in Copper?, Renewable Energy World; Jan 15, 2016; https://www.renewableenergyworld.com/articles/2016/01/will-the-transition-to-renewable-energy-be-paved-in-copper.html Archived 2018-06-22 at the Wayback Machine
- ^ García-Olivares, Antonio, Joaquim Ballabrera-Poy, Emili García-Ladona, and Antonio Turiel. A global renewable mix with proven technologies and common materials, Energy Policy, 41 (2012): 561-57, http://imedea.uib-csic.es/master/cambioglobal/Modulo_I_cod101601/Ballabrera_Diciembre_2011/Articulos/Garcia-Olivares.2011.pdf
- ^ A kilo more of copper increases environmental performance by 100 to 1,000 times; Renewable Energy Magazine; April 14, 2011; http://www.renewableenergymagazine.com/article/a-kilo-more-of-copper-increases-environmental
- ^ Copper at the core of renewable energies; European Copper Institute; European Copper Institute; 18 pages; http://www.eurocopper.org/files/presskit/press_kit_copper_in_renewables_final_29_10_2008.pdf Archived 2012-05-23 at the Wayback Machine
- ^ Copper in energy systems; Copper Development Association Inc.; http://www.copper.org/environment/green/energy.html
- ^ The Rise Of Solar: A Unique Opportunity For Copper; Solar Industry Magazine; April 2017; Zolaika Strong; https://issues.solarindustrymag.com/article/rise-solar-unique-opportunity-copper
- ^ Pops, Horace, 1995. Physical Metallurgy of Electrical Conductors, in Nonferrous Wire Handbook, Volume 3: Principles and Practice, The Wire Association International
- ^ The World Copper Factbook, 2017; http://www.icsg.org/index.php/component/jdownloads/finish/170/2462
- ^ Copper Mineral Commodity Summary (USGS, 2017) https://minerals.usgs.gov/minerals/pubs/commodity/copper/ mcs-2017-coppe.pdf
- ^ Global Mineral Resource Assessment (USGS, 2014) http://pubs.usgs.gov/fs/2014/3004/pdf/fs2014-3004.pdf
- ^ Long-Term Availability of Copper; International Copper Association; http://copperalliance.org/wordpress/wp-content/uploads/2018/02/ICA-long-term-availability-201802-A4-HR.pdf Archived 2018-06-29 at the Wayback Machine
- ^ Will the Transition to Renewable Energy Be Paved in Copper?, Renewable Energy World; Jan 15, 2016; by Zolaikha Strong; https://www.renewableenergyworld.com/articles/2016/01/will-the-transition-to-renewable-energy-be-paved-in-copper.html Archived 2018-06-22 at the Wayback Machine
- ^ Seale, Wayne (2007). The role of copper, brass, and bronze in architecture and design; Metal Architecture, May 2007
- ^ Copper roofing in detail; Copper in Architecture; Copper Development Association, U.K., www.cda.org.uk/arch
- ^ Architecture, European Copper Institute; http://eurocopper.org/copper/copper-architecture.html Archived 9 October 2012 at the Wayback Machine
- ^ Kronborg completed; Agency for Palaces and Cultural Properties, København, «Kronborg completed — Agency for Palaces and Cultural Properties». Archived from the original on 24 October 2012. Retrieved 12 September 2012.
- ^ Berg, Jan. «Why did we paint the library’s roof?». Archived from the original on 25 June 2007. Retrieved 20 September 2007.
- ^ Architectural considerations; Copper in Architecture Design Handbook, http://www.copper.org/applications/architecture/arch_dhb/fundamentals/arch_considerations.htm[permanent dead link]
- ^ Peters, Larry E. (2004). Preventing corrosion on copper roofing systems; Professional Roofing, October 2004, http://www.professionalroofing.net
- ^ Oxidation Reaction: Why is the Statue of Liberty Blue-Green? Engage Students in Engineering; www.EngageEngineering.org; Chun Wu, PhD, Mount Marty College; Funded by the National Science Foundation (NSF) under Grant No. 083306. «Archived copy» (PDF). Archived from the original (PDF) on 25 October 2013. Retrieved 25 October 2013.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Fitzgerald, K.P.; Nairn, J.; Atrens, A. (1998). «The chemistry of copper patination». Corrosion Science. 40 (12): 2029–50. doi:10.1016/S0010-938X(98)00093-6.
- ^ Application Areas: Architecture – Finishes – patina; http://www.copper.org/applications/architecture/finishes.html
- ^ Glossary of copper terms, Copper Development Association (UK): «Glossary of copper terms». Archived from the original on 20 August 2012. Retrieved 14 September 2012.
- ^ Finishes – natural weathering; Copper in Architecture Design Handbook, Copper Development Association Inc., «Copper.org: Architecture Design Handbook: Finishes». Archived from the original on 16 October 2012. Retrieved 12 September 2012.
- ^ Davis, Joseph R. (2001). Copper and Copper Alloys. ASM International. pp. 3–6, 266. ISBN 978-0-87170-726-0.
- ^ Edding, Mario E., Flores, Hector, and Miranda, Claudio, (1995), Experimental Usage of Copper-Nickel Alloy Mesh in Mariculture. Part 1: Feasibility of usage in a temperate zone; Part 2: Demonstration of usage in a cold zone; Final report to the International Copper Association Ltd.
- ^ Corrosion Behaviour of Copper Alloys used in Marine Aquaculture Archived 24 September 2013 at the Wayback Machine. (PDF) . copper.org. Retrieved on 8 November 2011.
- ^ Copper Touch Surfaces Archived 23 July 2012 at the Wayback Machine. Copper Touch Surfaces. Retrieved on 8 November 2011.
- ^ «EPA Registers Copper Surfaces for Residual Use Against Coronavirus». United States Environmental Protection Agency. 10 February 2021. Retrieved 11 October 2021.
- ^ Montero, David A.; Arellano, Carolina; Pardo, Mirka; Vera, Rosa; Gálvez, Ricardo; Cifuentes, Marcela; Berasain, María A.; Gómez, Marisol; Ramírez, Claudio; Vidal, Roberto M. (5 January 2019). «Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities». Antimicrobial Resistance and Infection Control. 8 (1): 3. doi:10.1186/s13756-018-0456-4. ISSN 2047-2994. PMC 6321648. PMID 30627427.
- ^ a b «EPA registers copper-containing alloy products». United States Environmental Protection Agency. May 2008. Archived from the original on 29 September 2015.
- ^ Biurrun, Amaya; Caballero, Luis; Pelaz, Carmen; León, Elena; Gago, Alberto (1999). «Treatment of a Legionella pneumophila‐Colonized Water Distribution System Using Copper‐Silver Ionization and Continuous Chlorination» (PDF). Infection Control and Hospital Epidemiology. 20 (6): 426–428. doi:10.1086/501645. JSTOR 30141645. PMID 10395146. S2CID 32388649. Archived from the original (PDF) on 17 February 2019.
- ^ Zaleski, Andrew, As hospitals look to prevent infections, a chorus of researchers make a case for copper surfaces, STAT, 24 September 2020
- ^ Chilean subway protected with Antimicrobial Copper – Rail News from Archived 24 July 2012 at the Wayback Machine. rail.co. Retrieved on 8 November 2011.
- ^ Codelco to provide antimicrobial copper for new metro lines (Chile)[dead link]. Construpages.com.ve. Retrieved on 8 November 2011.
- ^ PR 811 Chilean Subway Installs Antimicrobial Copper Archived 23 November 2011 at the Wayback Machine. (PDF). antimicrobialcopper.com. Retrieved on 8 November 2011.
- ^ «Copper and Cupron». Cupron.
- ^ «Global copper market under supplied, demand on the rise – report». Mining.com. 6 January 2019. Retrieved 13 January 2019.
- ^ «Will the Transition to Renewable Energy Be Paved in Copper?». www.renewableenergyworld.com. 15 January 2015. Archived from the original on 22 June 2018. Retrieved 13 January 2019.
- ^ «Copper and cars: Boom goes beyond electric vehicles». MINING.com. 18 June 2018. Retrieved 13 January 2019.
- ^ «Impact of electric cars in medium-term copper demand ‘overrated’, experts say». MINING.com. 12 April 2018. Retrieved 13 January 2019.
- ^ «Why are Premiums for Copper Bullion So High?». Provident Metals. 20 August 2012. Retrieved 23 January 2019.
- ^ Chace, Zoe. «Penny Hoarders Hope for the Day The Penny Dies». NPR.org. NPR. Retrieved 23 January 2019.
- ^ Walker, W.R.; Keats, D.M. (1976). «An investigation of the therapeutic value of the ‘copper bracelet’-dermal assimilation of copper in arthritic/rheumatoid conditions». Agents and Actions. 6 (4): 454–459. PMID 961545.
- ^ Richmond SJ, Gunadasa S, Bland M, Macpherson H (2013). «Copper bracelets and magnetic wrist straps for rheumatoid arthritis – analgesic and anti-inflammatory effects: a randomised double-blind placebo controlled crossover trial». PLOS ONE. 8 (9): e71529. Bibcode:2013PLoSO…871529R. doi:10.1371/journal.pone.0071529. PMC 3774818. PMID 24066023.
- ^ Richmond, Stewart J.; Brown, Sally R.; Campion, Peter D.; Porter, Amanda J.L.; Moffett, Jennifer A. Klaber; Jackson, David A.; Featherstone, Valerie A.; Taylor, Andrew J. (2009). «Therapeutic effects of magnetic and copper bracelets in osteoarthritis: A randomised placebo-controlled crossover trial». Complementary Therapies in Medicine. 17 (5–6): 249–256. doi:10.1016/j.ctim.2009.07.002. ISSN 0965-2299. PMID 19942103.
- ^ University of Arkansas for Medical Sciences:
Find the Truth Behind Medical Myths Archived 6 January 2014 at the Wayback MachineWhile it’s never been proven that copper can be absorbed through the skin by wearing a bracelet, research has shown that excessive copper can result in poisoning, causing vomiting and, in severe cases, liver damage.
- ^
Truth in Advertising
Tommie Copper - ^ a b Geoffrey Michael Gadd (March 2010). «Metals, minerals and microbes: geomicrobiology and bioremediation». Microbiology. 156 (3): 609–643. doi:10.1099/mic.0.037143-0. PMID 20019082.
- ^ Harbhajan Singh (2006). Mycoremediation: Fungal Bioremediation. p. 509. ISBN 978-0-470-05058-3.
- ^ Vest, Katherine E.; Hashemi, Hayaa F.; Cobine, Paul A. (2013). «Chapter 13 The Copper Metallome in Eukaryotic Cells». In Banci, Lucia (ed.). Metallomics and the Cell. Metal Ions in Life Sciences. Vol. 12. Springer. pp. 451–78. doi:10.1007/978-94-007-5561-1_13. ISBN 978-94-007-5560-4. PMID 23595680. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ «Fun facts». Horseshoe crab. University of Delaware. Archived from the original on 22 October 2008. Retrieved 13 July 2008.
- ^ a b S.J. Lippard, J.M. Berg «Principles of bioinorganic chemistry» University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- ^ Decker, H. & Terwilliger, N. (2000). «COPs and Robbers: Putative evolution of copper oxygen-binding proteins». Journal of Experimental Biology. 203 (Pt 12): 1777–1782. doi:10.1242/jeb.203.12.1777. PMID 10821735.
- ^
Schneider, Lisa K.; Wüst, Anja; Pomowski, Anja; Zhang, Lin; Einsle, Oliver (2014). «Chapter 8. No Laughing Matter: The Unmaking of the Greenhouse Gas Dinitrogen Monoxide by Nitrous Oxide Reductase«. In Peter M.H. Kroneck; Martha E. Sosa Torres (eds.). The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. Vol. 14. Springer. pp. 177–210. doi:10.1007/978-94-017-9269-1_8. ISBN 978-94-017-9268-4. PMID 25416395. - ^ Denoyer, Delphine; Clatworthy, Sharnel A.S.; Cater, Michael A. (2018). «Chapter 16. Copper Complexes in Cancer Therapy». In Sigel, Astrid; Sigel, Helmut; Freisinger, Eva; Sigel, Roland K.O. (eds.). Metallo-Drugs: Development and Action of Anticancer Agents. Metal Ions in Life Sciences. Vol. 18. Berlin: de Gruyter GmbH. pp. 469–506. doi:10.1515/9783110470734-022. ISBN 978-3-11-047073-4. PMID 29394035.
- ^ a b «Amount of copper in the normal human body, and other nutritional copper facts». Archived from the original on 10 April 2009. Retrieved 3 April 2009.
- ^ Adelstein, S. J.; Vallee, B. L. (1961). «Copper metabolism in man». New England Journal of Medicine. 265 (18): 892–897. doi:10.1056/NEJM196111022651806. PMID 13859394.
- ^ M.C. Linder; Wooten, L.; Cerveza, P.; Cotton, S.; Shulze, R.; Lomeli, N. (1 May 1998). «Copper transport». The American Journal of Clinical Nutrition. 67 (5): 965S–971S. doi:10.1093/ajcn/67.5.965S. PMID 9587137.
- ^ Frieden, E.; Hsieh, H.S. (1976). Ceruloplasmin: The copper transport protein with essential oxidase activity. Advances in Enzymology – and Related Areas of Molecular Biology. Vol. 44. pp. 187–236. doi:10.1002/9780470122891.ch6. ISBN 978-0-470-12289-1. JSTOR 20170553. PMID 775938.
- ^ S.S. Percival; Harris, E.D. (1 January 1990). «Copper transport from ceruloplasmin: Characterization of the cellular uptake mechanism». American Journal of Physiology. Cell Physiology. 258 (1): C140–C146. doi:10.1152/ajpcell.1990.258.1.c140. PMID 2301561.
- ^ Dietary Reference Intakes: RDA and AI for Vitamins and Elements Archived 13 November 2018 at the Wayback Machine Food and Nutrition Board, Institute of Medicine, National Academies Press, 2011. Retrieved 18 April 2018.
- ^ Copper. IN: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Copper. National Academy Press. 2001, PP. 224–257.
- ^ «Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies» (PDF). 2017.
- ^ Tolerable Upper Intake Levels For Vitamins And Minerals (PDF), European Food Safety Authority, 2006
- ^ «Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR p. 33982» (PDF).
- ^ «Daily Value Reference of the Dietary Supplement Label Database (DSLD)». Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- ^ Bonham, Maxine; O’Connor, Jacqueline M.; Hannigan, Bernadette M.; Strain, J.J. (2002). «The immune system as a physiological indicator of marginal copper status?». British Journal of Nutrition. 87 (5): 393–403. doi:10.1079/BJN2002558. PMID 12010579.
- ^ Li, Yunbo; Trush, Michael; Yager, James (1994). «DNA damage caused by reactive oxygen species originating from a copper-dependent oxidation of the 2-hydroxy catechol of estradiol». Carcinogenesis. 15 (7): 1421–1427. doi:10.1093/carcin/15.7.1421. PMID 8033320.
- ^ Gordon, Starkebaum; John, M. Harlan (April 1986). «Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine». J. Clin. Invest. 77 (4): 1370–6. doi:10.1172/JCI112442. PMC 424498. PMID 3514679.
- ^ «Pesticide Information Profile for Copper Sulfate». Cornell University. Retrieved 10 July 2008.
- ^ Hunt, Charles E. & William W. Carlton (1965). «Cardiovascular Lesions Associated with Experimental Copper Deficiency in the Rabbit». Journal of Nutrition. 87 (4): 385–394. doi:10.1093/jn/87.4.385. PMID 5841854.
- ^ Ayyat M.S.; Marai I.F.M.; Alazab A.M. (1995). «Copper-Protein Nutrition of New Zealand White Rabbits under Egyptian Conditions». World Rabbit Science. 3 (3): 113–118. doi:10.4995/wrs.1995.249.
- ^ Brewer GJ (March 2012). «Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease». BioFactors (Review). 38 (2): 107–113. doi:10.1002/biof.1005. hdl:2027.42/90519. PMID 22438177. S2CID 16989047.
- ^ «Copper: Alzheimer’s Disease». Examine.com. Retrieved 21 June 2015.
- ^ NIOSH Pocket Guide to Chemical Hazards. «#0151». National Institute for Occupational Safety and Health (NIOSH).
- ^ NIOSH Pocket Guide to Chemical Hazards. «#0150». National Institute for Occupational Safety and Health (NIOSH).
- ^ OEHHA Copper
- ^ Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). «Hazardous Compounds in Tobacco Smoke». International Journal of Environmental Research and Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. ISSN 1660-4601. PMC 3084482. PMID 21556207.
- ^ Pourkhabbaz, A.; Pourkhabbaz, H. (2012). «Investigation of Toxic Metals in the Tobacco of Different Iranian Cigarette Brands and Related Health Issues». Iranian Journal of Basic Medical Sciences. 15 (1): 636–644. PMC 3586865. PMID 23493960.
- ^ Bernhard, David; Rossmann, Andrea; Wick, Georg (2005). «Metals in cigarette smoke». IUBMB Life. 57 (12): 805–809. doi:10.1080/15216540500459667. PMID 16393783. S2CID 35694266.
Notes
|
|
|
|
|
| in pure water, or acidic or alkali conditions. Copper in neutral water is more noble than hydrogen. | in water containing sulfide | in 10 M ammonia solution | in a chloride solution |
Further reading
- Massaro, Edward J., ed. (2002). Handbook of Copper Pharmacology and Toxicology. Humana Press. ISBN 978-0-89603-943-8.
- «Copper: Technology & Competitiveness (Summary) Chapter 6: Copper Production Technology» (PDF). Office of Technology Assessment. 2005.
- Current Medicinal Chemistry, Volume 12, Number 10, May 2005, pp. 1161–1208(48) Metals, Toxicity and Oxidative Stress
- William D. Callister (2003). Materials Science and Engineering: an Introduction (6th ed.). Wiley, New York. Table 6.1, p. 137. ISBN 978-0-471-73696-7.
- Material: Copper (Cu), bulk, MEMS and Nanotechnology Clearinghouse.
- Kim BE; Nevitt T; Thiele DJ (2008). «Mechanisms for copper acquisition, distribution and regulation». Nat. Chem. Biol. 4 (3): 176–85. doi:10.1038/nchembio.72. PMID 18277979.
External links
Wikiquote has quotations related to Copper.
Wikimedia Commons has media related to Copper.
Look up copper in Wiktionary, the free dictionary.
Wikisource has original text related to this article:
- Copper at The Periodic Table of Videos (University of Nottingham)
- Copper and compounds fact sheet from the National Pollutant Inventory of Australia
- Copper.org – official website of the Copper Development Association with an extensive site of properties and uses of copper
- Price history of copper, according to the IMF
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Copper | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | red-orange metallic luster | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Cu) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Copper in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1357.77 K (1084.62 °C, 1984.32 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2835 K (2562 °C, 4643 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.96 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 8.02 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.26 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 300.4 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.440 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −2, 0,[2] +1, +2, +3, +4 (a mildly basic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 128 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 132±4 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 140 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of copper |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | (annealed) 3810 m/s (at r.t.) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 16.5 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 401 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 16.78 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −5.46×10−6 cm3/mol[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 110–128 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 48 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 140 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 3.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 343–369 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 235–878 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-50-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Cyprus, principal mining place in Roman era (Cyprium) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Middle East (9000 BC) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | «Cu»: from Latin cuprum | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of copper
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Copper is a chemical element with the symbol Cu (from Latin: cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement.
Copper is one of the few metals that can occur in nature in a directly usable metallic form (native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create bronze, c. 3500 BC.[5]
In the Roman era, copper was mined principally on Cyprus, the origin of the name of the metal, from aes cyprium (metal of Cyprus), later corrupted to cuprum (Latin). Coper (Old English) and copper were derived from this, the later spelling first used around 1530.[6]
Commonly encountered compounds are copper(II) salts, which often impart blue or green colors to such minerals as azurite, malachite, and turquoise, and have been used widely and historically as pigments.
Copper used in buildings, usually for roofing, oxidizes to form a green verdigris (or patina). Copper is sometimes used in decorative art, both in its elemental metal form and in compounds as pigments. Copper compounds are used as bacteriostatic agents, fungicides, and wood preservatives.
Copper is essential to all living organisms as a trace dietary mineral because it is a key constituent of the respiratory enzyme complex cytochrome c oxidase. In molluscs and crustaceans, copper is a constituent of the blood pigment hemocyanin, replaced by the iron-complexed hemoglobin in fish and other vertebrates. In humans, copper is found mainly in the liver, muscle, and bone.[7] The adult body contains between 1.4 and 2.1 mg of copper per kilogram of body weight.[8]
Characteristics
Physical
Copper just above its melting point keeps its pink luster color when enough light outshines the orange incandescence color
Copper, silver, and gold are in group 11 of the periodic table; these three metals have one s-orbital electron on top of a filled d-electron shell and are characterized by high ductility, and electrical and thermal conductivity. The filled d-shells in these elements contribute little to interatomic interactions, which are dominated by the s-electrons through metallic bonds. Unlike metals with incomplete d-shells, metallic bonds in copper are lacking a covalent character and are relatively weak. This observation explains the low hardness and high ductility of single crystals of copper.[9] At the macroscopic scale, introduction of extended defects to the crystal lattice, such as grain boundaries, hinders flow of the material under applied stress, thereby increasing its hardness. For this reason, copper is usually supplied in a fine-grained polycrystalline form, which has greater strength than monocrystalline forms.[10]
The softness of copper partly explains its high electrical conductivity (59.6×106 S/m) and high thermal conductivity, second highest (second only to silver) among pure metals at room temperature.[11] This is because the resistivity to electron transport in metals at room temperature originates primarily from scattering of electrons on thermal vibrations of the lattice, which are relatively weak in a soft metal.[9] The maximum permissible current density of copper in open air is approximately 3.1×106 A/m2 of cross-sectional area, above which it begins to heat excessively.[12]
Copper is one of a few metallic elements with a natural color other than gray or silver.[13] Pure copper is orange-red and acquires a reddish tarnish when exposed to air. The is due to the low plasma frequency of the metal, which lies in the red part of the visible spectrum, causing it to absorb the higher-frequency green and blue colors.[14]
As with other metals, if copper is put in contact with another metal, galvanic corrosion will occur.[15]
Chemical
Unoxidized copper wire (left) and oxidized copper wire (right)
The East Tower of the Royal Observatory, Edinburgh, showing the contrast between the refurbished copper installed in 2010 and the green color of the original 1894 copper.
Copper does not react with water, but it does slowly react with atmospheric oxygen to form a layer of brown-black copper oxide which, unlike the rust that forms on iron in moist air, protects the underlying metal from further corrosion (passivation). A green layer of verdigris (copper carbonate) can often be seen on old copper structures, such as the roofing of many older buildings[16] and the Statue of Liberty.[17] Copper tarnishes when exposed to some sulfur compounds, with which it reacts to form various copper sulfides.[18]
Isotopes
There are 29 isotopes of copper. 63
Cu
and 65
Cu
are stable, with 63
Cu
comprising approximately 69% of naturally occurring copper; both have a spin of 3⁄2.[19] The other isotopes are radioactive, with the most stable being 67
Cu
with a half-life of 61.83 hours.[19] Seven metastable isotopes have been characterized; 68m
Cu
is the longest-lived with a half-life of 3.8 minutes. Isotopes with a mass number above 64 decay by β−, whereas those with a mass number below 64 decay by β+. 64
Cu
, which has a half-life of 12.7 hours, decays both ways.[20]
62
Cu
and 64
Cu
have significant applications. 62
Cu
is used in 62
Cu
Cu-PTSM as a radioactive tracer for positron emission tomography.[21]
Occurrence
Native copper from the Keweenaw Peninsula, Michigan, about 2.5 inches (6.4 cm) long
Copper is produced in massive stars[22] and is present in the Earth’s crust in a proportion of about 50 parts per million (ppm).[23] In nature, copper occurs in a variety of minerals, including native copper, copper sulfides such as chalcopyrite, bornite, digenite, covellite, and chalcocite, copper sulfosalts such as tetrahedite-tennantite, and enargite, copper carbonates such as azurite and malachite, and as copper(I) or copper(II) oxides such as cuprite and tenorite, respectively.[11] The largest mass of elemental copper discovered weighed 420 tonnes and was found in 1857 on the Keweenaw Peninsula in Michigan, US.[23] Native copper is a polycrystal, with the largest single crystal ever described measuring 4.4 × 3.2 × 3.2 cm.[24] Copper is the 25th most abundant element in Earth’s crust, representing 50 ppm compared with 75 ppm for zinc, and 14 ppm for lead.[25]
Typical background concentrations of copper do not exceed 1 ng/m3 in the atmosphere; 150 mg/kg in soil; 30 mg/kg in vegetation; 2 μg/L in freshwater and 0.5 μg/L in seawater.[26]
Production
Most copper is mined or extracted as copper sulfides from large open pit mines in porphyry copper deposits that contain 0.4 to 1.0% copper. Sites include Chuquicamata, in Chile, Bingham Canyon Mine, in Utah, United States, and El Chino Mine, in New Mexico, United States. According to the British Geological Survey, in 2005, Chile was the top producer of copper with at least one-third of the world share followed by the United States, Indonesia and Peru.[11] Copper can also be recovered through the in-situ leach process. Several sites in the state of Arizona are considered prime candidates for this method.[27] The amount of copper in use is increasing and the quantity available is barely sufficient to allow all countries to reach developed world levels of usage.[28] An alternative source of copper for collection currently being researched are polymetallic nodules, which are located at the depths of the Pacific Ocean approximately 3000–6500 meters below sea level. These nodules contain other valuable metals such as cobalt and nickel.[29]
Reserves and prices
Price of Copper 1959-2022
Copper has been in use at least 10,000 years, but more than 95% of all copper ever mined and smelted has been extracted since 1900.[30] As with many natural resources, the total amount of copper on Earth is vast, with around 1014 tons in the top kilometer of Earth’s crust, which is about 5 million years’ worth at the current rate of extraction. However, only a tiny fraction of these reserves is economically viable with present-day prices and technologies. Estimates of copper reserves available for mining vary from 25 to 60 years, depending on core assumptions such as the growth rate.[31] Recycling is a major source of copper in the modern world.[30] Because of these and other factors, the future of copper production and supply is the subject of much debate, including the concept of peak copper, analogous to peak oil.[citation needed]
The price of copper has historically been unstable,[32] and its price increased from the 60-year low of US$0.60/lb (US$1.32/kg) in June 1999 to $3.75 per pound ($8.27/kg) in May 2006. It dropped to $2.40/lb ($5.29/kg) in February 2007, then rebounded to $3.50/lb ($7.71/kg) in April 2007.[33][better source needed] In February 2009, weakening global demand and a steep fall in commodity prices since the previous year’s highs left copper prices at $1.51/lb ($3.32/kg).[34] Between September 2010 and February 2011, the price of copper rose from £5,000 a metric ton to £6,250 a metric ton.[35]
Methods
Scheme of flash smelting process
The concentration of copper in ores averages only 0.6%, and most commercial ores are sulfides, especially chalcopyrite (CuFeS2), bornite (Cu5FeS4) and, to a lesser extent, covellite (CuS) and chalcocite (Cu2S).[36] Conversely, the average concentration of copper in polymetallic nodules is estimated at 1.3%. The methods of extracting copper as well as other metals found in these nodules include sulphuric leaching, smelting and an application of the Cuprion process.[37][38] For minerals found in land ores, they are concentrated from crushed ores to the level of 10–15% copper by froth flotation or bioleaching.[39] Heating this material with silica in flash smelting removes much of the iron as slag. The process exploits the greater ease of converting iron sulfides into oxides, which in turn react with the silica to form the silicate slag that floats on top of the heated mass. The resulting copper matte, consisting of Cu2S, is roasted to convert the sulfides into oxides:[36]
- 2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
The cuprous oxide reacts with cuprous sulfide to converted to blister copper upon heating:
- 2 Cu2O + Cu2S → 6 Cu + 2 SO2
The Sudbury matte process converted only half the sulfide to oxide and then used this oxide to remove the rest of the sulfur as oxide. It was then electrolytically refined and the anode mud exploited for the platinum and gold it contained. This step exploits the relatively easy reduction of copper oxides to copper metal. Natural gas is blown across the blister to remove most of the remaining oxygen and electrorefining is performed on the resulting material to produce pure copper:[40]
- Cu2+ + 2 e− → Cu

Recycling
Like aluminium,[41] copper is recyclable without any loss of quality, both from raw state and from manufactured products.[42] In volume, copper is the third most recycled metal after iron and aluminium.[43] An estimated 80% of all copper ever mined is still in use today.[44] According to the International Resource Panel’s Metal Stocks in Society report, the global per capita stock of copper in use in society is 35–55 kg. Much of this is in more-developed countries (140–300 kg per capita) rather than less-developed countries (30–40 kg per capita).
The process of recycling copper is roughly the same as is used to extract copper but requires fewer steps. High-purity scrap copper is melted in a furnace and then reduced and cast into billets and ingots; lower-purity scrap is refined by electroplating in a bath of sulfuric acid.[45]
Alloys
Copper alloys are widely used in the production of coinage; seen here are two examples — post-1964 American dimes, which are composed of the alloy cupronickel[46] and a pre-1968 Canadian dime, which is composed of an alloy of 80 percent silver and 20 percent copper.[47]
Numerous copper alloys have been formulated, many with important uses. Brass is an alloy of copper and zinc. Bronze usually refers to copper-tin alloys, but can refer to any alloy of copper such as aluminium bronze. Copper is one of the most important constituents of silver and karat gold solders used in the jewelry industry, modifying the color, hardness and melting point of the resulting alloys.[48] Some lead-free solders consist of tin alloyed with a small proportion of copper and other metals.[49]
The alloy of copper and nickel, called cupronickel, is used in low-denomination coins, often for the outer cladding. The US five-cent coin (currently called a nickel) consists of 75% copper and 25% nickel in homogeneous composition. Prior to the introduction of cupronickel, which was widely adopted by countries in the latter half of the 20th century,[50] alloys of copper and silver were also used, with the United States using an alloy of 90% silver and 10% copper until 1965, when circulating silver was removed from all coins with the exception of the Half dollar — these were debased to an alloy of 40% silver and 60% copper between 1965 and 1970.[51] The alloy of 90% copper and 10% nickel, remarkable for its resistance to corrosion, is used for various objects exposed to seawater, though it is vulnerable to the sulfides sometimes found in polluted harbors and estuaries.[52] Alloys of copper with aluminium (about 7%) have a golden color and are used in decorations.[23] Shakudō is a Japanese decorative alloy of copper containing a low percentage of gold, typically 4–10%, that can be patinated to a dark blue or black color.[53]
Compounds
Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively.[54] Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes.[55]
Binary compounds
As with other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements, the principal examples being oxides, sulfides, and halides. Both cuprous and cupric oxides are known. Among the numerous copper sulfides, important examples include copper(I) sulfide and copper(II) sulfide.[citation needed]
Cuprous halides with fluorine, chlorine, bromine, and iodine are known, as are cupric halides with fluorine, chlorine, and bromine. Attempts to prepare copper(II) iodide yield only copper(I) iodide and iodine.[54]
- 2 Cu2+ + 4 I− → 2 CuI + I2
Coordination chemistry
Copper forms coordination complexes with ligands. In aqueous solution, copper(II) exists as [Cu(H
2O)
6]2+
. This complex exhibits the fastest water exchange rate (speed of water ligands attaching and detaching) for any transition metal aquo complex. Adding aqueous sodium hydroxide causes the precipitation of light blue solid copper(II) hydroxide. A simplified equation is:
Pourbaix diagram for copper in uncomplexed media (anions other than OH- not considered). Ion concentration 0.001 m (mol/kg water). Temperature 25 °C.
- Cu2+ + 2 OH− → Cu(OH)2
Aqueous ammonia results in the same precipitate. Upon adding excess ammonia, the precipitate dissolves, forming tetraamminecopper(II):
- Cu(H
2O)
4(OH)
2 + 4 NH3 → [Cu(H
2O)
2(NH
3)
4]2+
+ 2 H2O + 2 OH−
Many other oxyanions form complexes; these include copper(II) acetate, copper(II) nitrate, and copper(II) carbonate. Copper(II) sulfate forms a blue crystalline pentahydrate, the most familiar copper compound in the laboratory. It is used in a fungicide called the Bordeaux mixture.[56]
Polyols, compounds containing more than one alcohol functional group, generally interact with cupric salts. For example, copper salts are used to test for reducing sugars. Specifically, using Benedict’s reagent and Fehling’s solution the presence of the sugar is signaled by a color change from blue Cu(II) to reddish copper(I) oxide.[57] Schweizer’s reagent and related complexes with ethylenediamine and other amines dissolve cellulose.[58] Amino acids such as cystine form very stable chelate complexes with copper(II)[59][60][61] including in the form of metal-organic biohybrids (MOBs). Many wet-chemical tests for copper ions exist, one involving potassium ferrocyanide, which gives a brown precipitate with copper(II) salts.[citation needed]
Organocopper chemistry
Compounds that contain a carbon-copper bond are known as organocopper compounds. They are very reactive towards oxygen to form copper(I) oxide and have many uses in chemistry. They are synthesized by treating copper(I) compounds with Grignard reagents, terminal alkynes or organolithium reagents;[62] in particular, the last reaction described produces a Gilman reagent. These can undergo substitution with alkyl halides to form coupling products; as such, they are important in the field of organic synthesis. Copper(I) acetylide is highly shock-sensitive but is an intermediate in reactions such as the Cadiot-Chodkiewicz coupling[63] and the Sonogashira coupling.[64] Conjugate addition to enones[65] and carbocupration of alkynes[66] can also be achieved with organocopper compounds. Copper(I) forms a variety of weak complexes with alkenes and carbon monoxide, especially in the presence of amine ligands.[67]
Copper(III) and copper(IV)
Copper(III) is most often found in oxides. A simple example is potassium cuprate, KCuO2, a blue-black solid.[68] The most extensively studied copper(III) compounds are the cuprate superconductors. Yttrium barium copper oxide (YBa2Cu3O7) consists of both Cu(II) and Cu(III) centres. Like oxide, fluoride is a highly basic anion[69] and is known to stabilize metal ions in high oxidation states. Both copper(III) and even copper(IV) fluorides are known, K3CuF6 and Cs2CuF6, respectively.[54]
Some copper proteins form oxo complexes, which also feature copper(III).[70] With tetrapeptides, purple-colored copper(III) complexes are stabilized by the deprotonated amide ligands.[71]
Complexes of copper(III) are also found as intermediates in reactions of organocopper compounds.[72] For example, in the Kharasch–Sosnovsky reaction.[citation needed]
History
A timeline of copper illustrates how this metal has advanced human civilization for the past 11,000 years.[73]
Prehistoric
Copper Age
A corroded copper ingot from Zakros, Crete, shaped in the form of an animal skin (oxhide) typical in that era.
Many tools during the Chalcolithic Era included copper, such as the blade of this replica of Ötzi’s axe
Copper occurs naturally as native metallic copper and was known to some of the oldest civilizations on record. The history of copper use dates to 9000 BC in the Middle East;[74] a copper pendant was found in northern Iraq that dates to 8700 BC.[75] Evidence suggests that gold and meteoric iron (but not smelted iron) were the only metals used by humans before copper.[76] The history of copper metallurgy is thought to follow this sequence: First, cold working of native copper, then annealing, smelting, and, finally, lost-wax casting. In southeastern Anatolia, all four of these techniques appear more or less simultaneously at the beginning of the Neolithic c. 7500 BC.[77]
Copper smelting was independently invented in different places. It was probably discovered in China before 2800 BC, in Central America around 600 AD, and in West Africa about the 9th or 10th century AD.[78] Investment casting was invented in 4500–4000 BC in Southeast Asia[74] and carbon dating has established mining at Alderley Edge in Cheshire, UK, at 2280 to 1890 BC.[79] Ötzi the Iceman, a male dated from 3300 to 3200 BC, was found with an axe with a copper head 99.7% pure; high levels of arsenic in his hair suggest an involvement in copper smelting.[80] Experience with copper has assisted the development of other metals; in particular, copper smelting led to the discovery of iron smelting.[80] Production in the Old Copper Complex in Michigan and Wisconsin is dated between 6000 and 3000 BC.[81][82] Natural bronze, a type of copper made from ores rich in silicon, arsenic, and (rarely) tin, came into general use in the Balkans around 5500 BC.[83]
Bronze Age
Alloying copper with tin to make bronze was first practiced about 4000 years after the discovery of copper smelting, and about 2000 years after «natural bronze» had come into general use.[84] Bronze artifacts from the Vinča culture date to 4500 BC.[85] Sumerian and Egyptian artifacts of copper and bronze alloys date to 3000 BC.[86] The Bronze Age began in Southeastern Europe around 3700–3300 BC, in Northwestern Europe about 2500 BC. It ended with the beginning of the Iron Age, 2000–1000 BC in the Near East, and 600 BC in Northern Europe. The transition between the Neolithic period and the Bronze Age was formerly termed the Chalcolithic period (copper-stone), when copper tools were used with stone tools. The term has gradually fallen out of favor because in some parts of the world, the Chalcolithic and Neolithic are coterminous at both ends. Brass, an alloy of copper and zinc, is of much more recent origin. It was known to the Greeks, but became a significant supplement to bronze during the Roman Empire.[86]
Ancient and post-classical
In alchemy the symbol for copper was also the symbol for the goddess and planet Venus.
In Greece, copper was known by the name chalkos (χαλκός). It was an important resource for the Romans, Greeks and other ancient peoples. In Roman times, it was known as aes Cyprium, aes being the generic Latin term for copper alloys and Cyprium from Cyprus, where much copper was mined. The phrase was simplified to cuprum, hence the English copper. Aphrodite (Venus in Rome) represented copper in mythology and alchemy because of its lustrous beauty and its ancient use in producing mirrors; Cyprus, the source of copper, was sacred to the goddess. The seven heavenly bodies known to the ancients were associated with the seven metals known in antiquity, and Venus was assigned to copper, both because of the connection to the goddess and because Venus was the brightest heavenly body after the Sun and Moon and so corresponded to the most lustrous and desirable metal after gold and silver.[87]
Copper was first mined in ancient Britain as early as 2100 BC. Mining at the largest of these mines, the Great Orme, continued into the late Bronze Age. Mining seems to have been largely restricted to supergene ores, which were easier to smelt. The rich copper deposits of Cornwall seem to have been largely untouched, in spite of extensive tin mining in the region, for reasons likely social and political rather than technological.[88]
In North America, copper mining began with marginal workings by Native Americans. Native copper is known to have been extracted from sites on Isle Royale with primitive stone tools between 800 and 1600.[89] Copper metallurgy was flourishing in South America, particularly in Peru around 1000 AD. Copper burial ornamentals from the 15th century have been uncovered, but the metal’s commercial production did not start until the early 20th century.[citation needed]
The cultural role of copper has been important, particularly in currency. Romans in the 6th through 3rd centuries BC used copper lumps as money. At first, the copper itself was valued, but gradually the shape and look of the copper became more important. Julius Caesar had his own coins made from brass, while Octavianus Augustus Caesar’s coins were made from Cu-Pb-Sn alloys. With an estimated annual output of around 15,000 t, Roman copper mining and smelting activities reached a scale unsurpassed until the time of the Industrial Revolution; the provinces most intensely mined were those of Hispania, Cyprus and in Central Europe.[90][91]
The gates of the Temple of Jerusalem used Corinthian bronze treated with depletion gilding.[clarification needed][citation needed] The process was most prevalent in Alexandria, where alchemy is thought to have begun.[92] In ancient India, copper was used in the holistic medical science Ayurveda for surgical instruments and other medical equipment. Ancient Egyptians (~2400 BC) used copper for sterilizing wounds and drinking water, and later to treat headaches, burns, and itching.[citation needed]
Modern
18th-century copper kettle from Norway made from Swedish copper
The Great Copper Mountain was a mine in Falun, Sweden, that operated from the 10th century to 1992. It satisfied two-thirds of Europe’s copper consumption in the 17th century and helped fund many of Sweden’s wars during that time.[93] It was referred to as the nation’s treasury; Sweden had a copper backed currency.[94]
Chalcography of the city of Vyborg at the turn of the 17th and 18th centuries. The year 1709 carved on the printing plate.
Copper is used in roofing,[16] currency, and for photographic technology known as the daguerreotype. Copper was used in Renaissance sculpture, and was used to construct the Statue of Liberty; copper continues to be used in construction of various types. Copper plating and copper sheathing were widely used to protect the under-water hulls of ships, a technique pioneered by the British Admiralty in the 18th century.[95] The Norddeutsche Affinerie in Hamburg was the first modern electroplating plant, starting its production in 1876.[96] The German scientist Gottfried Osann invented powder metallurgy in 1830 while determining the metal’s atomic mass; around then it was discovered that the amount and type of alloying element (e.g., tin) to copper would affect bell tones.[citation needed]
During the rise in demand for copper for the Age of Electricity, from the 1880s until the Great Depression of the 1930s, the United States produced one third to half the world’s newly mined copper.[97] Major districts included the Keweenaw district of northern Michigan, primarily native copper deposits, which was eclipsed by the vast sulphide deposits of Butte, Montana in the late 1880s, which itself was eclipsed by porphyry deposits of the Souhwest United States, especially at Bingham Canyon, Utah and Morenci, Arizona. Introduction of open pit steam shovel mining and innovations in smelting, refining, flotation concentration and other processing steps led to mass production. Early in the twentieth century, Arizona ranked first, followed by Montana, then Utah and Michigan.[98]
Flash smelting was developed by Outokumpu in Finland and first applied at Harjavalta in 1949; the energy-efficient process accounts for 50% of the world’s primary copper production.[99]
The Intergovernmental Council of Copper Exporting Countries, formed in 1967 by Chile, Peru, Zaire and Zambia, operated in the copper market as OPEC does in oil, though it never achieved the same influence, particularly because the second-largest producer, the United States, was never a member; it was dissolved in 1988.[100]
Applications
Copper fittings for soldered plumbing joints
The major applications of copper are electrical wire (60%), roofing and plumbing (20%), and industrial machinery (15%). Copper is used mostly as a pure metal, but when greater hardness is required, it is put into such alloys as brass and bronze (5% of total use).[23] For more than two centuries, copper paint has been used on boat hulls to control the growth of plants and shellfish.[101] A small part of the copper supply is used for nutritional supplements and fungicides in agriculture.[56][102] Machining of copper is possible, although alloys are preferred for good machinability in creating intricate parts.
Wire and cable
Despite competition from other materials, copper remains the preferred electrical conductor in nearly all categories of electrical wiring except overhead electric power transmission where aluminium is often preferred.[103][104] Copper wire is used in power generation, power transmission, power distribution, telecommunications, electronics circuitry, and countless types of electrical equipment.[105] Electrical wiring is the most important market for the copper industry.[106] This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used for electrical wire and cable conductors.[107] Many electrical devices rely on copper wiring because of its multitude of inherent beneficial properties, such as its high electrical conductivity, tensile strength, ductility, creep (deformation) resistance, corrosion resistance, low thermal expansion, high thermal conductivity, ease of soldering, malleability, and ease of installation.
For a short period from the late 1960s to the late 1970s, copper wiring was replaced by aluminium wiring in many housing construction projects in America. The new wiring was implicated in a number of house fires and the industry returned to copper.[108]
Electronics and related devices
Copper electrical busbars distributing power to a large building
Integrated circuits and printed circuit boards increasingly feature copper in place of aluminium because of its superior electrical conductivity; heat sinks and heat exchangers use copper because of its superior heat dissipation properties. Electromagnets, vacuum tubes, cathode ray tubes, and magnetrons in microwave ovens use copper, as do waveguides for microwave radiation.[109]
Electric motors
Copper’s superior conductivity enhances the efficiency of electrical motors.[110] This is important because motors and motor-driven systems account for 43%–46% of all global electricity consumption and 69% of all electricity used by industry.[111] Increasing the mass and cross section of copper in a coil increases the efficiency of the motor. Copper motor rotors, a new technology designed for motor applications where energy savings are prime design objectives,[112][113] are enabling general-purpose induction motors to meet and exceed National Electrical Manufacturers Association (NEMA) premium efficiency standards.[114]
Renewable energy production
Renewable energy sources such as solar, wind, tidal, hydro, biomass, and geothermal have become significant sectors of the energy market.[115][116] The rapid growth of these sources in the 21st century has been prompted by increasing costs of fossil fuels as well as their environmental impact issues that significantly lowered their use.
Copper plays an important role in these renewable energy systems.[117][118][119][120][121] Copper usage averages up to five times more in renewable energy systems than in traditional power generation, such as fossil fuel and nuclear power plants.[122] Since copper is an excellent thermal and electrical conductor among engineering metals (second only to silver),[123] electrical systems that utilize copper generate and transmit energy with high efficiency and with minimum environmental impacts.
When choosing electrical conductors, facility planners and engineers factor capital investment costs of materials against operational savings due to their electrical energy efficiencies over their useful lives, plus maintenance costs. Copper often fares well in these calculations. A factor called «copper usage intensity,” is a measure of the quantity of copper necessary to install one megawatt of new power-generating capacity.
Copper wires for recycling
When planning for a new renewable power facility, engineers and product specifiers seek to avoid supply shortages of selected materials. According to the United States Geological Survey, in-ground copper reserves have increased more than 700% since 1950, from almost 100 million tonnes to 720 million tonnes in 2017, despite the fact that world refined usage has more than tripled in the last 50 years.[124] Copper resources are estimated to exceed 5,000 million tonnes.[125][126]
Bolstering the supply from copper extraction is the fact that more than 30 percent of copper installed during the last decade came from recycled sources.[127] Its recycling rate is higher than any other metal.[128]
This article discusses the role of copper in various renewable energy generation systems.
Architecture
Old copper utensils in a Jerusalem restaurant
Copper has been used since ancient times as a durable, corrosion resistant, and weatherproof architectural material.[129][130][131][132] Roofs, flashings, rain gutters, downspouts, domes, spires, vaults, and doors have been made from copper for hundreds or thousands of years. Copper’s architectural use has been expanded in modern times to include interior and exterior wall cladding, building expansion joints, radio frequency shielding, and antimicrobial and decorative indoor products such as attractive handrails, bathroom fixtures, and counter tops. Some of copper’s other important benefits as an architectural material include low thermal movement, light weight, lightning protection, and recyclability
The metal’s distinctive natural green patina has long been coveted by architects and designers. The final patina is a particularly durable layer that is highly resistant to atmospheric corrosion, thereby protecting the underlying metal against further weathering.[133][134][135] It can be a mixture of carbonate and sulfate compounds in various amounts, depending upon environmental conditions such as sulfur-containing acid rain.[136][137][138][139] Architectural copper and its alloys can also be ‘finished’ to take on a particular look, feel, or color. Finishes include mechanical surface treatments, chemical coloring, and coatings.[140]
Copper has excellent brazing and soldering properties and can be welded; the best results are obtained with gas metal arc welding.[141]
Antibiofouling
Copper is biostatic, meaning bacteria and many other forms of life will not grow on it. For this reason it has long been used to line parts of ships to protect against barnacles and mussels. It was originally used pure, but has since been superseded by Muntz metal and copper-based paint. Similarly, as discussed in copper alloys in aquaculture, copper alloys have become important netting materials in the aquaculture industry because they are antimicrobial and prevent biofouling, even in extreme conditions[142] and have strong structural and corrosion-resistant[143] properties in marine environments.
Antimicrobial
Copper-alloy touch surfaces have natural properties that destroy a wide range of microorganisms (e.g., E. coli O157:H7, methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus, Clostridium difficile, influenza A virus, adenovirus, SARS-Cov-2, and fungi).[144][145] Indians have been using copper vessels since ancient times for storing water, even before modern science realized its antimicrobial properties.[146] Some copper alloys were proven to kill more than 99.9% of disease-causing bacteria within just two hours when cleaned regularly.[147] The United States Environmental Protection Agency (EPA) has approved the registrations of these copper alloys as «antimicrobial materials with public health benefits»;[147] that approval allows manufacturers to make legal claims to the public health benefits of products made of registered alloys. In addition, the EPA has approved a long list of antimicrobial copper products made from these alloys, such as bedrails, handrails, over-bed tables, sinks, faucets, door knobs, toilet hardware, computer keyboards, health club equipment, and shopping cart handles (for a comprehensive list, see: Antimicrobial copper-alloy touch surfaces#Approved products). Copper doorknobs are used by hospitals to reduce the transfer of disease, and Legionnaires’ disease is suppressed by copper tubing in plumbing systems.[148] Antimicrobial copper alloy products are now being installed in healthcare facilities in the U.K., Ireland, Japan, Korea, France, Denmark, and Brazil, as well as being called for in the US,[149] and in the subway transit system in Santiago, Chile, where copper-zinc alloy handrails were installed in some 30 stations between 2011 and 2014.[150][151][152]
Textile fibers can be blended with copper to create antimicrobial protective fabrics.[153][unreliable source?]
Speculative investing
Copper may be used as a speculative investment due to the predicted increase in use from worldwide infrastructure growth, and the important role it has in producing wind turbines, solar panels, and other renewable energy sources.[154][155] Another reason predicted demand increases is the fact that electric cars contain an average of 3.6 times as much copper as conventional cars, although the effect of electric cars on copper demand is debated.[156][157] Some people invest in copper through copper mining stocks, ETFs, and futures. Others store physical copper in the form of copper bars or rounds although these tend to carry a higher premium in comparison to precious metals.[158] Those who want to avoid the premiums of copper bullion alternatively store old copper wire, copper tubing or American pennies made before 1982.[159]
Folk medicine
Copper is commonly used in jewelry, and according to some folklore, copper bracelets relieve arthritis symptoms.[160] In one trial for osteoarthritis and one trial for rheumatoid arthritis, no differences is found between copper bracelet and control (non-copper) bracelet.[161][162] No evidence shows that copper can be absorbed through the skin. If it were, it might lead to copper poisoning.[163]
Compression clothing
Recently, some compression clothing with inter-woven copper has been marketed with health claims similar to the folk medicine claims. Because compression clothing is a valid treatment for some ailments, the clothing may have that benefit, but the added copper may have no benefit beyond a placebo effect.[164]
Degradation
Chromobacterium violaceum and Pseudomonas fluorescens can both mobilize solid copper as a cyanide compound.[165] The ericoid mycorrhizal fungi associated with Calluna, Erica and Vaccinium can grow in metalliferous soils containing copper.[165] The ectomycorrhizal fungus Suillus luteus protects young pine trees from copper toxicity. A sample of the fungus Aspergillus niger was found growing from gold mining solution and was found to contain cyano complexes of such metals as gold, silver, copper, iron, and zinc. The fungus also plays a role in the solubilization of heavy metal sulfides.[166]
Biological role
Rich sources of copper include oysters, beef and lamb liver, Brazil nuts, blackstrap molasses, cocoa, and black pepper. Good sources include lobster, nuts and sunflower seeds, green olives, avocados, and wheat bran.
Biochemistry
Copper proteins have diverse roles in biological electron transport and oxygen transportation, processes that exploit the easy interconversion of Cu(I) and Cu(II).[167] Copper is essential in the aerobic respiration of all eukaryotes. In mitochondria, it is found in cytochrome c oxidase, which is the last protein in oxidative phosphorylation. Cytochrome c oxidase is the protein that binds the O2 between a copper and an iron; the protein transfers 8 electrons to the O2 molecule to reduce it to two molecules of water. Copper is also found in many superoxide dismutases, proteins that catalyze the decomposition of superoxides by converting it (by disproportionation) to oxygen and hydrogen peroxide:
- Cu2+-SOD + O2− → Cu+-SOD + O2 (reduction of copper; oxidation of superoxide)
- Cu+-SOD + O2− + 2H+ → Cu2+-SOD + H2O2 (oxidation of copper; reduction of superoxide)
The protein hemocyanin is the oxygen carrier in most mollusks and some arthropods such as the horseshoe crab (Limulus polyphemus).[168] Because hemocyanin is blue, these organisms have blue blood rather than the red blood of iron-based hemoglobin. Structurally related to hemocyanin are the laccases and tyrosinases. Instead of reversibly binding oxygen, these proteins hydroxylate substrates, illustrated by their role in the formation of lacquers.[169] The biological role for copper commenced with the appearance of oxygen in earth’s atmosphere.[170] Several copper proteins, such as the «blue copper proteins», do not interact directly with substrates; hence they are not enzymes. These proteins relay electrons by the process called electron transfer.[169]
Photosynthesis functions by an elaborate electron transport chain within the thylakoid membrane. A central link in this chain is plastocyanin, a blue copper protein.
A unique tetranuclear copper center has been found in nitrous-oxide reductase.[171]
Chemical compounds which were developed for treatment of Wilson’s disease have been investigated for use in cancer therapy.[172]
Nutrition
Copper is an essential trace element in plants and animals, but not all microorganisms. The human body contains copper at a level of about 1.4 to 2.1 mg per kg of body mass.[173]
Absorption
Copper is absorbed in the gut, then transported to the liver bound to albumin.[174] After processing in the liver, copper is distributed to other tissues in a second phase, which involves the protein ceruloplasmin, carrying the majority of copper in blood. Ceruloplasmin also carries the copper that is excreted in milk, and is particularly well-absorbed as a copper source.[175] Copper in the body normally undergoes enterohepatic circulation (about 5 mg a day, vs. about 1 mg per day absorbed in the diet and excreted from the body), and the body is able to excrete some excess copper, if needed, via bile, which carries some copper out of the liver that is not then reabsorbed by the intestine.[176][177]
Dietary recommendations
The U.S. Institute of Medicine (IOM) updated the estimated average requirements (EARs) and recommended dietary allowances (RDAs) for copper in 2001. If there is not sufficient information to establish EARs and RDAs, an estimate designated Adequate Intake (AI) is used instead. The AIs for copper are: 200 μg of copper for 0–6-month-old males and females, and 220 μg of copper for 7–12-month-old males and females. For both sexes, the RDAs for copper are: 340 μg of copper for 1–3 years old, 440 μg of copper for 4–8 years old, 700 μg of copper for 9–13 years old, 890 μg of copper for 14–18 years old and 900 μg of copper for ages 19 years and older. For pregnancy, 1,000 μg. For lactation, 1,300 μg.[178] As for safety, the IOM also sets tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. In the case of copper the UL is set at 10 mg/day. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes.[179]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women and men ages 18 and older the AIs are set at 1.3 and 1.6 mg/day, respectively. AIs for pregnancy and lactation is 1.5 mg/day. For children ages 1–17 years the AIs increase with age from 0.7 to 1.3 mg/day. These AIs are higher than the U.S. RDAs.[180] The European Food Safety Authority reviewed the same safety question and set its UL at 5 mg/day, which is half the U.S. value.[181]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For copper labeling purposes 100% of the Daily Value was 2.0 mg, but as of May 27, 2016 it was revised to 0.9 mg to bring it into agreement with the RDA.[182][183] A table of the old and new adult daily values is provided at Reference Daily Intake.
Deficiency
Because of its role in facilitating iron uptake, copper deficiency can produce anemia-like symptoms, neutropenia, bone abnormalities, hypopigmentation, impaired growth, increased incidence of infections, osteoporosis, hyperthyroidism, and abnormalities in glucose and cholesterol metabolism. Conversely, Wilson’s disease causes an accumulation of copper in body tissues.
Severe deficiency can be found by testing for low plasma or serum copper levels, low ceruloplasmin, and low red blood cell superoxide dismutase levels; these are not sensitive to marginal copper status. The «cytochrome c oxidase activity of leucocytes and platelets» has been stated as another factor in deficiency, but the results have not been confirmed by replication.[184]
Toxicity
Gram quantities of various copper salts have been taken in suicide attempts and produced acute copper toxicity in humans, possibly due to redox cycling and the generation of reactive oxygen species that damage DNA.[185][186] Corresponding amounts of copper salts (30 mg/kg) are toxic in animals.[187] A minimum dietary value for healthy growth in rabbits has been reported to be at least 3 ppm in the diet.[188] However, higher concentrations of copper (100 ppm, 200 ppm, or 500 ppm) in the diet of rabbits may favorably influence feed conversion efficiency, growth rates, and carcass dressing percentages.[189]
Chronic copper toxicity does not normally occur in humans because of transport systems that regulate absorption and excretion. Autosomal recessive mutations in copper transport proteins can disable these systems, leading to Wilson’s disease with copper accumulation and cirrhosis of the liver in persons who have inherited two defective genes.[173]
Elevated copper levels have also been linked to worsening symptoms of Alzheimer’s disease.[190][191]
Human exposure
In the US, the Occupational Safety and Health Administration (OSHA) has designated a permissible exposure limit (PEL) for copper dust and fumes in the workplace as a time-weighted average (TWA) of 1 mg/m3.[192] The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 1 mg/m3, time-weighted average. The IDLH (immediately dangerous to life and health) value is 100 mg/m3.[193]
Copper is a constituent of tobacco smoke.[194][195] The tobacco plant readily absorbs and accumulates heavy metals, such as copper from the surrounding soil into its leaves. These are readily absorbed into the user’s body following smoke inhalation.[196] The health implications are not clear.[197]
See also
- Copper in renewable energy
- Copper nanoparticle
- Erosion corrosion of copper water tubes
- Cold water pitting of copper tube
- List of countries by copper production
- Metal theft
- Operation Tremor
- Anaconda Copper
- Antofagasta PLC
- Codelco
- El Boleo mine
- Grasberg mine
References
- ^ «Standard Atomic Weights: Copper». CIAAW. 1969.
- ^ Moret, Marc-Etienne; Zhang, Limei; Peters, Jonas C. (2013). «A Polar Copper–Boron One-Electron σ-Bond». J. Am. Chem. Soc. 135 (10): 3792–3795. doi:10.1021/ja4006578. PMID 23418750.
- ^ Lide, D. R., ed. (2005). «Magnetic susceptibility of the elements and inorganic compounds». CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5. Archived from the original (PDF) on 3 March 2011.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Robert McHenry, ed. (1992). «Bronze». The New Encyclopædia Britannica. Vol. 3 (15 ed.). Chicago: Encyclopædia Britannica, Incorporated. p. 612. ISBN 978-0-85229-553-3. OCLC 25228234.
- ^ «Copper». Merriam-Webster Dictionary. 2018. Retrieved 22 August 2018.
- ^ Johnson, MD PhD, Larry E., ed. (2008). «Copper». Merck Manual Home Health Handbook. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Archived from the original on 7 March 2016. Retrieved 7 April 2013.
- ^ «Copper in human health».
- ^ a b Trigg, George L.; Immergut, Edmund H. (1992). Encyclopedia of Applied Physics. Vol. 4: Combustion to Diamagnetism. VCH. pp. 267–272. ISBN 978-3-527-28126-8. Retrieved 2 May 2011.
- ^ Smith, William F. & Hashemi, Javad (2003). Foundations of Materials Science and Engineering. McGraw-Hill Professional. p. 223. ISBN 978-0-07-292194-6.
- ^ a b c Hammond, C. R. (2004). The Elements, in Handbook of Chemistry and Physics (81st ed.). CRC Press. ISBN 978-0-8493-0485-9.
- ^ Resistance Welding Manufacturing Alliance (2003). Resistance Welding Manual (4th ed.). Resistance Welding Manufacturing Alliance. pp. 18–12. ISBN 978-0-9624382-0-2.
- ^ Chambers, William; Chambers, Robert (1884). Chambers’s Information for the People. Vol. L (5th ed.). W. & R. Chambers. p. 312. ISBN 978-0-665-46912-1.
- ^ Ramachandran, Harishankar (14 March 2007). «Why is Copper Red?» (PDF). IIT Madras. Retrieved 27 December 2022.
- ^ «Galvanic Corrosion». Corrosion Doctors. Retrieved 29 April 2011.
- ^ a b Grieken, Rene van; Janssens, Koen (2005). Cultural Heritage Conservation and Environmental Impact Assessment by Non-Destructive Testing and Micro-Analysis. CRC Press. p. 197. ISBN 978-0-203-97078-2.
- ^ «Copper.org: Education: Statue of Liberty: Reclothing the First Lady of Metals – Repair Concerns». Copper.org. Retrieved 11 April 2011.
- ^ Rickett, B. I.; Payer, J. H. (1995). «Composition of Copper Tarnish Products Formed in Moist Air with Trace Levels of Pollutant Gas: Hydrogen Sulfide and Sulfur Dioxide/Hydrogen Sulfide». Journal of the Electrochemical Society. 142 (11): 3723–3728. Bibcode:1995JElS..142.3723R. doi:10.1149/1.2048404.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), «The NUBASE evaluation of nuclear and decay properties», Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729….3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ «Interactive Chart of Nuclides». National Nuclear Data Center. Archived from the original on 25 August 2013. Retrieved 8 April 2011.
- ^ Okazawad, Hidehiko; Yonekura, Yoshiharu; Fujibayashi, Yasuhisa; Nishizawa, Sadahiko; Magata, Yasuhiro; Ishizu, Koichi; Tanaka, Fumiko; Tsuchida, Tatsuro; Tamaki, Nagara; Konishi, Junji (1994). «Clinical Application and Quantitative Evaluation of Generator-Produced Copper-62-PTSM as a Brain Perfusion Tracer for PET» (PDF). Journal of Nuclear Medicine. 35 (12): 1910–1915. PMID 7989968.
- ^ Romano, Donatella; Matteucci, Fransesca (2007). «Contrasting copper evolution in ω Centauri and the Milky Way». Monthly Notices of the Royal Astronomical Society: Letters. 378 (1): L59–L63. arXiv:astro-ph/0703760. Bibcode:2007MNRAS.378L..59R. doi:10.1111/j.1745-3933.2007.00320.x. S2CID 14595800.
- ^ a b c d Emsley, John (2003). Nature’s building blocks: an A–Z guide to the elements. Oxford University Press. pp. 121–125. ISBN 978-0-19-850340-8. Retrieved 2 May 2011.
- ^ Rickwood, P. C. (1981). «The largest crystals» (PDF). American Mineralogist. 66: 885.
- ^ Emsley, John (2003). Nature’s building blocks: an A–Z guide to the elements. Oxford University Press. pp. 124, 231, 449, 503. ISBN 978-0-19-850340-8. Retrieved 2 May 2011.
- ^ Rieuwerts, John (2015). The Elements of Environmental Pollution. London and New York: Earthscan Routledge. p. 207. ISBN 978-0-415-85919-6. OCLC 886492996.
- ^ Randazzo, Ryan (19 June 2011). «A new method to harvest copper». Azcentral.com. Retrieved 25 April 2014.
- ^ Gordon, R.B.; Bertram, M.; Graedel, T.E. (2006). «Metal stocks and sustainability». Proceedings of the National Academy of Sciences. 103 (5): 1209–1214. Bibcode:2006PNAS..103.1209G. doi:10.1073/pnas.0509498103. PMC 1360560. PMID 16432205.
- ^ Beaudoin, Yannick C.; Baker, Elaine (December 2013). Deep Sea Minerals: Manganese Nodules, a physical, biological, environmental and technical review. Secretariat of the Pacific Community. pp. 7–18. ISBN 978-82-7701-119-6. Retrieved 8 February 2021.
- ^ a b Leonard, Andrew (3 March 2006). «Peak copper?». Salon. Retrieved 8 March 2022.
- ^ Brown, Lester (2006). Plan B 2.0: Rescuing a Planet Under Stress and a Civilization in Trouble. New York: W.W. Norton. p. 109. ISBN 978-0-393-32831-8.
- ^ Schmitz, Christopher (1986). «The Rise of Big Business in the World, Copper Industry 1870–1930». Economic History Review. 2. 39 (3): 392–410. doi:10.1111/j.1468-0289.1986.tb00411.x. JSTOR 2596347.
- ^ «Copper Trends: Live Metal Spot Prices». Archived from the original on 1 May 2012.
- ^ Ackerman, R. (2 April 2009). «A Bottom in Sight For Copper». Forbes. Archived from the original on 8 December 2012.
- ^ Employment Appeal Tribunal, AEI Cables Ltd. v GMB and others, 5 April 2013, accessed 5 February 2021
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Su, Kun; Ma, Xiaodong; Parianos, John; Zhao, Baojun (2020). «Thermodynamic and Experimental Study on Efficient Extraction of Valuable Metals from Polymetallic Nodules». Minerals. 10 (4): 360. Bibcode:2020Mine…10..360S. doi:10.3390/min10040360.
- ^ International Seabed Authority. «Polymetallic Nodules» (PDF). International Seabed Authority. Retrieved 8 February 2021.
- ^ Watling, H.R. (2006). «The bioleaching of sulphide minerals with emphasis on copper sulphides – A review» (PDF). Hydrometallurgy. 84 (1): 81–108. doi:10.1016/j.hydromet.2006.05.001. Archived from the original (PDF) on 18 August 2011.
- ^ Samans, Carl (1949). Engineering metals and their alloys. New York: Macmillan. OCLC 716492542.
- ^ Burton, Julie McCulloch (2015). Pen to Paper: Making Fun of Life. iUniverse. ISBN 978-1-4917-5394-1.
- ^ Bahadir, Ali Mufit; Duca, Gheorghe (2009). The Role of Ecological Chemistry in Pollution Research and Sustainable Development. Springer. ISBN 978-90-481-2903-4.
- ^ Green, Dan (2016). The Periodic Table in Minutes. Quercus. ISBN 978-1-68144-329-4.
- ^ «International Copper Association». Archived from the original on 5 March 2012. Retrieved 22 July 2009.
- ^ «Overview of Recycled Copper» Copper.org. (25 August 2010). Retrieved on 8 November 2011.
- ^ «Dime». US Mint. Retrieved 9 July 2019.[permanent dead link]
- ^ «Pride and skill – the 10-cent coin». Royal Canadian Mint. Retrieved 9 July 2019.
- ^ «Gold Jewellery Alloys». World Gold Council. Archived from the original on 14 April 2009. Retrieved 6 June 2009.
- ^ Balver Zinn Solder Sn97Cu3 Archived 7 July 2011 at the Wayback Machine. (PDF) . balverzinn.com. Retrieved on 8 November 2011.
- ^ Deane, D. V. «Modern Coinage Systems» (PDF). British Numismatic Society. Retrieved 1 July 2019.
- ^ «What is 90% Silver?». American Precious Metals Exchange (APMEX). Archived from the original on 28 July 2020. Retrieved 1 July 2019.
- ^ Corrosion Tests and Standards. ASTM International. 2005. p. 368.
- ^ Oguchi, Hachiro (1983). «Japanese Shakudō: its history, properties and production from gold-containing alloys». Gold Bulletin. 16 (4): 125–132. doi:10.1007/BF03214636.
- ^ a b c Holleman, A.F.; Wiberg, N. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- ^ Trammell, Rachel; Rajabimoghadam, Khashayar; Garcia-Bosch, Isaac (30 January 2019). «Copper-Promoted Functionalization of Organic Molecules: from Biologically Relevant Cu/O2 Model Systems to Organometallic Transformations». Chemical Reviews. 119 (4): 2954–3031. doi:10.1021/acs.chemrev.8b00368. PMC 6571019. PMID 30698952.
- ^ a b Wiley-Vch (2 April 2007). «Nonsystematic (Contact) Fungicides». Ullmann’s Agrochemicals. p. 623. ISBN 978-3-527-31604-5.
- ^ Ralph L. Shriner, Christine K.F. Hermann, Terence C. Morrill, David Y. Curtin, Reynold C. Fuson «The Systematic Identification of Organic Compounds» 8th edition, J. Wiley, Hoboken. ISBN 0-471-21503-1
- ^ Saalwächter, Kay; Burchard, Walther; Klüfers, Peter; Kettenbach, G.; Mayer, Peter; Klemm, Dieter; Dugarmaa, Saran (2000). «Cellulose Solutions in Water Containing Metal Complexes». Macromolecules. 33 (11): 4094–4107. Bibcode:2000MaMol..33.4094S. CiteSeerX 10.1.1.951.5219. doi:10.1021/ma991893m.
- ^ Deodhar, S., Huckaby, J., Delahoussaye, M. and DeCoster, M.A., 2014, August. High-aspect ratio bio-metallic nanocomposites for cellular interactions. In IOP Conference Series: Materials Science and Engineering (Vol. 64, No. 1, p. 012014). https://iopscience.iop.org/article/10.1088/1757-899X/64/1/012014/meta.
- ^ Kelly, K.C., Wasserman, J.R., Deodhar, S., Huckaby, J. and DeCoster, M.A., 2015. Generation of scalable, metallic high-aspect ratio nanocomposites in a biological liquid medium. JoVE (Journal of Visualized Experiments), (101), p.e52901. https://www.jove.com/t/52901/generation-scalable-metallic-high-aspect-ratio-nanocomposites.
- ^ Karan, A., Darder, M., Kansakar, U., Norcross, Z. and DeCoster, M.A., 2018. Integration of a Copper-Containing Biohybrid (CuHARS) with Cellulose for Subsequent Degradation and Biomedical Control. International journal of environmental research and public health, 15(5), p.844. https://www.mdpi.com/1660-4601/15/5/844
- ^ «Modern Organocopper Chemistry» Norbert Krause, Ed., Wiley-VCH, Weinheim, 2002. ISBN 978-3-527-29773-3.
- ^ Berná, José; Goldup, Stephen; Lee, Ai-Lan; Leigh, David; Symes, Mark; Teobaldi, Gilberto; Zerbetto, Fransesco (26 May 2008). «Cadiot–Chodkiewicz Active Template Synthesis of Rotaxanes and Switchable Molecular Shuttles with Weak Intercomponent Interactions». Angewandte Chemie. 120 (23): 4464–4468. Bibcode:2008AngCh.120.4464B. doi:10.1002/ange.200800891.
- ^ Rafael Chinchilla & Carmen Nájera (2007). «The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry». Chemical Reviews. 107 (3): 874–922. doi:10.1021/cr050992x. PMID 17305399.
- ^ «An Addition of an Ethylcopper Complex to 1-Octyne: (E)-5-Ethyl-1,4-Undecadiene» (PDF). Organic Syntheses. 64: 1. 1986. doi:10.15227/orgsyn.064.0001. Archived from the original (PDF) on 19 June 2012.
- ^ Kharasch, M.S.; Tawney, P.O. (1941). «Factors Determining the Course and Mechanisms of Grignard Reactions. II. The Effect of Metallic Compounds on the Reaction between Isophorone and Methylmagnesium Bromide». Journal of the American Chemical Society. 63 (9): 2308–2316. doi:10.1021/ja01854a005.
- ^ Imai, Sadako; Fujisawa, Kiyoshi; Kobayashi, Takako; Shirasawa, Nobuhiko; Fujii, Hiroshi; Yoshimura, Tetsuhiko; Kitajima, Nobumasa; Moro-oka, Yoshihiko (1998). «63Cu NMR Study of Copper(I) Carbonyl Complexes with Various Hydrotris(pyrazolyl)borates: Correlation between 63Cu Chemical Shifts and CO Stretching Vibrations». Inorganic Chemistry. 37 (12): 3066–3070. doi:10.1021/ic970138r.
- ^ G. Brauer, ed. (1963). «Potassium Cuprate (III)». Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). NY: Academic Press. p. 1015.
- ^ Schwesinger, Reinhard; Link, Reinhard; Wenzl, Peter; Kossek, Sebastian (2006). «Anhydrous phosphazenium fluorides as sources for extremely reactive fluoride ions in solution». Chemistry: A European Journal. 12 (2): 438–45. doi:10.1002/chem.200500838. PMID 16196062.
- ^ Lewis, E.A.; Tolman, W.B. (2004). «Reactivity of Dioxygen-Copper Systems». Chemical Reviews. 104 (2): 1047–1076. doi:10.1021/cr020633r. PMID 14871149.
- ^ McDonald, M.R.; Fredericks, F.C.; Margerum, D.W. (1997). «Characterization of Copper(III)–Tetrapeptide Complexes with Histidine as the Third Residue». Inorganic Chemistry. 36 (14): 3119–3124. doi:10.1021/ic9608713. PMID 11669966.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1187. ISBN 978-0-08-037941-8.
- ^ A Timeline of Copper Technologies, Copper Development Association, https://www.copper.org/education/history/timeline/
- ^ a b «CSA – Discovery Guides, A Brief History of Copper». Csa.com. Archived from the original on 3 February 2015. Retrieved 12 September 2008.
- ^ Rayner W. Hesse (2007). Jewelrymaking through History: an Encyclopedia. Greenwood Publishing Group. p. 56. ISBN 978-0-313-33507-5.No primary source is given in that book.
- ^ «Copper». Elements.vanderkrogt.net. Retrieved 12 September 2008.
- ^ Renfrew, Colin (1990). Before civilization: the radiocarbon revolution and prehistoric Europe. Penguin. ISBN 978-0-14-013642-5. Retrieved 21 December 2011.
- ^ Cowen, R. «Essays on Geology, History, and People: Chapter 3: Fire and Metals». Retrieved 7 July 2009.
- ^ Timberlake, S. & Prag A.J.N.W. (2005). The Archaeology of Alderley Edge: Survey, excavation and experiment in an ancient mining landscape. Oxford: John and Erica Hedges Ltd. p. 396. doi:10.30861/9781841717159. ISBN 9781841717159.
- ^ a b «CSA – Discovery Guides, A Brief History of Copper». CSA Discovery Guides. Archived from the original on 3 February 2015. Retrieved 29 April 2011.
- ^ Pleger, Thomas C. «A Brief Introduction to the Old Copper Complex of the Western Great Lakes: 4000–1000 BC», Proceedings of the Twenty-Seventh Annual Meeting of the Forest History Association of Wisconsin, Oconto, Wisconsin, 5 October 2002, pp. 10–18.
- ^ Emerson, Thomas E. and McElrath, Dale L. Archaic Societies: Diversity and Complexity Across the Midcontinent, SUNY Press, 2009 ISBN 1-4384-2701-8.
- ^ Dainian, Fan. Chinese Studies in the History and Philosophy of Science and Technology. p. 228.
- ^ Wallach, Joel. Epigenetics: The Death of the Genetic Theory of Disease Transmission.
- ^ Radivojević, Miljana; Rehren, Thilo (December 2013). «Tainted ores and the rise of tin bronzes in Eurasia, c. 6500 years ago». Antiquity Publications Ltd.
- ^ a b McNeil, Ian (2002). Encyclopaedia of the History of Technology. London; New York: Routledge. pp. 13, 48–66. ISBN 978-0-203-19211-5.
- ^ Rickard, T.A. (1932). «The Nomenclature of Copper and its Alloys». Journal of the Royal Anthropological Institute. 62: 281–290. doi:10.2307/2843960. JSTOR 2843960.
- ^ Timberlake, Simon (11 June 2017). «New ideas on the exploitation of copper, tin, gold, and lead ores in Bronze Age Britain: The mining, smelting, and movement of metal». Materials and Manufacturing Processes. 32 (7–8): 709–727. doi:10.1080/10426914.2016.1221113. S2CID 138178474.
- ^ Martin, Susan R. (1995). «The State of Our Knowledge About Ancient Copper Mining in Michigan». The Michigan Archaeologist. 41 (2–3): 119. Archived from the original on 7 February 2016.
- ^ Hong, S.; Candelone, J.-P.; Patterson, C.C.; Boutron, C.F. (1996). «History of Ancient Copper Smelting Pollution During Roman and Medieval Times Recorded in Greenland Ice». Science. 272 (5259): 246–249 (247f.). Bibcode:1996Sci…272..246H. doi:10.1126/science.272.5259.246. S2CID 176767223.
- ^ de Callataÿ, François (2005). «The Graeco-Roman Economy in the Super Long-Run: Lead, Copper, and Shipwrecks». Journal of Roman Archaeology. 18: 361–372 (366–369). doi:10.1017/S104775940000742X. S2CID 232346123.
- ^ Savenije, Tom J.; Warman, John M.; Barentsen, Helma M.; van Dijk, Marinus; Zuilhof, Han; Sudhölter, Ernst J.R. (2000). «Corinthian Bronze and the Gold of the Alchemists» (PDF). Macromolecules. 33 (2): 60–66. Bibcode:2000MaMol..33…60S. doi:10.1021/ma9904870. Archived from the original (PDF) on 29 September 2007.
- ^ Lynch, Martin (2004). Mining in World History. p. 60. ISBN 978-1-86189-173-0.
- ^ «Gold: prices, facts, figures and research: A brief history of money». Retrieved 22 April 2011.
- ^ «Copper and Brass in Ships». Retrieved 6 September 2016.
- ^ Stelter, M.; Bombach, H. (2004). «Process Optimization in Copper Electrorefining». Advanced Engineering Materials. 6 (7): 558–562. doi:10.1002/adem.200400403. S2CID 138550311.
- ^ Gardner, E. D.; et al. (1938). Copper Mining in North America. Washington, D. C.: U. S. Bureau of Mines. Retrieved 19 March 2019.
- ^ Hyde, Charles (1998). Copper for America, the United States Copper Industry from Colonial Times to the 1990s. Tucson, Arizona: University of Arizona Press. p. passim. ISBN 0-8165-1817-3.
- ^ «Outokumpu Flash Smelting» (PDF). Outokumpu. p. 2. Archived from the original (PDF) on 24 July 2011.
- ^ Karen A. Mingst (1976). «Cooperation or illusion: an examination of the intergovernmental council of copper exporting countries». International Organization. 30 (2): 263–287. doi:10.1017/S0020818300018270. S2CID 154183817.
- ^ Ryck Lydecker. «Is Copper Bottom Paint Sinking?». BoatUS Magazine. Retrieved 3 June 2016.
- ^ «Copper». American Elements. 2008. Archived from the original on 8 June 2008. Retrieved 12 July 2008.
- ^ Pops, Horace, 2008, «Processing of wire from antiquity to the future», Wire Journal International, June, pp. 58–66
- ^ The Metallurgy of Copper Wire, http://www.litz-wire.com/pdf%20files/Metallurgy_Copper_Wire.pdf Archived 1 September 2013 at the Wayback Machine
- ^ Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, pp. 141–192 and pp. 331–375.
- ^ «Copper, Chemical Element – Overview, Discovery and naming, Physical properties, Chemical properties, Occurrence in nature, Isotopes». Chemistryexplained.com. Retrieved 16 October 2012.
- ^ Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, p.348
- ^ «Aluminum Wiring Hazards and Pre-Purchase Inspections». www.heimer.com. Archived from the original on 28 May 2016. Retrieved 3 June 2016.
- ^ «Accelerator: Waveguides (SLAC VVC)». SLAC Virtual Visitor Center. Retrieved 29 April 2011.
- ^ IE3 energy-saving motors, Engineer Live, http://www.engineerlive.com/Design-Engineer/Motors_and_Drives/IE3_energy-saving_motors/22687/
- ^ Energy‐efficiency policy opportunities for electric motor‐driven systems, International Energy Agency, 2011 Working Paper in the Energy Efficiency Series, by Paul Waide and Conrad U. Brunner, OECD/IEA 2011
- ^ Fuchsloch, J. and E.F. Brush, (2007), «Systematic Design Approach for a New Series of Ultra‐NEMA Premium Copper Rotor Motors», in EEMODS 2007 Conference Proceedings, 10–15 June, Beijing.
- ^ Copper motor rotor project; Copper Development Association; «Copper.org: Copper Motor Rotor Project». Archived from the original on 13 March 2012. Retrieved 7 November 2012.
- ^ NEMA Premium Motors, The Association of Electrical Equipment and Medical Imaging Manufacturers; «NEMA — NEMA Premium Motors». Archived from the original on 2 April 2010. Retrieved 12 October 2009.
- ^ International Energy Agency, IEA sees renewable energy growth accelerating over next 5 years, http://www.iea.org/newsroomandevents/pressreleases/2012/july/name,28200,en.html
- ^ Global trends in renewable energy investment 2012, by REN21 (Renewable Energy Policy Network for the 21st Century); http://www.ren21.net/gsr
- ^ Will the Transition to Renewable Energy Be Paved in Copper?, Renewable Energy World; Jan 15, 2016; https://www.renewableenergyworld.com/articles/2016/01/will-the-transition-to-renewable-energy-be-paved-in-copper.html Archived 2018-06-22 at the Wayback Machine
- ^ García-Olivares, Antonio, Joaquim Ballabrera-Poy, Emili García-Ladona, and Antonio Turiel. A global renewable mix with proven technologies and common materials, Energy Policy, 41 (2012): 561-57, http://imedea.uib-csic.es/master/cambioglobal/Modulo_I_cod101601/Ballabrera_Diciembre_2011/Articulos/Garcia-Olivares.2011.pdf
- ^ A kilo more of copper increases environmental performance by 100 to 1,000 times; Renewable Energy Magazine; April 14, 2011; http://www.renewableenergymagazine.com/article/a-kilo-more-of-copper-increases-environmental
- ^ Copper at the core of renewable energies; European Copper Institute; European Copper Institute; 18 pages; http://www.eurocopper.org/files/presskit/press_kit_copper_in_renewables_final_29_10_2008.pdf Archived 2012-05-23 at the Wayback Machine
- ^ Copper in energy systems; Copper Development Association Inc.; http://www.copper.org/environment/green/energy.html
- ^ The Rise Of Solar: A Unique Opportunity For Copper; Solar Industry Magazine; April 2017; Zolaika Strong; https://issues.solarindustrymag.com/article/rise-solar-unique-opportunity-copper
- ^ Pops, Horace, 1995. Physical Metallurgy of Electrical Conductors, in Nonferrous Wire Handbook, Volume 3: Principles and Practice, The Wire Association International
- ^ The World Copper Factbook, 2017; http://www.icsg.org/index.php/component/jdownloads/finish/170/2462
- ^ Copper Mineral Commodity Summary (USGS, 2017) https://minerals.usgs.gov/minerals/pubs/commodity/copper/ mcs-2017-coppe.pdf
- ^ Global Mineral Resource Assessment (USGS, 2014) http://pubs.usgs.gov/fs/2014/3004/pdf/fs2014-3004.pdf
- ^ Long-Term Availability of Copper; International Copper Association; http://copperalliance.org/wordpress/wp-content/uploads/2018/02/ICA-long-term-availability-201802-A4-HR.pdf Archived 2018-06-29 at the Wayback Machine
- ^ Will the Transition to Renewable Energy Be Paved in Copper?, Renewable Energy World; Jan 15, 2016; by Zolaikha Strong; https://www.renewableenergyworld.com/articles/2016/01/will-the-transition-to-renewable-energy-be-paved-in-copper.html Archived 2018-06-22 at the Wayback Machine
- ^ Seale, Wayne (2007). The role of copper, brass, and bronze in architecture and design; Metal Architecture, May 2007
- ^ Copper roofing in detail; Copper in Architecture; Copper Development Association, U.K., www.cda.org.uk/arch
- ^ Architecture, European Copper Institute; http://eurocopper.org/copper/copper-architecture.html Archived 9 October 2012 at the Wayback Machine
- ^ Kronborg completed; Agency for Palaces and Cultural Properties, København, «Kronborg completed — Agency for Palaces and Cultural Properties». Archived from the original on 24 October 2012. Retrieved 12 September 2012.
- ^ Berg, Jan. «Why did we paint the library’s roof?». Archived from the original on 25 June 2007. Retrieved 20 September 2007.
- ^ Architectural considerations; Copper in Architecture Design Handbook, http://www.copper.org/applications/architecture/arch_dhb/fundamentals/arch_considerations.htm[permanent dead link]
- ^ Peters, Larry E. (2004). Preventing corrosion on copper roofing systems; Professional Roofing, October 2004, http://www.professionalroofing.net
- ^ Oxidation Reaction: Why is the Statue of Liberty Blue-Green? Engage Students in Engineering; www.EngageEngineering.org; Chun Wu, PhD, Mount Marty College; Funded by the National Science Foundation (NSF) under Grant No. 083306. «Archived copy» (PDF). Archived from the original (PDF) on 25 October 2013. Retrieved 25 October 2013.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Fitzgerald, K.P.; Nairn, J.; Atrens, A. (1998). «The chemistry of copper patination». Corrosion Science. 40 (12): 2029–50. doi:10.1016/S0010-938X(98)00093-6.
- ^ Application Areas: Architecture – Finishes – patina; http://www.copper.org/applications/architecture/finishes.html
- ^ Glossary of copper terms, Copper Development Association (UK): «Glossary of copper terms». Archived from the original on 20 August 2012. Retrieved 14 September 2012.
- ^ Finishes – natural weathering; Copper in Architecture Design Handbook, Copper Development Association Inc., «Copper.org: Architecture Design Handbook: Finishes». Archived from the original on 16 October 2012. Retrieved 12 September 2012.
- ^ Davis, Joseph R. (2001). Copper and Copper Alloys. ASM International. pp. 3–6, 266. ISBN 978-0-87170-726-0.
- ^ Edding, Mario E., Flores, Hector, and Miranda, Claudio, (1995), Experimental Usage of Copper-Nickel Alloy Mesh in Mariculture. Part 1: Feasibility of usage in a temperate zone; Part 2: Demonstration of usage in a cold zone; Final report to the International Copper Association Ltd.
- ^ Corrosion Behaviour of Copper Alloys used in Marine Aquaculture Archived 24 September 2013 at the Wayback Machine. (PDF) . copper.org. Retrieved on 8 November 2011.
- ^ Copper Touch Surfaces Archived 23 July 2012 at the Wayback Machine. Copper Touch Surfaces. Retrieved on 8 November 2011.
- ^ «EPA Registers Copper Surfaces for Residual Use Against Coronavirus». United States Environmental Protection Agency. 10 February 2021. Retrieved 11 October 2021.
- ^ Montero, David A.; Arellano, Carolina; Pardo, Mirka; Vera, Rosa; Gálvez, Ricardo; Cifuentes, Marcela; Berasain, María A.; Gómez, Marisol; Ramírez, Claudio; Vidal, Roberto M. (5 January 2019). «Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities». Antimicrobial Resistance and Infection Control. 8 (1): 3. doi:10.1186/s13756-018-0456-4. ISSN 2047-2994. PMC 6321648. PMID 30627427.
- ^ a b «EPA registers copper-containing alloy products». United States Environmental Protection Agency. May 2008. Archived from the original on 29 September 2015.
- ^ Biurrun, Amaya; Caballero, Luis; Pelaz, Carmen; León, Elena; Gago, Alberto (1999). «Treatment of a Legionella pneumophila‐Colonized Water Distribution System Using Copper‐Silver Ionization and Continuous Chlorination» (PDF). Infection Control and Hospital Epidemiology. 20 (6): 426–428. doi:10.1086/501645. JSTOR 30141645. PMID 10395146. S2CID 32388649. Archived from the original (PDF) on 17 February 2019.
- ^ Zaleski, Andrew, As hospitals look to prevent infections, a chorus of researchers make a case for copper surfaces, STAT, 24 September 2020
- ^ Chilean subway protected with Antimicrobial Copper – Rail News from Archived 24 July 2012 at the Wayback Machine. rail.co. Retrieved on 8 November 2011.
- ^ Codelco to provide antimicrobial copper for new metro lines (Chile)[dead link]. Construpages.com.ve. Retrieved on 8 November 2011.
- ^ PR 811 Chilean Subway Installs Antimicrobial Copper Archived 23 November 2011 at the Wayback Machine. (PDF). antimicrobialcopper.com. Retrieved on 8 November 2011.
- ^ «Copper and Cupron». Cupron.
- ^ «Global copper market under supplied, demand on the rise – report». Mining.com. 6 January 2019. Retrieved 13 January 2019.
- ^ «Will the Transition to Renewable Energy Be Paved in Copper?». www.renewableenergyworld.com. 15 January 2015. Archived from the original on 22 June 2018. Retrieved 13 January 2019.
- ^ «Copper and cars: Boom goes beyond electric vehicles». MINING.com. 18 June 2018. Retrieved 13 January 2019.
- ^ «Impact of electric cars in medium-term copper demand ‘overrated’, experts say». MINING.com. 12 April 2018. Retrieved 13 January 2019.
- ^ «Why are Premiums for Copper Bullion So High?». Provident Metals. 20 August 2012. Retrieved 23 January 2019.
- ^ Chace, Zoe. «Penny Hoarders Hope for the Day The Penny Dies». NPR.org. NPR. Retrieved 23 January 2019.
- ^ Walker, W.R.; Keats, D.M. (1976). «An investigation of the therapeutic value of the ‘copper bracelet’-dermal assimilation of copper in arthritic/rheumatoid conditions». Agents and Actions. 6 (4): 454–459. PMID 961545.
- ^ Richmond SJ, Gunadasa S, Bland M, Macpherson H (2013). «Copper bracelets and magnetic wrist straps for rheumatoid arthritis – analgesic and anti-inflammatory effects: a randomised double-blind placebo controlled crossover trial». PLOS ONE. 8 (9): e71529. Bibcode:2013PLoSO…871529R. doi:10.1371/journal.pone.0071529. PMC 3774818. PMID 24066023.
- ^ Richmond, Stewart J.; Brown, Sally R.; Campion, Peter D.; Porter, Amanda J.L.; Moffett, Jennifer A. Klaber; Jackson, David A.; Featherstone, Valerie A.; Taylor, Andrew J. (2009). «Therapeutic effects of magnetic and copper bracelets in osteoarthritis: A randomised placebo-controlled crossover trial». Complementary Therapies in Medicine. 17 (5–6): 249–256. doi:10.1016/j.ctim.2009.07.002. ISSN 0965-2299. PMID 19942103.
- ^ University of Arkansas for Medical Sciences:
Find the Truth Behind Medical Myths Archived 6 January 2014 at the Wayback MachineWhile it’s never been proven that copper can be absorbed through the skin by wearing a bracelet, research has shown that excessive copper can result in poisoning, causing vomiting and, in severe cases, liver damage.
- ^
Truth in Advertising
Tommie Copper - ^ a b Geoffrey Michael Gadd (March 2010). «Metals, minerals and microbes: geomicrobiology and bioremediation». Microbiology. 156 (3): 609–643. doi:10.1099/mic.0.037143-0. PMID 20019082.
- ^ Harbhajan Singh (2006). Mycoremediation: Fungal Bioremediation. p. 509. ISBN 978-0-470-05058-3.
- ^ Vest, Katherine E.; Hashemi, Hayaa F.; Cobine, Paul A. (2013). «Chapter 13 The Copper Metallome in Eukaryotic Cells». In Banci, Lucia (ed.). Metallomics and the Cell. Metal Ions in Life Sciences. Vol. 12. Springer. pp. 451–78. doi:10.1007/978-94-007-5561-1_13. ISBN 978-94-007-5560-4. PMID 23595680. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ «Fun facts». Horseshoe crab. University of Delaware. Archived from the original on 22 October 2008. Retrieved 13 July 2008.
- ^ a b S.J. Lippard, J.M. Berg «Principles of bioinorganic chemistry» University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- ^ Decker, H. & Terwilliger, N. (2000). «COPs and Robbers: Putative evolution of copper oxygen-binding proteins». Journal of Experimental Biology. 203 (Pt 12): 1777–1782. doi:10.1242/jeb.203.12.1777. PMID 10821735.
- ^
Schneider, Lisa K.; Wüst, Anja; Pomowski, Anja; Zhang, Lin; Einsle, Oliver (2014). «Chapter 8. No Laughing Matter: The Unmaking of the Greenhouse Gas Dinitrogen Monoxide by Nitrous Oxide Reductase«. In Peter M.H. Kroneck; Martha E. Sosa Torres (eds.). The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. Vol. 14. Springer. pp. 177–210. doi:10.1007/978-94-017-9269-1_8. ISBN 978-94-017-9268-4. PMID 25416395. - ^ Denoyer, Delphine; Clatworthy, Sharnel A.S.; Cater, Michael A. (2018). «Chapter 16. Copper Complexes in Cancer Therapy». In Sigel, Astrid; Sigel, Helmut; Freisinger, Eva; Sigel, Roland K.O. (eds.). Metallo-Drugs: Development and Action of Anticancer Agents. Metal Ions in Life Sciences. Vol. 18. Berlin: de Gruyter GmbH. pp. 469–506. doi:10.1515/9783110470734-022. ISBN 978-3-11-047073-4. PMID 29394035.
- ^ a b «Amount of copper in the normal human body, and other nutritional copper facts». Archived from the original on 10 April 2009. Retrieved 3 April 2009.
- ^ Adelstein, S. J.; Vallee, B. L. (1961). «Copper metabolism in man». New England Journal of Medicine. 265 (18): 892–897. doi:10.1056/NEJM196111022651806. PMID 13859394.
- ^ M.C. Linder; Wooten, L.; Cerveza, P.; Cotton, S.; Shulze, R.; Lomeli, N. (1 May 1998). «Copper transport». The American Journal of Clinical Nutrition. 67 (5): 965S–971S. doi:10.1093/ajcn/67.5.965S. PMID 9587137.
- ^ Frieden, E.; Hsieh, H.S. (1976). Ceruloplasmin: The copper transport protein with essential oxidase activity. Advances in Enzymology – and Related Areas of Molecular Biology. Vol. 44. pp. 187–236. doi:10.1002/9780470122891.ch6. ISBN 978-0-470-12289-1. JSTOR 20170553. PMID 775938.
- ^ S.S. Percival; Harris, E.D. (1 January 1990). «Copper transport from ceruloplasmin: Characterization of the cellular uptake mechanism». American Journal of Physiology. Cell Physiology. 258 (1): C140–C146. doi:10.1152/ajpcell.1990.258.1.c140. PMID 2301561.
- ^ Dietary Reference Intakes: RDA and AI for Vitamins and Elements Archived 13 November 2018 at the Wayback Machine Food and Nutrition Board, Institute of Medicine, National Academies Press, 2011. Retrieved 18 April 2018.
- ^ Copper. IN: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Copper. National Academy Press. 2001, PP. 224–257.
- ^ «Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies» (PDF). 2017.
- ^ Tolerable Upper Intake Levels For Vitamins And Minerals (PDF), European Food Safety Authority, 2006
- ^ «Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR p. 33982» (PDF).
- ^ «Daily Value Reference of the Dietary Supplement Label Database (DSLD)». Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- ^ Bonham, Maxine; O’Connor, Jacqueline M.; Hannigan, Bernadette M.; Strain, J.J. (2002). «The immune system as a physiological indicator of marginal copper status?». British Journal of Nutrition. 87 (5): 393–403. doi:10.1079/BJN2002558. PMID 12010579.
- ^ Li, Yunbo; Trush, Michael; Yager, James (1994). «DNA damage caused by reactive oxygen species originating from a copper-dependent oxidation of the 2-hydroxy catechol of estradiol». Carcinogenesis. 15 (7): 1421–1427. doi:10.1093/carcin/15.7.1421. PMID 8033320.
- ^ Gordon, Starkebaum; John, M. Harlan (April 1986). «Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine». J. Clin. Invest. 77 (4): 1370–6. doi:10.1172/JCI112442. PMC 424498. PMID 3514679.
- ^ «Pesticide Information Profile for Copper Sulfate». Cornell University. Retrieved 10 July 2008.
- ^ Hunt, Charles E. & William W. Carlton (1965). «Cardiovascular Lesions Associated with Experimental Copper Deficiency in the Rabbit». Journal of Nutrition. 87 (4): 385–394. doi:10.1093/jn/87.4.385. PMID 5841854.
- ^ Ayyat M.S.; Marai I.F.M.; Alazab A.M. (1995). «Copper-Protein Nutrition of New Zealand White Rabbits under Egyptian Conditions». World Rabbit Science. 3 (3): 113–118. doi:10.4995/wrs.1995.249.
- ^ Brewer GJ (March 2012). «Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease». BioFactors (Review). 38 (2): 107–113. doi:10.1002/biof.1005. hdl:2027.42/90519. PMID 22438177. S2CID 16989047.
- ^ «Copper: Alzheimer’s Disease». Examine.com. Retrieved 21 June 2015.
- ^ NIOSH Pocket Guide to Chemical Hazards. «#0151». National Institute for Occupational Safety and Health (NIOSH).
- ^ NIOSH Pocket Guide to Chemical Hazards. «#0150». National Institute for Occupational Safety and Health (NIOSH).
- ^ OEHHA Copper
- ^ Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). «Hazardous Compounds in Tobacco Smoke». International Journal of Environmental Research and Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. ISSN 1660-4601. PMC 3084482. PMID 21556207.
- ^ Pourkhabbaz, A.; Pourkhabbaz, H. (2012). «Investigation of Toxic Metals in the Tobacco of Different Iranian Cigarette Brands and Related Health Issues». Iranian Journal of Basic Medical Sciences. 15 (1): 636–644. PMC 3586865. PMID 23493960.
- ^ Bernhard, David; Rossmann, Andrea; Wick, Georg (2005). «Metals in cigarette smoke». IUBMB Life. 57 (12): 805–809. doi:10.1080/15216540500459667. PMID 16393783. S2CID 35694266.
Notes
|
|
|
|
|
| in pure water, or acidic or alkali conditions. Copper in neutral water is more noble than hydrogen. | in water containing sulfide | in 10 M ammonia solution | in a chloride solution |
Further reading
- Massaro, Edward J., ed. (2002). Handbook of Copper Pharmacology and Toxicology. Humana Press. ISBN 978-0-89603-943-8.
- «Copper: Technology & Competitiveness (Summary) Chapter 6: Copper Production Technology» (PDF). Office of Technology Assessment. 2005.
- Current Medicinal Chemistry, Volume 12, Number 10, May 2005, pp. 1161–1208(48) Metals, Toxicity and Oxidative Stress
- William D. Callister (2003). Materials Science and Engineering: an Introduction (6th ed.). Wiley, New York. Table 6.1, p. 137. ISBN 978-0-471-73696-7.
- Material: Copper (Cu), bulk, MEMS and Nanotechnology Clearinghouse.
- Kim BE; Nevitt T; Thiele DJ (2008). «Mechanisms for copper acquisition, distribution and regulation». Nat. Chem. Biol. 4 (3): 176–85. doi:10.1038/nchembio.72. PMID 18277979.
External links
Wikiquote has quotations related to Copper.
Wikimedia Commons has media related to Copper.
Look up copper in Wiktionary, the free dictionary.
Wikisource has original text related to this article:
- Copper at The Periodic Table of Videos (University of Nottingham)
- Copper and compounds fact sheet from the National Pollutant Inventory of Australia
- Copper.org – official website of the Copper Development Association with an extensive site of properties and uses of copper
- Price history of copper, according to the IMF
Медь в таблице менделеева занимает 29 место, в 4 периоде.
| Символ | Cu |
| Номер | 29 |
| Атомный вес | 63.5460000 |
| Латинское название | Cuprum |
| Русское название | Медь |
Как самостоятельно построить электронную конфигурацию? Ответ здесь
Электронная схема меди
Cu: 1s2 2s2 2p6 3s2 3p6 4s1 3d10
Короткая запись:
Cu: [Ar]4s1 3d10
Такое же количество электронов как и атом меди имеют:
Ni-1, Zn+1, Ga+2, Ge+3
Порядок заполнения оболочек атома меди (Cu) электронами:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d →
5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
На подуровне ‘s’ может находиться до 2 электронов, на ‘s’ — до 6, на
‘d’ — до 10 и на ‘f’ до 14
Медь имеет 29 электронов,
заполним электронные оболочки в описанном выше порядке:
Элемент Cu является исключением!
2 электрона на 1s-подуровне
2 электрона на 2s-подуровне
6 электронов на 2p-подуровне
2 электрона на 3s-подуровне
6 электронов на 3p-подуровне
1 электрон на 4s-подуровне
10 электронов на 3d-подуровне
Степень окисления меди
Атомы меди в соединениях имеют степени окисления 4, 3, 2, 1, 0.
Степень окисления — это условный заряд атома в соединении: связь в молекуле
между атомами основана на разделении электронов, таким образом, если у атома виртуально увеличивается
заряд, то степень окисления отрицательная (электроны несут отрицательный заряд), если заряд уменьшается,
то степень окисления положительная.
Ионы меди
Валентность Cu
Атомы меди в соединениях проявляют валентность IV, III, II, I.
Валентность меди характеризует способность атома Cu к образованию хмических связей.
Валентность следует из строения электронной оболочки атома, электроны, участвующие в образовании
химических соединений называются валентными электронами. Более обширное определение валентности это:
Число химических связей, которыми данный атом соединён с другими атомами
Валентность не имеет знака.
Квантовые числа Cu
Квантовые числа определяются последним электроном в конфигурации,
для атома Cu эти числа имеют значение N = 3, L = 2, Ml = 2, Ms = -½
Видео заполнения электронной конфигурации (gif):
Результат:
Энергия ионизации
Чем ближе электрон к центру атома — тем больше энергии необходимо, что бы его оторвать.
Энергия, затрачиваемая на отрыв электрона от атома называется энергией ионизации и обозначается Eo.
Если не указано иное, то энергия ионизации — это энергия отрыва первого электрона, также существуют энергии
ионизации для каждого последующего электрона.
Энергия ионизации Cu:
Eo = 746 кДж/моль
— Что такое ион читайте в статье.
Перейти к другим элементам таблицы менделеева
Где Cu в таблице менделеева?
Таблица Менделеева
Скачать таблицу менделеева в хорошем качестве
Медь
1. Положение меди в периодической системе химических элементов
2. Электронное строение меди
3. Физические свойства
4. Нахождение в природе
5. Способы получения
6. Качественные реакции
7. Химические свойства
Оксид меди (II)
- Способы получения
- Химические свойства
Оксид меди (I)
- Химические свойства
Гидроксид меди (II)
- Химические свойства
Соли меди
Медь
Положение в периодической системе химических элементов
Медь расположена в 11 группе (или в побочной подгруппе II группы в короткопериодной ПСХЭ) и в четвертом периоде периодической системы химических элементов Д.И. Менделеева.
Электронное строение меди
Электронная конфигурация меди в основном состоянии:
+29Cu 1s22s22p63s23p63d104s1 1s 2s
2p
3s 3p
4s
3d
У атома меди уже в основном энергетическом состоянии происходит провал (проскок) электрона с 4s-подуровня на 3d-подуровень.
Физические свойства
Медь – твердый металл золотисто-розового цвета (розового цвета при отсутствии оксидной плёнки). Медь относительно легко поддается механической обработке. В природе встречается в том числе в чистом виде и широко применяется в различных отраслях науки, техники и производства.
Изображение с портала zen.yandex.com/media/id/5d426107ae56cc00ad977411/uralskaia-boginia-liubvi-5d6bcceda660d700b075a12d
Температура плавления 1083,4оС, температура кипения 2567оС, плотность меди 8,92 г/см3.
Медь — ценный металл в сфере вторичной переработки. Сдав лом меди в пункт приема, Вы можете получить хорошее денежное вознаграждение. Подробнее про прием лома меди.
Нахождение в природе
Медь встречается в земной коре (0,0047-0,0055 масс.%), в речной и морской воде. В природе медь встречается как в соединениях, так и в самородном виде. В промышленности используют халькопирит CuFeS2, также известный как медный колчедан, халькозин Cu2S и борнит Cu5FeS4. Также распространены и другие минералы меди: ковеллин CuS, куприт Cu2O, азурит Cu3(CO3)2(OH)2, малахит Cu2(OH)2CO3. Иногда медь встречается в самородном виде, масса которых может достигать 400 тонн.
Способы получения меди
Медь получают из медных руд и минералов. Основные методы получения меди — электролиз, пирометаллургический и гидрометаллургический.
- Гидрометаллургический метод: растворение медных минералов в разбавленных растворах серной кислоты, с последующим вытеснением металлическим железом.
Например, вытеснение меди из сульфата железом:
CuSO4 + Fe = Cu + FeSO4
Видеоопыт взаимодействия сульфата меди (II) с железом можно посмотреть здесь.
- Пирометаллургический метод: получение меди из сульфидных руд. Это сложный процесс, который включает большое количество реакций. Основные стадии процесса:
1) Обжиг сульфидов:
2CuS + 3O2 = 2CuO + 2SO2
2) восстановление меди из оксида, например, водородом:
CuO + H2 = Cu + H2O
- Электролиз растворов солей меди:
2CuSO4 + 2H2O → 2Cu + O2 + 2H2SO4
Качественные реакции на ионы меди (II)
Качественная реакция на ионы меди +2 – взаимодействие солей меди (II) с щелочами. При этом образуется голубой осадок гидроксида меди(II).
Например, сульфат меди (II) взаимодействует с гидроксидом натрия:
CuSO4 + 2NaOH → Cu(OH)2 + Na2SO4
Соли меди (II) окрашивают пламя в зеленый цвет.
Химические свойства меди
В соединениях медь может проявлять степени окисления +1 и +2.
1. Медь — химически малоактивный металл. При нагревании медь может реагировать с некоторыми неметаллами: кислородом, серой, галогенами.
1.1. При нагревании медь реагирует с достаточно сильными окислителями, например, с кислородом, образуя CuО, Cu2О в зависимости от условий:
4Cu + О2 → 2Cu2О
2Cu + О2 → 2CuО
1.2. Медь реагирует с серой с образованием сульфида меди (II):
Cu + S → CuS
Видеоопыт взаимодействия меди с серой можно посмотреть здесь.
1.3. Медь взаимодействует с галогенами. При этом образуются галогениды меди (II):
Cu + Cl2 = CuCl2
Сu + Br2 = CuBr2
Но, обратите внимание:
2Cu + I2 = 2CuI
Видеоопыт взаимодействия меди с хлором можно посмотреть здесь.
1.4. С азотом, углеродом и кремнием медь не реагирует:
Cu + N2 ≠
Cu + C ≠
Cu + Si ≠
1.5. Медь не взаимодействует с водородом.
Cu + H2 ≠
1.6. Медь взаимодействует с кислородом с образованием оксида:
2Cu + O2 → 2CuO
2. Медь взаимодействует и со сложными веществами:
2.1. Медь в сухом воздухе и при комнатной температуре не окисляется, но во влажном воздухе, в присутствии оксида углерода (IV) покрывается зеленым налетом карбоната гидроксомеди (II):
2Cu + H2O + CO2 + O2 = (CuOH)2CO3
2.2. В ряду напряжений медь находится правее водорода и поэтому не может вытеснить водород из растворов минеральных кислот (разбавленной серной кислоты и др.).
Например, медь не реагирует с разбавленной серной кислотой:
Cu + H2SO4 (разб.) ≠
Видеоопыт взаимодействия меди с соляной кислотой можно посмотреть здесь.
2.3. При этом медь реагирует при нагревании с концентрированной серной кислотой. При нагревании реакция идет, образуются оксид серы (IV), сульфат меди (II) и вода:
Cu + 2H2SO4(конц.) → CuSO4 + SO2 + 2H2O
2.4. Медь реагирует даже при обычных условиях с азотной кислотой.
С концентрированной азотной кислотой:
Cu + 4HNO3(конц.) = Cu(NO3)2 + 2NO2 + 2H2O
С разбавленной азотной кислотой:
3Cu + 8HNO3(разб.) = 3Cu(NO3)2 + 2NO + 4H2O
Реакция меди с азотной кислотой
2.5. Растворы щелочей на медь практически не действуют.
2.6. Медь вытесняет металлы, стоящие правее в ряду напряжений, из растворов их солей.
Например, медь реагирует с нитратом ртути (II) с образованием нитрата меди (II) и ртути:
Hg(NO3)2 + Cu = Cu(NO3)2 + Hg
2.7. Медь окисляется оксидом азота (IV) и солями железа (III)
2Cu + NO2 = Cu2O + NO
2FeCl3 + Cu = 2FeCl2 + CuCl2
Оксид меди (II)
Оксид меди (II) CuO – твердое кристаллическое вещество черного цвета.
Способы получения оксида меди (II)
Оксид меди (II) можно получить различными методами:
1. Термическим разложением гидроксида меди (II) при 200°С:
Cu(OH)2 → CuO + H2O
2. В лаборатории оксид меди (II) получают окислением меди при нагревании на воздухе при 400–500°С:
2Cu + O2 2CuO
3. В лаборатории оксид меди (II) также получают прокаливанием солей (CuOH)2CO3, Cu(NO3)2:
(CuOH)2CO3 2CuO + CO2 + H2O
2Cu(NO3)2 2CuO + 4NO2 + O2
Химические свойства оксида меди (II)
Оксид меди (II) – основный оксид (при этом у него есть слабо выраженные амфотерные свойства). При этом он является довольно сильным окислителем.
1. При взаимодействии оксида меди (II) с сильными и растворимыми кислотами образуются соли.
Например, оксид меди (II) взаимодействует с соляной кислотой:
СuO + 2HBr = CuBr2 + H2O
CuO + 2HCl = CuCl2 + H2O
Видеоопыт взаимодействия оксида меди (II) с серной кислотой можно посмотреть здесь.
2. Оксид меди (II) вступает в реакцию с кислотными оксидами.
Например, оксид меди (II) взаимодействует с оксидом серы (VI) с образованием сульфата меди (II):
CuO + SO3 → CuSO4
3. Оксид меди (II) не взаимодействует с водой.
4. В окислительно-восстановительных реакциях соединения меди (II) проявляют окислительные свойства:
Например, оксид меди (II) окисляет аммиак:
3CuO + 2NH3 → 3Cu + N2 + 3H2O
Оксид меди (II) можно восстановить углеродом, водородом или угарным газом при нагревании:
СuO + C → Cu + CO
Видеоопыт взаимодействия оксида меди (II) с водородом можно посмотреть здесь.
Более активные металлы вытесняют медь из оксида.
Например, алюминий восстанавливает оксид меди (II):
3CuO + 2Al = 3Cu + Al2O3
Оксид меди (I)
Оксид меди (I) Cu2O – твердое кристаллическое вещество коричнево-красного цвета.
Способы получения оксида меди (I)
В лаборатории оксид меди (I) получают восстановлением свежеосажденного гидроксида меди (II), например, альдегидами или глюкозой:
CH3CHO + 2Cu(OH)2 → CH3COOH + Cu2O↓ + 2H2O
CH2ОН(CHOН)4СНО + 2Cu(OH)2 → CH2ОН(CHOН)4СООН + Cu2O↓ + 2H2O
Химические свойства оксида меди (I)
1. Оксид меди (I) обладает основными свойствами.
При действии на оксид меди (I) галогеноводородных кислот получают галогениды меди (I) и воду:
Например, соляная кислота с оксидом меди (I) образует хлорид меди (I):
Cu2O + 2HCl = 2CuCl↓ + H2O
2. При растворении Cu2O в концентрированной серной, азотной кислотах образуются только соли меди (II):
Cu2O + 3H2SO4(конц.) = 2CuSO4 + SO2 + 3H2O
Cu2O + 6HNO3(конц.) = 2Cu(NO3)2 + 2NO2 + 3H2O
5Cu2O + 13H2SO4 + 2KMnO4 = 10CuSO4 + 2MnSO4 + K2SO4 + 13H2O
3. Устойчивыми соединениями меди (I) являются нерастворимые соединения (CuCl, Cu2S) или комплексные соединения [Cu(NH3)2]+. Последние получают растворением в концентрированном растворе аммиака оксида меди (I), хлорида меди (I):
Cu2O + 4NH3 + H2O = 2[Cu(NH3)2]OH
CuCl + 2NH3 = [Cu(NH3)2]Cl
Аммиачные растворы солей меди (I) взаимодействуют с ацетиленом:
СH ≡ CH + 2[Cu(NH3)2]Cl → СuC ≡ CCu + 2NH4Cl + 2NH3
4. В окислительно-восстановительных реакциях соединения меди (I) проявляют окислительно-восстановительную двойственность:
Например, при взаимодействии с угарным газом, более активными металлами или водородом оксид меди (II) проявляет свойства окислителя:
Cu2O + CO = 2Cu + CO2
Cu2O + H2 = 2Cu + H2O
3Cu2O + 2Al = 6Cu + Al2O3
А под действием окислителей, например, кислорода — свойства восстановителя:
2Cu2O + O2 = 4CuO
Гидроксид меди (II)
Способы получения гидроксида меди (II)
1. Гидроксид меди (II) можно получить действием раствора щелочи на соли меди (II).
Например, хлорид меди (II) реагирует с водным раствором гидроксида натрия с образованием гидроксида меди (II) и хлорида натрия:
CuCl2 + 2NaOH → Cu(OH)2 + 2NaCl
Химические свойства
Гидроксид меди (II) Сu(OН)2 проявляет слабо выраженные амфотерные свойства (с преобладанием основных).
1. Взаимодействует с кислотами.
Например, взаимодействует с бромоводородной кислотой с образованием бромида меди (II) и воды:
Сu(OН)2 + 2HBr = CuBr2 + 2H2O
Cu(OН)2 + 2HCl = CuCl2 + 2H2O
2. Гидроксид меди (II) легко взаимодействует с раствором аммиака, образуя сине-фиолетовое комплексное соединение:
Сu(OH)2 + 4(NH3 · H2O) = [Cu(NH3)4](OH)2 + 4H2O
Cu(OH)2 + 4NH3 = [Cu(NH3)4](OH)2
3. При взаимодействии гидроксида меди (II) с концентрированными (более 40%) растворами щелочей образуется комплексное соединение:
Cu(OH)2 + 2NaOH(конц.) = Na2[Cu(OH)4]
Но этой реакции в ЕГЭ по химии пока нет!
4. При нагревании гидроксид меди (II) разлагается:
Сu(OH)2 → CuO + H2O
Соли меди
Соли меди (I)
В окислительно-восстановительных реакциях соединения меди (I) проявляют окислительно-восстановительную двойственность. Как восстановители они реагируют с окислителями.
Например, хлорид меди (I) окисляется концентрированной азотной кислотой:
CuCl + 3HNO3(конц.) = Cu(NO3)2 + HCl + NO2 + H2O
Также хлорид меди (I) реагирует с хлором:
2CuCl + Cl2 = 2CuCl2
Хлорид меди (I) окисляется кислородом в присутствии соляной кислоты:
4CuCl + O2 + 4HCl = 4CuCl2 + 2H2O
Прочие галогениды меди (I) также легко окисляются другими сильными окислителями:
2CuI + 4H2SO4 + 2MnO2 = 2CuSO4 + 2MnSO4 + I2 + 4H2O
Иодид меди (I) реагирует с концентрированной серной кислотой:
4CuI + 5H2SO4(конц.гор.) = 4CuSO4 + 2I2 + H2S + 4H2O
Сульфид меди (I) реагирует с азотной кислотой. При этом образуются различные продукты окисления серы на холоде и при нагревании:
Cu2S + 8HNO3(конц.хол.) = 2Cu(NO3)2 + S + 4NO2 + 4H2O
Cu2S + 12HNO3(конц.гор.) = Cu(NO3)2 + CuSO4 + 10NO2 + 6H2O
Для соединений меди (I) возможна реакция диспропорционирования:
2CuCl = Cu + CuCl2
Комплексные соединения типа [Cu(NH3)2]+ получают растворением в концентрированном растворе аммиака:
CuCl + 3NH3 + H2O → [Cu(NH3)2]OH + NH4Cl
Соли меди (II)
В окислительно-восстановительных реакциях соединения меди (II) проявляют окислительные свойства.
Например, соли меди (II) окисляют иодиды и сульфиты:
2CuCl2 + 4KI = 2CuI + I2 + 4KCl
2CuCl2 + Na2SO3 + 2NaOH = 2CuCl + Na2SO4 + 2NaCl + H2O
Бромиды и иодиды меди (II) можно окислить перманганатом калия:
5CuBr2 + 2KMnO4 + 8H2SO4 = 5CuSO4 + K2SO4 + 2MnSO4 + 5Br2 + 8H2O
Соли меди (II) также окисляют сульфиты:
2CuSO4 + Na2SO3 + 2H2O = Cu2O + Na2SO4 + 2H2SO4
Более активные металлы вытесняют медь из солей.
Например, сульфат меди (II) реагирует с железом:
CuSO4 + Fe = FeSO4 + Cu
Cu(NO3)2 + Fe = Fe(NO3)2 + Cu
Сульфид меди (II) можно окислить концентрированной азотной кислотой. При нагревании возможно образование сульфата меди (II):
CuS + 8HNO3(конц.гор.) = CuSO4 + 8NO2 + 4H2O
Еще одна форма этой реакции:
CuS + 10HNO3(конц.) = Cu(NO3)2 + H2SO4 + 8NO2↑ + 4H2O
При горении сульфида меди (II) образуется оксид меди (II) и диоксид серы:
2CuS + 3O2 2CuO + 2SO2↑
Соли меди (II) вступают в обменные реакции, как и все соли.
Например, растворимые соли меди (II) реагируют с сульфидами:
CuBr2 + Na2S = CuS↓ + 2NaBr
При взаимодействии солей меди (II) с щелочами образуется голубой осадок гидроксида меди (II):
CuSO4 + 2NaOH = Cu(OH)2↓ + Na2SO4
Электролиз раствора нитрата меди (II):
2Cu(NO3)2 + 2Н2О → 2Cu + O2 + 4HNO3
Некоторые соли меди при нагревании разлагаются, например, нитрат меди (II):
2Cu(NO3)2 → 2CuO + 4NO2 + O2
Основный карбонат меди разлагается на оксид меди (II), углекислый газ и воду:
(CuOH)2CO3 → 2CuO + CO2 + H2O
При взаимодействии солей меди (II) с избытком аммиака образуются аммиачные комплексы:
CuCl2 + 4NH3 = [Cu(NH3)4]Cl2
При смешивании растворов солей меди (II) и карбонатов происходит гидролиз и по катиону слабого основания, и по аниону слабой кислоты:
2CuSO4 + 2Na2CO3 + H2O = (CuOH)2CO3↓ + 2Na2SO4 + CO2
Медь и соединения меди
1) Через раствор хлорида меди (II) с помощью графитовых электродов пропускали постоянный электрический ток. Выделившийся на катоде продукт электролиза растворили в концентрированной азотной кислоте. Образовавшийся при этом газ собрали и пропустили через раствор гидроксида натрия. Выделившийся на аноде газообразный продукт электролиза пропустили через горячий раствор гидроксида натрия. Напишите уравнения описанных реакций.
2) Вещество, полученное на катоде при электролизе расплава хлорида меди (II), реагирует с серой. Полученный продукт обработали концентрированной азотной кислотой, и выделившийся газ пропустили через раствор гидроксида бария. Напишите уравнения описанных реакций.
3) Неизвестная соль бесцветна и окрашивает пламя в желтый цвет. При легком нагревании этой соли с концентрированной серной кислотой отгоняется жидкость, в которой растворяется медь; последнее превращение сопровождается выделением бурого газа и образованием соли меди. При термическом распаде обеих солей одним из продуктов разложения является кислород. Напишите уравнения описанных реакций.
4) При взаимодействии раствора соли А со щелочью было получено студенистое нерастворимое в воде вещество голубого цвета, которое растворили в бесцветной жидкости Б с образованием раствора синего цвета. Твердый продукт, оставшийся после осторожного выпаривания раствора, прокалили; при этом выделились два газа, один из которых бурого цвета, а второй входит в состав атмосферного воздуха, и осталось твердое вещество черного цвета, которое растворяется в жидкости Б с образованием вещества А. Напишите уравнения описанных реакций.
5) Медную стружку растворили в разбавленной азотной кислоте, и раствор нейтрализовали едким кали. Выделившееся вещество голубого цвета отделили, прокалили (цвет вещества изменился на черный), смешали с коксом и повторно прокалили. Напишите уравнения описанных реакций.
6) В раствор нитрата ртути (II) добавили медную стружку. После окончания реакции раствор профильтровали, и фильтрат по каплям прибавляли к раствору, содержащему едкий натр и гидроксид аммония. При этом наблюдали кратковременное образование осадка, который растворился с образованием раствора ярко-синего цвета. При добавлении в полученный раствор избытка раствора серной кислоты происходило изменение цвета. Напишите уравнения описанных реакций.
7) Оксид меди (I) обработали концентрированной азотной кислотой, раствор осторожно выпарили и твердый остаток прокалили. Газообразные продукты реакции пропустили через большое количество воды и в образовавшийся раствор добавили магниевую стружку, в результате выделился газ, используемый в медицине. Напишите уравнения описанных реакций.

9) Соль, полученную при растворении меди в разбавленной азотной кислоте, подвергли электролизу, используя графитовые электроды. Вещество, выделившееся на аноде, ввели во взаимодействие с натрием, а полученный продукт реакции поместили в сосуд с углекислым газом. Напишите уравнения описанных реакций.
10) Твердый продукт термического разложения малахита растворили при нагревании в концентрированной азотной кислоте. Раствор осторожно выпарили, и твердый остаток прокалили, получив вещество черного цвета, которое нагрели в избытке аммиака (газ). Напишите уравнения описанных реакций.
11) К порошкообразному веществу черного цвета добавили раствор разбавленной серной кислоты и нагрели. В полученный раствор голубого цвета приливали раствор едкого натра до прекращения выделения осадка. Осадок отфильтровали и нагрели. Продукт реакции нагревали в атмосфере водорода, в результате чего получилось вещество красного цвета. Напишите уравнения описанных реакций.
12) Неизвестное вещество красного цвета нагрели в хлоре, и продукт реакции растворили в воде. В полученный раствор добавили щелочь, выпавший осадок голубого цвета отфильтровали и прокалили. При нагревании продукта прокаливании, который имеет черный цвет, с коксом было получено исходное вещество красного цвета. Напишите уравнения описанных реакций.
13) Раствор, полученный при взаимодействии меди с концентрированной азотной кислотой, выпарили и осадок прокалили. Газообразные продукты полностью поглощены водой, а над твердым остатком пропустили водород. Напишите уравнения описанных реакций.
14) Черный порошок, который образовался при сжигании металла красного цвета в избытке воздуха, растворили в 10%-серной кислоте. В полученный раствор добавили щелочь, и выпавший осадок голубого цвета отделили и растворили в избытке раствора аммиака. Напишите уравнения описанных реакций.
15) Вещество черного цвета получили, прокаливая осадок, который образуется при взаимодействии гидроксида натрия и сульфата меди (II). При нагревании этого вещества с углем получают металл красного цвета, который растворяется в концентрированной серной кислоте. Напишите уравнения описанных реакций.
16) Металлическую медь обработали при нагревании йодом. Полученный продукт растворили в концентрированной серной кислоте при нагревании. Образовавшийся раствор обработали раствором гидроксидом калия. Выпавший осадок прокалили. Напишите уравнения описанных реакций.
17) К раствору хлорида меди (II) добавили избыток раствора соды. Выпавший осадок прокалили, а полученный продукт нагрели в атмосфере водорода. Полученный порошок растворили в разбавленной азотной кислоте. Напишите уравнения описанных реакций.
18) Медь растворили в разбавленной азотной кислоте. К полученному раствору добавили избыток раствора аммиака, наблюдая сначала образование осадка, а затем – его полное растворение с образованием темно-синего раствора. Полученный раствор обработали серной кислотой до появления характерной голубой окраски солей меди. Напишите уравнения описанных реакций.
19) Медь растворили в концентрированной азотной кислоте. К полученному раствору добавили избыток раствора аммиака, наблюдая сначала образование осадка, а затем – его полное растворение с образованием темно-синего раствора. Полученный раствор обработали избытком соляной кислоты. Напишите уравнения описанных реакций.
20) Газ, полученный при взаимодействии железных опилок с раствором соляной кислоты, пропустили над нагретым оксидом меди (II) до полного восстановления металла. полученный металл растворили в концентрированной азотной кислоте. Образовавшийся раствор подвергли электролизу с инертными электродами. Напишите уравнения описанных реакций.
21) Йод поместили в пробирку с концентрированной горячей азотной кислотой. Выделившийся газ пропустили через воду в присутствии кислорода. В полученный раствор добавили гидроксид меди (II). Образовавшийся раствор выпарили и сухой твердый остаток прокалили. Напишите уравнения описанных реакций.
22) Оранжевый оксид меди поместили в концентрированную серную кислоту и нагрели. К полученному голубому раствору прилили избыток раствора гидроксида калия. выпавший синий осадок отфильтровали, просушили и прокалили. Полученное при этом твердое черное вещество в стеклянную трубку, нагрели и пропустили над ним аммиак. Напишите уравнения описанных реакций.
23) Оксид меди (II) обработали раствором серной кислоты. При электролизе образующегося раствора на инертном аноде выделяется газ. Газ смешали с оксидом азота (IV) и поглотили с водой. К разбавленному раствору полученной кислоты добавили магний, в результате чего в растворе образовалось две соли, а выделение газообразного продукта не происходило. Напишите уравнения описанных реакций.
24) Оксид меди (II) нагрели в токе угарного газа. Полученное вещество сожгли в атмосфере хлора. Продукт реакции растворили в в воде. Полученный раствор разделили на две части. К одной части добавили раствор иодида калия, ко второй – раствор нитрата серебра. И в том, и в другом случае наблюдали образование осадка. Напишите уравнения описанных реакций.
25) Нитрат меди (II) прокалили, образовавшееся твердое вещество растворили в разбавленной серной кислоте. Раствор полученной соли подвергли электролизу. Выделившееся на катоде вещество растворили в концентрированной азотной кислоте. Растворение протекает с выделением бурого газа. Напишите уравнения описанных реакций.
26) Щавелевую кислоту нагрели с небольшим количеством концентрированной серной кислоты. Выделившийся газ пропустили через раствор гидроксида кальция. В котором выпал осадок. Часть газа не поглотилась, его пропустили над твердым веществом черного цвета, полученным при прокаливании нитрата меди (II). В результате образовалось твердое вещество темно-красного цвета. Напишите уравнения описанных реакций.
27) Концентрированная серная кислота прореагировала с медью. Выделившийся при газ полностью поглотили избытком раствора гидроксида калия. Продукт окисления меди смешали с расчетным количеством гидроксида натрия до прекращения выпадения осадка. Последний растворили в избытке соляной кислоты. Напишите уравнения описанных реакций.
Ответы и решения
1.
CuCl2 Cu + Сl2
на катоде на аноде
Cu + 4HNO3(конц.) = Cu(NO3)2 + 2NO2↑ + 2H2O
2Cu(NO3)2 =2CuO + 4NO2↑ + O2↑
6NaOH(гор.) + 3Cl2 = NaClO3 + 5NaCl + 3H2O
2.
CuCl2 = Cu + Сl2
на катоде на аноде
Cu + S = CuS
CuS + 8HNO3(конц.гор.) = CuSO4 + 8NO2↑ + 4H2O
или CuS + 10HNO3(конц.) = Cu(NO3)2 + H2SO4 + 8NO2↑ + 4H2O
4NO2 + 2Ba(OH)2 = Ba(NO3)2 + Ba(NO2)2 + 2H2O
3.
NaNO3(тв.) + H2SO4(конц.) = HNO3 + NaHSO4
Cu + 4HNO3(конц.) = Cu(NO3)2 + 2NO2↑ + 2H2O
2Cu(NO3)2 = 2CuO + 4NO2↑ + O2↑
2NaNO3 = 2NaNO2 + O2↑
4.
Cu(NO3)2 + 2NaOH = Cu(OH)2↓ + 2NaNO3
Cu(OH)2 + 2HNO3 = Cu(NO3)2 + 2H2O
2Cu(NO3)2 = 2CuO + 4NO2↑ + O2↑
CuO + 2HNO3 = Cu(NO3)2 + H2O
5. 3Cu + 8HNO3(разб.) = 3Cu(NO3)2 + 2NO2↑ + 4H2O
Cu(NO3)2 + 2КOH = Cu(OH)2↓ + 2КNO3
Cu(OH)2 = CuO + H2O
CuO + C Cu + CO
6. Hg(NO3)2 + Cu = Cu(NO3)2 + Hg
Cu(NO3)2 + 2NaOH = Cu(OH)2↓ + 2NaNO3
Сu(OH)2 + 4(NH3 · H2O) = [Cu(NH3)4](OH)2 + 4H2O
[Cu(NH3)4](OH)2 + 5H2SO4 = CuSO4 + 4NH4HSO4 + 2H2O
7. Cu2O + 6HNO3(конц.) = 2Cu(NO3)2 + 2NO2 + 3H2O
2Cu(NO3)2 = 2CuO + 4NO2↑ + O2↑
4NO2 + O2 + 2H2O = 4HNO3
10HNO3 + 4Mg = 4Mg(NO3)2 + N2O + 5H2O
8. (CuOH)2CO3 = 2CuO + CO2 + H2O
CuO + H2 = Cu + H2O
Cu + 2H2SO4(конц.) = CuSO4 + SO2 + 2H2O
CuSO4 + Cu + 2NaCl = 2CuCl↓ + Na2SO4
9.
3Cu + 8HNO3(разб.) = 3Cu(NO3)2 + 2NO2↑ + 4H2O
2Cu(NO3)2 + 2H2O = 2Cu + O2 + 4HNO3
на катоде на аноде
2Na + O2 = Na2O2
2Na2O2 + CO2 = 2Na2CO3 + O2
10.
(CuOH)2CO3 = 2CuO + CO2 + H2O
CuO + 2HNO3 = Cu(NO3)2 + H2O
2Cu(NO3)2 = 2CuO + 4NO2↑ + O2↑
3CuO + 2NH3 = 3Cu + N2 + 3H2O
11.
CuO + H2SO4 = CuSO4 + H2O
CuSO4 + 2NaOH = Cu(OH)2 + Na2SO4
Cu(OH)2 = CuO + H2O
CuO + H2 = Cu + H2O
12.
Cu + Cl2 = CuCl2
CuCl2 + 2NaOH = Cu(OH)2↓ + 2NaCl
Cu(OH)2 = CuO + H2O
CuO + C = Cu + CO
13.
Cu + 4HNO3(конц.) = Cu(NO3)2 + 2NO2↑ + 2H2O
2Cu(NO3)2 = 2CuO + 4NO2↑ + O2↑
4NO2 + O2 + 2H2O = 4HNO3
CuO + H2 = Cu + H2O
14.
2Cu + O2 = 2CuO
CuO + H2SO4 = CuSO4 + H2O
CuSO4 + NaOH = Cu(OH)2↓ + Na2SO4
Сu(OH)2 + 4(NH3 · H2O) = [Cu(NH3)4](OH)2 + 4H2O
15.
СuSO4 + 2NaOH = Cu(OH)2 + Na2SO4
Cu(OH)2 = CuO + H2O
CuO + C = Cu + CO
Cu + 2H2SO4(конц.) = CuSO4 + SO2 + 2H2O
16.
2Cu + I2 = 2CuI
2CuI + 4H2SO4 = 2CuSO4 + I2 + 2SO2 + 4H2O
СuSO4 + 2KOH = Cu(OH)2 + K2SO4
Cu(OH)2 = CuO + H2O
17.
2CuCl2 + 2Na2CO3 + H2O = (CuOH)2CO3 + CO2 + 4NaCl
(CuOH)2CO3 = 2CuO + CO2 + H2O
CuO + H2 = Cu + H2O
3Cu + 8HNO3(разб.) = 3Cu(NO3)2 + 2NO2↑ + 4H2O
18.
3Cu + 8HNO3(разб.) = 3Cu(NO3)2 + 2NO2↑ + 4H2O
Сu(NO3)2 + 2NH3· H2O = Cu(OH)2↓ + 2NH4NO3
Cu(OH)2 + 4NH3· H2O = [Cu(NH3)4](OH)2 + 4H2O
[Cu(NH3)4](OH)2 + 3H2SO4 = CuSO4 + 2(NH4)2SO4 + 2H2O
19) Cu + 4HNO3(конц.) = Cu(NO3)2 + 2NO2↑ + 2H2O
Сu(NO3)2 + 2NH3· H2O = Cu(OH)2↓ + 2NH4NO3
Cu(OH)2 + 4NH3· H2O = [Cu(NH3)4](OH)2 + 4H2O
[Cu(NH3)4](OH)2 + 6HCl = CuCl2 + 4NH4Cl + 2H2O
20.
Fe + 2HCl = FeCl2 + H2
CuO + H2 = Cu + H2O
Cu + 4HNO3(конц.) = Cu(NO3)2 + 2NO2↑ + 2H2O
2Cu(NO3)2 + 2H2O = 2Cu + O2 + 4HNO3
21.
I2 + 10HNO3 = 2HIO3 + 10NO2 + 4H2O
4NO2 + 2H2O + O2 = 4HNO3
Cu(OH)2 + 2HNO3 = Cu(NO3)2 + 2H2O
2Cu(NO3)2 = 2CuO + 4NO2↑ + O2↑
22.
Cu2O + 3H2SO4 = 2CuSO4 + SO2 + 3H2O
СuSO4 + 2KOH = Cu(OH)2 + K2SO4
Cu(OH)2 = CuO + H2O
3CuO + 2NH3 = 3Cu + N2 + 3H2O
23.
CuO + H2SO4 = CuSO4 + H2O
2CuSO4 + 2H2O = 2Cu + O2 + 2H2SO4
4NO2 + O2 + 2H2O = 4HNO3
10HNO3 + 4Mg = 4Mg(NO3)2 + NH4NO3 + 3H2O
24.
CuO + CO = Cu + CO2
Cu + Cl2 = CuCl2
2CuCl2 + 2KI = 2CuCl↓ + I2 + 2KCl
CuCl2 + 2AgNO3 = 2AgCl↓ + Cu(NO3)2
.
25.
2Cu(NO3)2 = 2CuO + 4NO2↑ + O2↑
CuO + H2SO4 = CuSO4 + H2O
2CuSO4 + 2H2O = 2Cu + O2 + 2H2SO4
Cu + 4HNO3(конц.) = Cu(NO3)2 + 2NO2↑ + 2H2O
26.
H2C2O4 = CO↑ + CO2↑ + H2O
CO2 + Ca(OH)2 = CaCO3 + H2O
2Cu(NO3)2 =2CuO + 4NO2↑ + O2↑
CuO + CO = Cu + CO2
27.
Cu + 2H2SO4(конц.) = CuSO4 + SO2 + 2H2O
SO2 + 2KOH = K2SO3 + H2O
СuSO4 + 2NaOH = Cu(OH)2 + Na2SO4
Cu(OH)2 + 2HCl = CuCl2 + 2H2O
Название: Медь (cuprum)
Порядковый номер: 29
Группа: i
Период: 4
Электронное строение: 3d10 4s1
Атомная масса: 63,546
Электроотрицательность: 1,9
Температура плавления: 1083?С
Температура кипения: 2567?С
Плотность (г/см3): 8,96
Характерные степени окисления: +1 +2
Цвет: Красно-коричневый
Кем открыт: неизвестно
Год открытия: до н.э.
Страна открытия: неизвестно
Название: Медь (cuprum)
Порядковый номер: 29
Группа: i
Период: 4
Электронное строение: 3d10 4s1
Атомная масса: 63,546
Электроотрицательность: 1,9
Температура плавления: 1083?С
Температура кипения: 2567?С
Плотность (г/см3): 8,96
Характерные степени окисления: +1 +2
Цвет: Красно-коричневый
Кем открыт: неизвестно
Год открытия: до н.э.
Страна открытия: неизвестно
Общая характеристика меди как элемента
Химический знак – Cu.
Относительная атомная масса – 63,546.
В соединениях медь двухвалентна, степень окисления в соединениях с неметаллами равна +2.
Медь как вещество
Способы получения меди:
- Восстановлением из оксида меди(II) при нагревании:
CuO + H2→ Cu + H2O
- Вытеснением из раствора соли более активным металлом:
CuCl2 + 2Al → 3Cu↓ + 2AlCl3
- Электролизным методом:
2CuSO4 + 2H2O → 2Cu↓ + O2↑ + 2H2SO4
- Гидрометаллургическим методом (растворение минералов меди в разбавленной кислоте, например в серной кислоте, и последующее вытеснение металлическим железом):
CuSO4 + Fe → Cu↓ + FeSO4
Физические свойства меди:
- В чистом виде это вязкий, мягкий и ковкий металл красно-розового цвета.
- Не изменяется на воздухе при отсутствии влаги и углекислого газа. Если же в воздухе медь вступает в реакцию с кислородом, водой и углекислым газом, то образуется непрочная патина зелёного цвета – продукт окисления.
- При нагревании тускнеет (образование оксидной пленки).
- Обладает исключительно хорошей тепло- и электропроводностью.
- Температура плавления равна 1084,5°С, температура кипения равна 2540°С.
Химические свойства меди:
Медь – слабый восстановитель (благородный металл). Переводится в раствор кислотами-неокислителями или гидратом аммиака в присутствии кислорода, цианидом калия.
Реагирует с концентрированной серной и азотной кислотами, «царской водкой», кислородом, галогенами, халькогенами, оксидами неметаллов. При нагревании вступает во взаимодействие с галогеноводородами.
Не реагирует с водой, разбавленной хлороводородной кислотой.
1. Реагирует с концентрированной серной и азотной кислотами:
Cu + H2SO4(хол.) → CuO + SO2↑ + H2O
Cu + 4HNO3 → Cu(NO3)2 + 2NO2↑ + 2H2O
2. Окисляется «царской водкой»:
Cu + 2HNO3 + 6HCl → 3CuCl2 + 2NO↑ + 4H2O
3. Окисляется кислородом при нагревании:
4Cu + O2(недост.)→ 2Cu2O
2Cu + O2(изб.)→ 2CuO
4. При комнатной температуре реагирует с галогенами (хлором и бромом) и серой:
Cu + Cl2 → CuCl2
Cu + S → CuS
5. При нагревании вступает во взаимодействие с оксидами неметаллов:
4Cu + SO2→ Cu2S + 2CuO
Cu + 2N2O4→ Cu(NO3)2 + 2NO↑
Применение меди:
Металлообрабатывающая, машиностроительная, химическая, стекольная, военная промышленности, сельское хозяйство и ряд других отраслей – основные потребители меди.
Медь широко применяется в электротехнике для изготовления силовых и других кабелей, проводов или проводников. Применяют и в различных теплоотводных и теплообменных устройствах: радиаторах охлаждения, кондиционирования и отопления, компьютерных кулерах. В ювелирном деле часто используются сплавы меди с золотом для увеличения прочности изделий к деформациям и истиранию. Медь широко применяется как катализатор, как бактерицидное средство и в архитектурном деле.
Источники
1. https://ru.wikipedia.org/wiki.
2. Лидин, Р. А. Химические свойства неорганических веществ / Р. А. Лидин, В. А. Молочко, Л. Л. Андреева. – М.: Химия, 2000.
3. Таубе П. Р. От водорода до … / П. Р. Таубе, Е. И. Руденко. – М.: Высшая школа, 1968.
История меди
Медь называют одним из первых металлов, которые человек освоил в древности и пользуется им до сегодняшнего дня. Добыча меди была доступной, потому что руду необходимо было плавить при сравнительно невысокой температуре. Первой рудой, из которой стали добывать медь, была малахитовая руда (calorizator). Каменный век в истории человечества сменился именно медным, когда предметы быта, орудия труда и оружие из меди получили самое широкое распространение.
Общая характеристика меди
Медь является элементом XI группы IV периода периодической системы химических элементов Д.И. Менделеева, имеет атомный номер 29 и атомную массу 63,546. Принятое обозначение – Cu (от латинского Cuprum).
Нахождение в природе
Медь достаточно широко представлена в земной коре, в осадочных породах, в водах морских и пресных водоёмах, в сланцах. Распространена как в виде соединений, так и в самостоятельном варианте.
Физические и химические свойства
Медь является пластичным, так называемым переходным металлом, имеет золотисто-розовый цвет. При контакте с воздухом на поверхности меди образуется оксидная плёнка, придающая металлу желтовато-красный оттенок. Известны основные сплавы меди – с цинком (латунь), с оловом (бронза), с никелем (мельхиор).
Суточная потребность в меди
Потребность в меди у взрослого человека составляет 2 мг в день (около 0,035 мг/ 1 кг веса).
Продукты питания богатые медью
Медь – один из самых важных микроэлементов для организма, поэтому продукты питания, богатые медью, должны быть в рационе каждого. Это:
- орехи, злаки, бобовые
- рыба, морепродукты
- крупы (особенно рис и гречка), макаронные изделия, ржаной хлеб
- говяжья печень, сырой яичный желток
- кисломолочные продукты
- овощи, фрукты, ягоды и зелень
- питьевая вода.
Признаки нехватки меди
Признаками недостаточного количества меди в организме служат: анемия и ухудшение дыхания, потеря аппетита, расстройства желудка, нервозность, депрессивные состояния, быстрая утомляемость, нарушения пигментации кожи и волос, ломкость и выпадение волос, сыпи на кожных покровах, частые инфекции. Возможны внутренние кровотечения.
Признаки избытка меди
Переизбыток меди характеризуется бессонницей, нарушениями мозговой активности, эпилепсией, проблемами с менструальным циклом.
Взаимодействия с другими
Предполагается, что медь и цинк конкурируют друг с другом в процессе усваивания в пищеварительном тракте, поэтому избыток одного из этих элементов в пище может вызвать недостаток другого элемента.
Применение меди в жизни
Медь имеет огромное значение в народном хозяйстве, её основное применение – электротехника, но металл широко используется для чеканки монет, часто – в произведениях искусства. Медь также используется в медицине, архитектуре и строительстве.
Полезные свойства меди и его влияние на организм
Требуется для превращения железа организма в гемоглобин. Делает возможным использование аминокислоты тирозин, позволяя ей проявлять свое действие как фактору пигментации волос и кожи. После усваивания меди кишечником она транспортируется к печени с помощью альбумина. Медь также участвует в процессах роста и размножения. Принимает участие в образовании коллагена и эластина и синтезе эндорфинов – гормонов «счастья».
Автор: Виктория Н. (специально для Calorizator.ru)
Копирование данной статьи целиком или частично запрещено.
Медь — элемент побочной подгруппы первой группы, четвертого периода периодической системы химических элементов Д. И. Менделеева, с атомным номером 29. Обозначается символом Cu (лат. Cuprum).
Атомный номер — 29
Атомная масса — 63,546
Плотность, кг/м³ — 8960
Температура плавления, °С — 1083
Теплоемкость, кДж/(кг·°С) — 0,385
Электроотрицательность — 1,9
Ковалентный радиус, Å — 1,17
1-й ионизац. потенциал, эв — 7,73
Медь встречается в природе как в соединениях, так и в самородном виде. Промышленное значение имеют халькопирит CuFeS2, также известный как медный колчедан, халькозин Cu2S и борнит Cu5FeS4. Вместе с ними встречаются и другие минералы меди: ковеллин CuS, куприт Cu2O, азурит Cu3(CO3)2(OH)2, малахит Cu2CO3(OH)2. Иногда медь встречается в самородном виде, масса отдельных скоплений может достигать 400 тонн. Сульфиды меди образуются в основном в среднетемпературных гидротермальных жилах. Также нередко встречаются месторождения меди в осадочных породах — медистые песчаники и сланцы. Наиболее известные из месторождений такого типа — Удокан в Читинской области, Джезказган в Казахстане, меденосный пояс Центральной Африки и Мансфельд в Германии.
Большая часть медной руды добывается открытым способом. Содержание меди в руде составляет от 0,4 до 1,0 %.
Физические свойства меди
Медь — золотисто-розовый пластичный металл, на воздухе быстро покрывается оксидной плёнкой, которая придаёт ей характерный интенсивный желтовато-красный оттенок. Медь обладает высокой тепло- и электропроводностью (занимает второе место по электропроводности после серебра). Имеет два стабильных изотопа — 63Cu и 65Cu, и несколько радиоактивных изотопов. Самый долгоживущий из них, 64Cu, имеет период полураспада 12,7 ч и два варианта распада с различными продуктами.
Цвет Меди красный, в изломе розовый, при просвечивании в тонких слоях зеленовато-голубой. Металл имеет гранецентрированную кубическую решетку с параметром а = 3,6074 Å; плотность 8,96 г/см3 (20 °С). Атомный радиус 1,28 Å; ионные радиусы Cu+ 0,98 Å; Сu2+ 0,80 Å; tпл1083 °С; tкип 2600 °С; удельная теплоемкость (при 20 °С) 385,48 дж/(кг·К), т.е. 0,092 кал/(г·°С). Наиболее важные и широко используемые свойства Меди: высокая теплопроводность — при 20 °С 394,279 вт/(м·К.), то есть 0,941 кал/(см·сек·°С); малое электрическое сопротивление — при 20 °С 1,68·10-8 ом·м. Термический коэффициент линейного расширения 17,0·10-6. Давление паров над Медью ничтожно, давление 133,322 н/м2 (т.е. 1 мм рт.ст.) достигается лишь при 1628 °С. Медь диамагнитна; атомная магнитная восприимчивость 5,27·10-6. Твердость Меди по Бринеллю 350 Мн/м2 (т. е. 35 кгс/мм2); предел прочности при растяжении 220 Мн/м2 (т. е. 22 кгс/мм2); относительное удлинение 60%, модуль упругости 132·103 Мн/м2(т.е. 13,2·103 кгс/мм2). Путем наклепа предел прочности может быть повышен до 400-450 Мн/м2, при этом удлинение уменьшается до 2% , а электропроводность уменьшается на 1-3.
Химические элементы: названия, символы и произношение символов
В таблице содержатся русские и латинские названия химических элементов, символы химических элементов и произношение символов. Для правильного произношения названий и символов в русских названиях и произношениях проставлены ударения.
| Русское название элемента | Латинское название элемента | Символ элемента | Произношение символа |
|---|---|---|---|
| Азо́т | Nitrogenium | N | эн |
| Акти́ний | Actinium | Ac | акти́ний |
| Алюми́ний | Aluminium | Al | алюми́ний |
| Амери́ций | Americium | Am | амери́ций |
| Арго́н | Argon | Ar | арго́н |
| Аста́т | Astatum | At | аста́т |
| Ба́рий | Barium | Ba | ба́рий |
| Бери́ллий | Beryllium | Be | бери́ллий |
| Бе́рклий | Berkelium | Bk | бе́рклий |
| Бор | Borum | B | бор |
| Бо́рий | Bohrium | Bh | бо́рий |
| Бром | Bromium | Br | бром |
| Вана́дий | Vanadium | V | вана́дий |
| Ви́смут | Bismuthum | Bi | ви́смут |
| Водоро́д | Hydrogenium | H | аш |
| Вольфра́м | Wolframium | W | вольфра́м |
| Гадоли́ний | Gadolinium | Gd | гадоли́ний |
| Га́ллий | Gallium | Ga | га́ллий |
| Га́фний | Hafnium | Hf | га́фний |
| Ге́лий | Helium | He | ге́лий |
| Герма́ний | Germanium | Ge | герма́ний |
| Го́льмий | Holmium | Ho | го́льмий |
| Дармшта́дтий | Darmstadtium | Ds | дармшта́дтий |
| Диспро́зий | Dysprosium | Dy | диспро́зий |
| Ду́бний | Dubnium | Db | ду́бний |
| Евро́пий | Europium | Eu | евро́пий |
| Желе́зо | Ferrum | Fe | фе́ррум |
| Зо́лото | Aurum | Au | а́урум |
| И́ндий | Indium | In | и́ндий |
| Йод | Iodium | I | йод |
| Ири́дий | Iridium | Ir | ири́дий |
| Итте́рбий | Ytterbium | Yb | итте́рбий |
| И́ттрий | Yttrium | Y | и́ттрий |
| Ка́дмий | Cadmium | Cd | ка́дмий |
| Ка́лий | Kalium | K | ка́лий |
| Калифо́рний | Californium | Cf | калифо́рний |
| Ка́льций | Calcium | Ca | ка́льций |
| Кислоро́д | Oxygenium | O | о |
| Ко́бальт | Cobaltum | Co | ко́бальт |
| Коперни́ций | Copernicium | Cn | коперни́ций |
| Кре́мний | Silicium | Si | сили́циум |
| Крипто́н | Krypton | Kr | крипто́н |
| Ксено́н | Xenon | Xe | ксено́н |
| Кю́рий | Curium | Cm | кю́рий |
| Ланта́н | Lanthanum | La | ланта́н |
| Ливермо́рий | Livermorium | Lv | ливермо́рий |
| Ли́тий | Lithium | Li | ли́тий |
| Лоуре́нсий | Lawrencium | Lr | лоуре́нсий |
| Люте́ций | Lutetium | Lu | люте́ций |
| Ма́гний | Magnesium | Mg | ма́гний |
| Ма́рганец | Manganum | Mn | ма́рганец |
| Медь | Cuprum | Cu | ку́прум |
| Мейтне́рий | Meitnerium | Mt | мейтне́рий |
| Менделе́вий | Mendelevium | Md | менделе́вий |
| Молибде́н | Molybdaenum | Mo | молибде́н |
| Моско́вий | Moscovium | Mc | моско́вий |
| Мышья́к | Arsenicum | As | арсе́никум |
| На́трий | Natrium | Na | на́трий |
| Неоди́м | Neodymium | Nd | неоди́м |
| Нео́н | Neon | Ne | нео́н |
| Непту́ний | Neptunium | Np | непту́ний |
| Ни́кель | Niccolum | Ni | ни́кель |
| Нио́бий | Niobium | Nb | нио́бий |
| Нихо́ний | Nihonium | Nh | нихо́ний |
| Нобе́лий | Nobelium | No | нобе́лий |
| Оганесо́н | Oganesson | Og | оганесо́н |
| О́лово | Stannum | Sn | ста́ннум |
| О́смий | Osmium | Os | о́смий |
| Палла́дий | Palladium | Pd | палла́дий |
| Пла́тина | Platinum | Pt | пла́тина |
| Плуто́ний | Plutonium | Pu | плуто́ний |
| Поло́ний | Polonium | Po | поло́ний |
| Празеоди́м | Praseodymium | Pr | празеоди́м |
| Проме́тий | Promethium | Pm | проме́тий |
| Протакти́ний | Protactinium | Pa | протакти́ний |
| Ра́дий | Radium | Ra | ра́дий |
| Радо́н | Radon | Rn | радо́н |
| Резерфо́рдий | Rutherfordium | Rf | резерфо́рдий |
| Ре́ний | Rhenium | Re | ре́ний |
| Рентге́ний | Roentgenium | Rg | рентге́ний |
| Ро́дий | Rhodium | Rh | ро́дий |
| Ртуть | Hydrargyrum | Hg | гидра́ргирум |
| Руби́дий | Rubidium | Rb | руби́дий |
| Руте́ний | Ruthenium | Ru | руте́ний |
| Сама́рий | Samarium | Sm | сама́рий |
| Свине́ц | Plumbum | Pb | плю́мбум |
| Селе́н | Selenium | Se | селе́н |
| Се́ра | Sulfur | S | эс |
| Серебро́ | Argentum | Ag | арге́нтум |
| Сибо́ргий | Seaborgium | Sg | сибо́ргий |
| Ска́ндий | Scandium | Sc | ска́ндий |
| Стро́нций | Strontium | Sr | стро́нций |
| Сурьма́ | Stibium | Sb | сти́биум |
| Та́ллий | Thallium | Tl | та́ллий |
| Танта́л | Tantalum | Ta | танта́л |
| Теллу́р | Tellurium | Te | теллу́р |
| Теннесси́н | Tennessium | Ts | теннесси́н |
| Те́рбий | Terbium | Tb | те́рбий |
| Техне́ций | Technetium | Tc | техне́ций |
| Тита́н | Titanium | Ti | тита́н |
| То́рий | Thorium | Th | то́рий |
| Ту́лий | Thulium | Tm | ту́лий |
| Углеро́д | Carboneum | C | цэ |
| Ура́н | Uranium | U | ура́н |
| Фе́рмий | Fermium | Fm | фе́рмий |
| Флеро́вий | Flerovium | Fl | флеро́вий |
| Фо́сфор | Phosphorus | P | пэ |
| Фра́нций | Francium | Fr | фра́нций |
| Фтор | Fluorum | F | фтор |
| Ха́ссий | Hassium | Hs | га́ссий |
| Хлор | Chlorum | Cl | хлор |
| Хром | Chromium | Cr | хром |
| Це́зий | Caesium | Cs | це́зий |
| Це́рий | Cerium | Ce | це́рий |
| Цинк | Zincum | Zn | цинк |
| Цирко́ний | Zirconium | Zr | цирко́ний |
| Эйнште́йний | Einsteinium | Es | эйнште́йний |
| Э́рбий | Erbium | Er | э́рбий |