ПЦР-тестирование (также известное как тестирование полимеразной цепной реакции) — это тип лабораторного исследования, который сообщает, есть ли у кого-то в настоящее время COVID-19. Этот вид диагностики в отличие от экспресс-тестов, которые проверяют наличие антител в крови, выявляет непосредственно наличие вируса в организме. ПЦР-тестирование проводится в лаборатории и может помочь найти даже крошечное количества вируса. Это достигается за счет усиления генетического материала вируса до уровня, на котором он может быть обнаружен. Для теста требуется образец от человека. Этот образец собирается врачом, обычно с помощью тампона, вводимого человеку в нос или горло.
ПЦР-тесты используются для непосредственного определения наличия антигена, а не наличия иммунного ответа организма или антител. Обнаруживая вирусную РНК, которая будет присутствовать в организме до того, как сформируются антитела или появятся симптомы заболевания, тесты могут определить, есть ли у кого-то вирус на очень ранней стадии.
Кому и когда нужно проходить диагностику
ПЦР-тест на COVID-19 необходим, если:
- У вас есть симптомы COVID-19, такие как жар, кашель, усталость или затрудненное дыхание.
- У вас нет симптомов, но у вас был тесный контакт (в пределах 1,5 м в общей сложности 15 минут или более) с кем-то, у кого положительный результат теста на вирус COVID-19 или есть подозрение на его наличие.
Ваш врач, другой медицинский работник или отдел общественного здравоохранения порекомендуют пройти тест. Определенные группы считаются высокоприоритетными для диагностического тестирования. К ним относятся люди с признаками и симптомами COVID-19, которые:
- Работают в медицинском учреждении или в качестве служб быстрого реагирования.
- Живут или работают в учреждениях долгосрочного ухода, таких как дома престарелых, или других местах, где люди проживают вместе, например, в тюрьмах или приютах.
- Находятся на лечении в больнице.
Другим людям может быть предоставлен приоритет для тестирования в зависимости от рекомендаций местного департамента здравоохранения по мониторингу COVID-19 в отдельных сообществах.
Как работает ПЦР
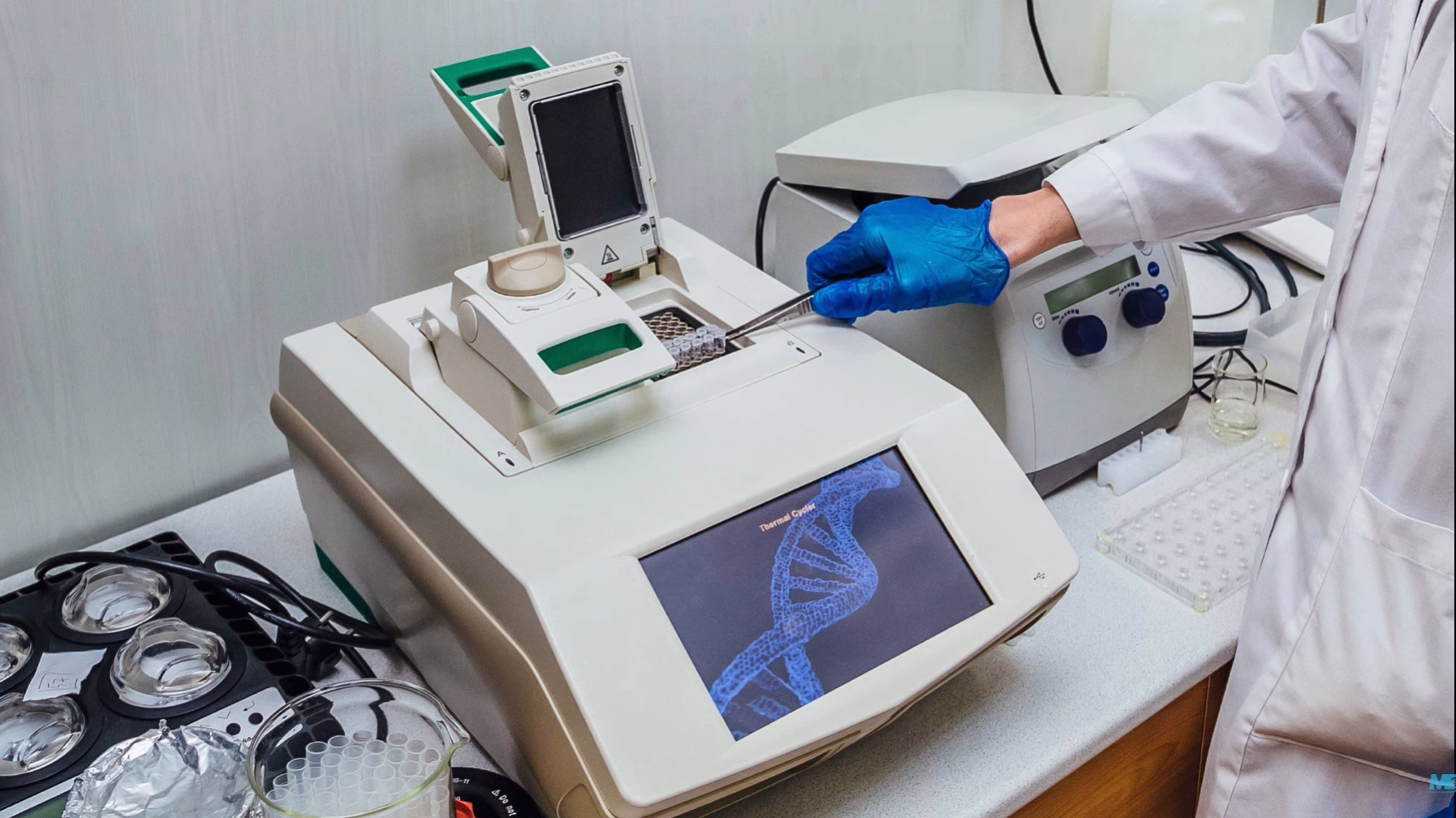
ПЦР проводится в 3 основных этапа. Эти три шага повторяются в течение 30 или 40 циклов. Циклы выполняются на автоматическом циклическом устройстве, которое быстро нагревает и охлаждает пробирки, содержащие реакционную смесь.
Каждый этап – денатаурация (изменение структуры), отжиг (соединение) и растяжение — происходит при разной температуре:
- Денатурация: при 94 ° C (201,2 F) двухцепочечная ДНК плавится и раскрывается на два фрагмента одноцепочечной ДНК.
- Отжиг: при средних температурах, около 54 ° C (129,2 F), праймеры образуют пары (отжиг) с одноцепочечной «матрицей» (матрица — это последовательность ДНК, которую нужно скопировать). На небольшой длине двухцепочечной ДНК (объединенный праймер и шаблон), полимераза присоединяется и начинает копировать шаблон.
- Удлинение: при 72 ° C (161,6 ° F) лучше всего работает полимераза, и строительные блоки ДНК, комплементарные матрице, соединяются с праймером, образуя двухцепочечную молекулу ДНК.
За один цикл одиночный сегмент двухцепочечной ДНК-матрицы амплифицируется в два отдельных фрагмента двухцепочечной ДНК. Эти две части затем доступны для усиления в следующем цикле. По мере повторения циклов создается все больше и больше копий, а количество копий шаблона увеличивается в геометрической прогрессии.
На последнем, четвертом этапе к рецепторным участкам на РНК присоединяется индикатор, который, затем, окрашивается на контрольном и/или тестовом участке тест-кассеты.
Как проводится диагностика
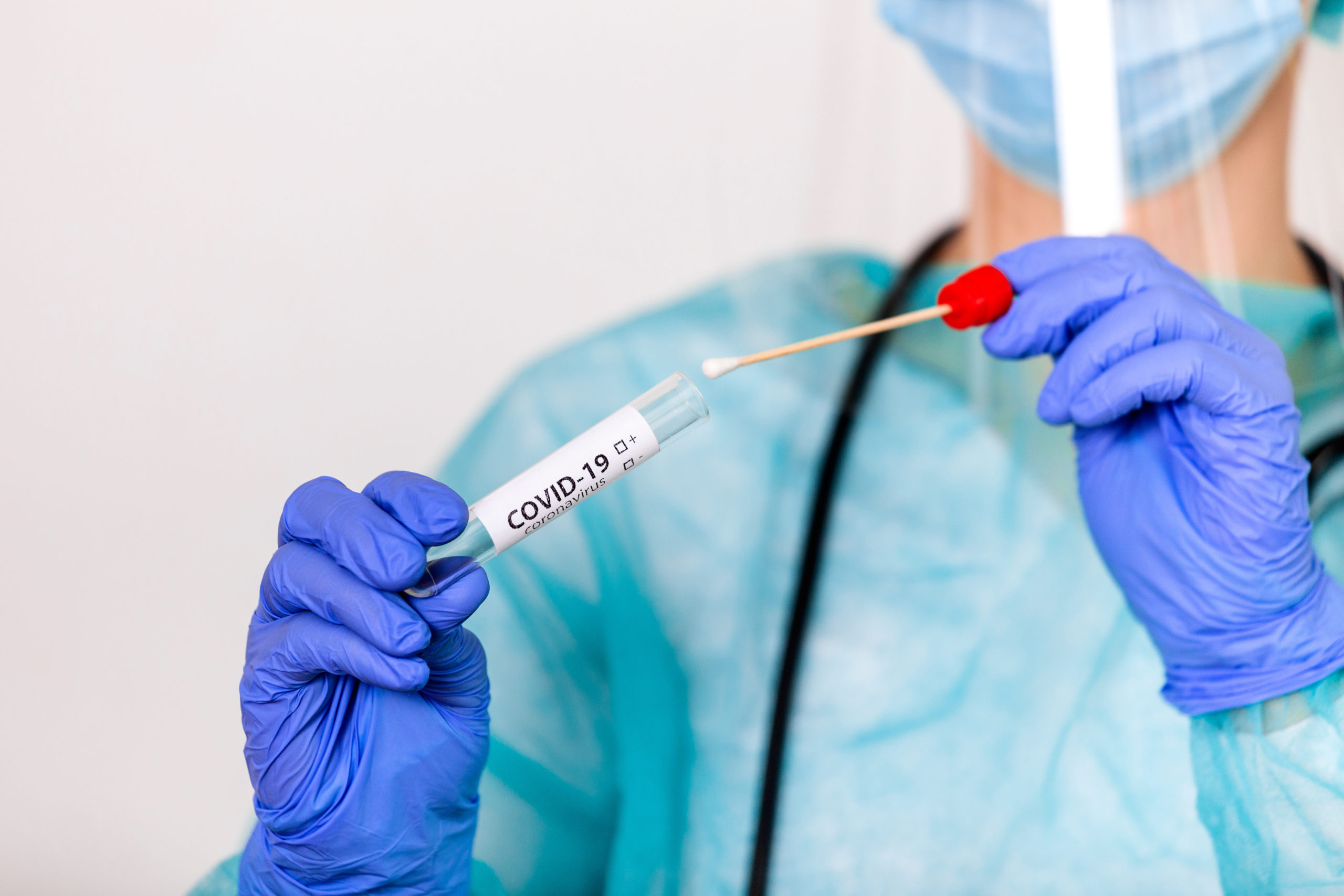
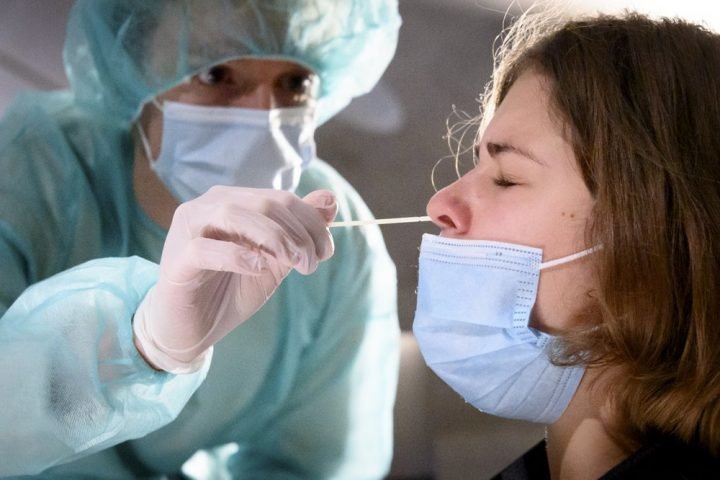
Для диагностического теста на COVID-19 медицинский работник берет образец слизи из носа, горла или образец слюны. Образец, необходимый для диагностического тестирования, можно взять в кабинете врача, в медицинском учреждении, в центре тестирования или на дому (а некоторых ситуациях выезд лаборанта возможен на дом). Рекомендуется использовать длинный мазок из носа (мазок из носоглотки), хотя мазок из зева приемлем. Врач или другой медицинский работник вставляет в нос пациента тонкую гибкую палочку с ватой на кончике или проводит тампоном по задней стенке горла, чтобы собрать образец слизи. Это может быть несколько неприятно. Что касается пробы из носа, мазок может производиться в обеих ноздрях, чтобы собрать достаточно слизи для анализа. Тампон ненадолго остается на месте, прежде чем его осторожно поворачивают при извлечении. Образец герметично закрывается в пробирке и отправляется в лабораторию для анализа.
Что влияет на результат тестирования
Технические проблемы, включая загрязнение во время отбора проб (например, тампон случайно касается загрязненной перчатки или поверхности), загрязнение ампликонами ПЦР, загрязнение реагентов, перекрестное загрязнение образца и перекрестные реакции с другими вирусами или генетическим материалом — основные причины ложных результатов исследования.
Но кроме технических проблем на информативность исследования влияет правильная подготовка. Пациент должен быть проинформирован о том, что за 4 часа до тестирования он не может курить, есть, жевать жвачку, чистить зубы или полоскать рот ополаскивателем.
Современная альтернатива ПЦР
Более современным и удобным способом тестирования на вирусные антигены является экспресс-тестирование, которое использует ту же индикаторную реакцию, что и классический ПЦР тест, но без первых трех этапов, связанных с повышением количества специфических отрезков вирусной РНК. Это стало возможным благодаря более чувствительному индикатору. Использование этих тестов заметно сокращает время тестирования (время проведения теста — всего 15 минут)
Приобрести экспресс тесты на выявление вирусных антигенов можно по ссылке:
ПЦР Тест мазок Сovid 19 для выявления антигена коронавируса
Экспресс-тест на COVID-19 (коронавирус) N-COV-19 RAPID (Великобритания)
Как выбрать тест на ковид? Информации в сети очень много, она сложная, потому что написана в основном врачами и лабораториями. А еще потому что не все пока понятно про коронавирус в принципе, и поэтому очень много обтекаемых фраз. А это не помогает пациенту разобраться.
Нас интересуют 2 простых вопроса:
- Как понять, болею ли я в данный момент/заразен ли я в данный момент?
- Как узнать, переболел ли я в прошлом/есть ли у меня иммунитет?
Мы коротко описали основные виды тестов на коронавирус, которые сейчас предлагают клиники и лаборатории. Совсем без специфической терминологии не обойтись, но мы постарались описать их максимально понятно. И что самое ценное, рассказали, какие виды тестов показательны в какой период заболевания, что означают положительные и отрицательные результаты того или иного метода и в каких случаях придется пройти повторное тестирование.
Молекулярная диагностика коронавируса (ПЦР)
Важнейшим лабораторным методом является ПЦР в биоматериале, взятом из дыхательных путей (мазок из носа и ротоглотки).
Суть метода ПЦР заключается в том, что генетическая информация вируса (РНК) многократно копируется в лабораторных условиях специальным прибором – амплификатором, увеличивая свою концентрацию в два раза в каждом цикле копирования. Это дает возможность выявить вирус или бактерию даже в тех случаях, когда его количество составляет лишь сотню клеток в миллилитре крови. Если генетическая информация тестируемой бактерии или вируса в пробе отсутствует, то она не копируется и не определяется.
Анализ методом ПЦР обычно проводится тем, у кого есть симптомы респираторного заболевания или тем, кто имел контакты с возможным источником инфекции.
Отсутствие генетического материала возбудителя (отрицательный результат) означает, что человек не инфицирован на момент взятия анализа. Для подтверждения или исключения наличия инфекции тест выполняется повторно через определенные промежутки времени.
ПЦР-диагностика используется для установления факта заболевания коронавирусом. Вирус можно обнаружить сразу после заражения, даже если у пациента еще нет проявлений болезни. Анализ уместно сделать, если вы контактировали с носителем инфекции или находились там, где было возможно заражение. Он также используется для подтверждения диагноза.
Однако молекулярные РНК-тесты не являются абсолютно надежными и могут у значительной части в действительности инфицированных пациентов дать отрицательный результат. Это зависит от достаточности содержания вируса в материале выбранной локализации на той или иной стадии инфекции, качества взятия материала, предела чувствительности теста, присутствия ингибиторов ПЦР и пр. Поэтому в диагностике особое значение придается характерной картине КТ. В дополнение к этим исследованиям и клинической оценке могут быть полезны исследования, направленные на выявление в крови специфических антител, вырабатываемых организмом против SARS-CoV-2.
Нет, мы не хотим вас запутать. Если тест отрицательный, но симптомы присутствуют, не надо читать статьи, нужно обратиться к врачу. Специфику течения любой инфекции никто не отменял. Врач будет использовать дополнительные методы исследования и ставить диагноз. Не занимайтесь самодиагностикой. Для этого нужно было закончить медицинский ВУЗ.
У нас в клинике вы можете пройти исследование РНК коронавирусов SARS-CoV-2 (COVID-19), SARS-CoV и MERS-CoV методом ПЦР (качественное определение). Тест-система разработана в ЦНИИ Эпидемиологии Роспотребнадзора. Чувствительность используемой тест-системы составляет 103 копии плазмид на миллилитр (10*3).
Тестирование на наличие антител к коронавирусу COVID-19
Аналогично другим вирусным инфекциям, Коронавирус стимулирует гуморальный и клеточный иммунный ответ (IgM и IgG) . После вторжения вируса в организм, в крови больного начинают появляться сначала иммуноглобулин класса M (IgM), позднее – иммуноглобулин класса G (IgG), после чего активируется иммунитет и клетки иммунной системы атакуют собственные клетки, зараженные вирусом.
Для получения ответа на вопрос инфицирован ли пациент в данный момент, подвергался ли человек воздействию вируса и развился ли у него иммунный ответ, необходимо применение тестов на выявление антител (Ig G, Ig M, Ig A) к конкретному вирусу. В основе таких тестов лежат методы иммуноферментного анализа (ИФА), иммунохроматографии (ИХА) и их аналогов.
Антитела класса М появляются в острой фазе заболевания и снижаются после выздоровления. Антитела класса G появляются на 7 сутки от начала заболевания и держатся длительное время, продолжительность этого времени еще неизвестна.
По наличию и уровню IgM антител в крови можно судить о текущей или недавно перенесенной инфекции. Антитела IgM появляются уже через несколько дней после первых проявлений болезни (на 2-3 сутки от начала заболевания), их концентрация достигает максимума на 7-10 сутки от начала заболевания и определяются в крови 1- 1,5 месяца. IgM первым вырабатывается среди всех иммуноглобулинов при контакте организма с инфекцией, поэтому их называют иммуноглобулинами первичного иммунного ответа. Их присутствие в крови свидетельствует об острой стадии инфекционного процесса.
Специфические IgG антитела обычно присутствуют в крови длительное время и после выздоровления и могут выполнять защитную роль. Поэтому исследование уровня специфических IgG к SARS-CoV-2 может помочь для прогноза вероятного наличия иммунной защиты в результате перенесенной инфекции. Таким образом, определение IgG не используется при ранней диагностике инфекции – он обнаруживается в крови через две недели от начала заболевания, пик его определяется через месяц и продолжительность определения его пока неизвестна. IgG определяет появление иммунитета в дальнейшем.
Для первичного прохождения исследования на антитела, рекомендуется выявление в крови одновременно IgM и IgG антител.
У нас в клинике вы можете пройти экспресс-тестирование за 15 минут методом ИХА или сдать кровь на анализ на антитела методом ИФА. Мы работаем только с аккредитованными Роспотребнадзором лабораториями.
Экспресс тест на коронавирус (ИХА)
Экспресс-тесты — это качественные или полуколичественные способы диагностики, которые дают ответ лишь на вопрос, имеются ли признаки присутствия коронавируса в организме пациента и, фактически, не дают возможность оценить количество возбудителя.
Экспресс тесты проводятся методом иммунохроматографии (ИХА), не требуют использования специального оборудования, но требуют присутствие медсестры, так как забор крови производится из пальца. Продолжительность процедуры анализа находится в пределах 10-30 минут
Положительный результат такого теста требует обязательной проверки методом ПЦР (полимеразной цепной реакции).
Существует два типа быстрых тестов на COVID-19:
- тесты непосредственного выявления антигена SARS-CoV-2, которые установят наличие компонентов самого вируса (например, белковой оболочки)
- тесты выявления антител (они наиболее распространенные в экспресс-диагностике) — это непрямые тесты по выявлению иммуноглобулинов в крови — IgM и IgG.
Процедура анализа чрезвычайно проста:
- Собрать в пробирку пробу крови или плазмы или сыворотки.
- Добавить каплю пробы в специальное углубление на панели с тестовой лентой.
- Капнуть в углубление 2-3 капли буферного раствора.
- Через 15 минут получаем результат — это появление окрашенных участков на тестовой ленте. Участки показывают или отрицательный результат, или наличие иммуноглобулинов IgM и IgG, как отдельно, так и обоих вместе.
Анализ на антитела к коронавирусу (ИФА)
Непрямой иммуноферментный анализ (ИФА) – полуколичественный анализ, им определяется количество выявленных антител IgM и IgG. Берется венозная кровь, а для исследования используется сыворотка крови.
Показания для назначения анализа на антитела к коронавирусу COVID-19:
- Диагностика заболевания.
- Определение иммунитета.
- Получение информации о перенесенном заболевании с бессимптомным течением.
- Отбор доноров для переливания крови пациентам с тяжелой формой заболевания.
- Определение стадии заболевания, периода заразности для окружающих.
В двух словах, чем отличается ИФА и ИХА? В каком случае достаточно эксперсс теста (ИХА), а когда нужно точно делать количественный (ИФА)?
Это сложный вопрос. Экспресс тесты — высокая специфичность (почти 100%), т.е. срабатывает только на COVID-19, но низкая чувствительность (71%). А метод ИФА более чувствительный, но менее специфичный. Это очень тонкие различия и пациенту они не нужны. Экспресс тест — это быстро, можно охватить большой коллектив, быстро получить ответ. А анализ из вены — более основательно.
Дополнительные методы диагностики
Кроме указанных выше специфических анализов, у больных коронавирусной инфекцией, и для лиц с подозрением на это заболевание, определяют газы, растворенные в крови, печеночные и почечные пробы, миоглобин, ферменты миокарда, скорость оседания эритроцитов, С-реактивный белок, общий анализ мочи и проводят другие исследования, которые позволяют уточнить состояние пациента и назначить нужное лечение.
Коротко обо всех методах диагностики коронавируса
Тест на коронавирус и антитела к нему – взаимодополняющие диагностики.
Тест на коронавирус- это определение вируса в мазке из ротоглотки методом ПЦР. Он используется для того, чтобы установить наличие коронавируса на самых ранних стадиях, даже если у вас нет никаких симптомов проявлений болезни. Но при этом используется обычно при наличие симптомов, либо при положительном анализе на антитела М.
Анализ на антитела к коронавирусу позволяет выявить как заболевших в острой стадии, так и уже переболевших коронавирусом COVID-19.
Наличие специфических антител класса M (иммуноглобулинов IgM) выявляются в крови в острой фазе заболевания и вскоре после выздоровления снижаются. Наличие специфических антител класса G (иммуноглобулинов IgG) в сыворотке крови говорит о факте инфицирования вирусом SARS-CoV-2 в прошлом и о сформированном специфическом иммунном ответе (наличие иммунитета).
Как понять, болею ли я сейчас и могу ли быть заразен?
- Если нет никаких клинических симптомов, то для первичного анализа, рекомендуется выявление в крови одновременно IgM и IgG антител. Любым методом: ИФА (кровь из вены) или ИХА (экспресс тест из пальца). Достаточно сделать один тест и получить отрицательный результат. В таком случае беспокоиться не о чем.
- Если вы решили пройти экспресс-тест и были выявлены антитела класса M, то обязательно нужно делать диагностику в мазке методом ПЦР, даже если нет симптомов.
- Если вы сдали кровь (ИФА) и были выявлены антитела класса M, то тоже обязательно нужно делать диагностику в мазке методом ПЦР, даже если нет симптомов.
- Если есть клинические симптомы, то прежде всего нужна диагностика в мазке методом ПЦР, а также желательна диагностика на антитела методом ИФА (кровь из вены).
Как понять, болел ли я в прошлом и есть ли иммунитет?
- Если нет никаких клинических симптомов, то для первичного анализа мы все равно рекомендуем сдать анализ на выявление в крови одновременно IgM и IgG антител. Любым методом: ИФА (кровь из вены) или ИХА (экспресс тест из пальца),
- чтобы исключить острую стадию заболевания без симптомов (отрицательный результат — отсутствие иммуноглобулинов М)
- чтобы узнать о наличие у вас иммунитета после перенесенного в прошлом заболевание (положительный результат — наличие иммуноглобулинов G)
Методы исследований и интерпретация результатов
А это самый полезный и заключительный раздел статьи, в котором мы поможем вам разобраться, какие результаты дают разные методы исследований и что они означают.
Для первичного прохождения исследования на антитела, рекомендуется выявление в крови одновременно IgM и IgG антител. Почему? Потому что отдельно результаты по IgM или IgG не всегда могут дать понимание текущей ситуации.
Интерпретация результатов отдельно IgM и IgG
|
Положительно |
Отрицательно |
|
|
Антитела IgM |
наличие текущей или недавней инфекции |
наличие инфекции (ранний период) или отсутствие инфекции |
|
Антитела IgG |
наличие текущей или имевшей место в прошлом инфекции |
наличие инфекции (ранний период), отсутствие инфекции или выздоровление при имевшей место в отдаленном прошлом инфекции |
Понятно, что ничего не понятно. Одновременно может быть все. Именно по этой причине мы рекомендуем начинать с теста на выявление в крови одновременно IgM и IgG антител. Любым методом: ИФА (кровь из вены) или ИХА (экспресс тест из пальца). При комбинации этих двух показателей все становится гораздо понятнее.
Комбинации результатов тестов на коронавирус разными методами
Может говорить об острой фазе инфекции COVID-19; не дает информации о наличие или отсутствие у пациента коронавируса; не дает информации, является ли он потенциальным распространителем вируса или нет.
Требуется проведение теста методом ПЦР.
Рекомендована самоизоляция на 2 недели.
Может говорить о поздней фазе инфекции.
Требуется повторить анализ через 2-4 недели.
Может говорить об острой фазе инфекции COVID-19 с выделением вируса и возможностью стать источником потенциального заражения.
Требуется консультация врача и самоизоляция на 2 недели.
Свидетельствует о факте контакта с вирусом в прошлом с формированием специфического иммунного ответа. Вероятность повторного заражения существенно снижена (наличие устойчивого иммунитета к коронавирусу пока не доказано). Вероятность выделения вируса минимальная. Дополнительные обследования при отсутствие симптомов не требуются.
Рекомендовано продолжать соблюдение социального дистанцирования и мер предосторожности, так как на сегодняшний день не накоплено достаточно данных, позволяющих полностью исключить возможность повторного заражения.
Свидетельствует о факте контакта с вирусом в прошлом с формированием специфического иммунного ответа. Вероятность повторного заражения существенно снижена (наличие устойчивого иммунитета к коронавирусу пока не доказано). Вероятность выделения вируса минимальная. Дополнительные обследования при отсутствие симптомов не требуются.
Рекомендовано продолжать соблюдение социального дистанцирования и мер предосторожности, так как на сегодняшний день не накоплено достаточно данных, позволяющих полностью исключить возможность повторного заражения.
Нет данных о контакте этого человека с коронавирусом. При наличие симптомов рекомендовано дополнительно сдать анализ методом ПЦР, а также повторить анализ на IgM и IgG антитела через 2-4 недели
В ЕС-Клинике вы можете пройти ПЦР-диагностику, экспресс-тестирование за 15 минут методом ИХА или сдать кровь на анализ на антитела методом ИФА. Мы проводим все виды тестирования на коронавирус и антитела IgG, IgM к SARS-COV-2 (COVID-19) для физических и юридических лиц. Берем анализ в клинике или с выездом на дом, в офис, на предприятие. Заявки принимаем круглосуточно по телефону +7(499)4500303
Что такое ПЦР тест на коронавирус и как правильно сдавать мазок
Согласно официальной статистике, выздоровление от COVID-19 может происходить как за семь, так и за сорок дней. Длительность лечения зависит не только от возраста и состояния организма заразившегося, но и от оперативности выявления вируса. Самым достоверным методом определения возбудителя инфекции является ПЦР тест. Его точность составляет 99%.
ПЦР тест на коронавирус – это анализ полимеразной цепной реакции (PCR), который проводится с помощью взятия мазка со слизистой рта и носоглотки и позволяет диагностировать даже бессимптомное течение болезни, посмотреть насколько эффективное лечение и подтвердить выздоровление.

Положительный ПЦР на коронавирус свидетельствует о наличии клеток SARS-CoV-2 (РНК) в организме и показывает вероятную стадию заболевания. Рассмотрим подробнее методы обнаружения КОВИД-19.
Лабораторная диагностика коронавирусной инфекции
Если человек ощущает легкое недомогание, как при ОРВИ, или первые симптомы заражения – это повод обратиться в поликлинику. Даже если нет никаких ковидных признаков, но был контакт с носителем coronavirus, не стоит медлить с обращением к врачу и сдачей анализов.
Существует два вида лабораторной диагностики, которую можно проводить уже в первый день инфицирования.
Общая
Она включает в себя основные клинические исследования:
- ОАК. Кровь анализируется на лейкопению (снижение числа лейкоцитов), лимфопению (уменьшение количества лимфоцитов), тромбоцитопению (недостаток тромбоцитов).
- Биохимический анализ крови. Такой метод позволяет оценить состояние внутренних органов, на которые влияет новая инфекция. Производится лабораторная диагностика печеночных ферментов электролитов, мочевины, билирубина, альбумина и глюкозы. Анализ этих показателей помогает определить, есть ли функциональные отклонения в организме, и правильно назначить лечение, учитывая выявленные нарушения.
- Пульсоксиметрия. Эта диагностическая процедура призвана оценить уровень кислорода в крови. Плюс метода в том, что цельность кожного покрова не нарушается. Замер происходит с помощью прибора пульсоксиметра. Если его показатели в пределах 95–98% – это норма.
-
Анализ уровня CRP. Этот маркер наличия коронавирусной инфекции показывает количество С-реактивного белка в организме.
Исследования доказали, что его концентрация значительно повышается при ковидном заболевании. У 73% пациентов уровень CRP оказался больше нормы. Такие показатели наблюдались при тяжелой и легкой форме течении инфекции. Анализ можно делать уже в первые дни заражения, потому что концентрация С-реактивного белка повышается спустя 6–8 часов от момента инфицирования.
- Определение уровня D-димера. Такой метод диагностики показывает, когда пациенту необходима госпитализация. Если концентрация увеличена в 3-4 раза от нормы, это означает, что начались осложнения. При этом анализе учитывается возраст пациента, поскольку после 50 лет уровень D-димера повышается. Хронические заболевания и беременность также влияют на показатель.
Специфическая
Среди таких методов диагностики чаще всего используются два вида.
Экспресс-тесты
Дают возможность узнать, выработался ли у человека иммунитет к вирусу. Для этого проводятся исследования на наличие двух антител: G и M. Если результат такого иммунохроматографического (ИХГ) теста положительный, это свидетельствует о том, что заболевание в организме протекает уже больше недели. ИХГ анализ помогает быстро выявить даже бессимптомный коронавирус. Кроме того, можно оценить уровень иммунной защиты организма.
Отличие экспресс-тестов от обыкновенных не только в быстром результате, но и в месте проведения. Делаются такие не в специальной лаборатории, а локально. Берется кровь из пальца либо из вены.
Это очень удобно, если необходимо протестировать много людей вне медицинского учреждения. Например, летом 2020 года власти Германии решили бесплатно тестировать таким методом иностранных туристов, которые прибывали в аэропорт.
Особенно важно провериться на наличие антител IgG и IgM тем категориям населения, которые контактируют со множеством людей и находятся в группе риска.
ПЦР тест
Этот вид диагностики коронавируса признан лучшим из-за надежности. Она составляет 99%. Такой тест не только помогает обнаружить носителя инфекции, но и вовремя выявить контактирующих.
В 1993 году автор методики полимеразной цепной реакции, Кэри Мюлис, получил Нобелевскую премию по химии. Ее суть в том, что берется фрагмент ДНК в малой концентрации и значительно искусственно увеличивается при помощи ферментов.
PCR считается самым чувствительным, потому что определяет наличие возбудителя и его количество, даже когда содержание минимальное. Анализ этим методом особенно актуальный при бессимптомном течении заболевания.
Сдавая ПЦР тест, можно получить еще несколько преимуществ:
- проверить на генетическом уровне собственное здоровье;
- выявить вирус на ранней стадии;
- обнаружить другие вредоносные микроорганизмы.
В основе PCR диагностики – технология инвитро (in vitro). Эта методика позволяет проводить эксперимент вне живого организма – в пробирке.
Подготовка к тесту на коронавирус методом ПЦР
PCR диагностика обычно проводится утром. Желательно сразу после сна. Чем точнее выполняются предписания, тем достовернее получается результат анализа.
Чтобы правильно сдать ПЦР на ковид, необходимо заранее подготовиться:
-
За 3-4 часа до прохождения исключить:
- прием еды, напитков;
- жевание резинки;
- использование капель в нос, солевых растворов и спреев;
- курение;
- чистку зубов;
- полоскание рта и горла.
Если мучает сильная жажда, то разрешается сделать всего несколько глотков обыкновенной воды не менее, чем за 30 минут до взятия мазков.
-
За 12 часов до сдачи исключить:
- леденцы для рассасывания;
- антисептики для полости рта;
- аэрозоли для горла.
-
За 1 сутки до тестирования отказаться от:
- употребления спиртных напитков, даже легких;
- приема антибиотиков.
Особенности проведения и результаты ПЦР диагностики ковида
Помимо лабораторных анализов, для точного диагноза проводятся опрос пациента, клиническое обследование. Также учитываются симптомы covid 19, которые проявляются и при острой респираторной вирусной инфекции. Это затруднение дыхания, высокая температура, кашель.
Как проводится тест:
- Из зева берется мазок на коронавирус. Для этого аппликатором со стерильным тампоном на конце несколько раз проводят по слизистой.
- Взятый биоматериал помещается в специальный пакет и отправляется в лабораторию на анализ.
По срокам ПЦР диагностика коронавируса проводится в течение 24–48 часов. Полный ее цикл занимает 2–4 суток. Сдавать анализ можно как в лаборатории, так и дома. В последнем случае оформляется выезд специальной бригады.

ПРЦ диагностика ковида может показать один из двух результатов:
-
Положительный.
При нем в тестах указывается несколько значений:
- <24 Ct – высокая вирусная нагрузка (острый вид заболевания);
- <24–<30 Ct – средняя;
- >30 Ct – низкая (выздоровление или начало заболевания).
- Отрицательный.
Чтобы получить расшифровку, необходимо в бланке найти строку «РНК SARS-CoV-2». Рядом с записью указано, обнаружен вирус или нет.
Ложноположительные и ложноотрицательные результаты ПЦР
Диагностика, лечение, вакцинация COVID-19
Вирусолог из Берлина, Кристиан Дростен, заметил сходство между тестированием этим методом и рыбалкой в домашних условиях. Если в аквариуме плавает карасик, но сачок пуст при попытке его выловить, это не значит, что в емкости никого нет.
То же самое можно сказать и про попытки выявить вирус в человеческом теле. Не обнаружив ковид19, нельзя утверждать, что потенциальный больной не инфицирован.
По данным азиатских исследователей, доля ложноотрицательных результатов ПЦР-тестов составляет от 40 до 60%. Это происходит потому, что вирус временно располагается в ротоглотке, откуда берется мазок для анализа, но не живет в ней. На момент диагностики инфекции может уже не быть в слизистой верхних дыхательных путей, если это не первые дни заражения.
Истинный результат при такой методике можно получить из мокроты пациента. Но ситуация усложняется тем, что кашель при коронавирусе чаще всего сухой. Поэтому при взятии биоматериала со слизистой даже качественные тест-системы могут не справиться, показав ложный результат.
Случаев, когда РНК обнаруживалась у совершенно здоровых людей, намного больше.
Причины искаженных данных ПЦР-тестов:
- Халатность со стороны людей, участвовавших в исследовании (курьеров, секретарей, специалистов). Например, несоблюдение температурного режима в месте, где хранились образцы для анализа, их размораживание при транспортировке. Также играет роль человеческий фактор. При взятии мазка можно перепутать пробу или ввести результат не в ту ячейку.
- Наличие нежизнеспособных клеток коронавируса у переболевшего. Когда ковид погибает, в организме человека еще имеется его генетический код. PCR обладает высокой чувствительностью, поэтому может выявиться повышение показателей.
- Неправильная подготовка со стороны пациента к прохождению теста.
Вывод
ПЦР-тест на ковид – надежный метод, позволяющий определить наличие ДНК вируса в организме с первого дня инфицирования. Подготавливаться к сдаче анализа необходимо заранее. Тогда шанс получить ложный результат сводится к минимуму.
Обратившись в нашу клинику, можно узнать о наличии заболевания даже при бессимптомном протекании. Это помогает обезопасить себя от возможных осложнений коронавируса, а близких – от заражения. Сотрудники знают, как правильно делать забор материала, чтобы исключить вероятность ложноотрицательного и ложноположительного результата.
В случае выявления заражения с каждым клиентом обсуждаются способы быстрого восстановления здоровья после основного курса лечения от КОВИД-19.
ПЦР-тест на коронавирус: что показывает и как делают
Сегодня наиболее достоверным способом выявления коронавирусной инфекции считается ПЦР-тест. Точность его составляет не менее 99%, даже если течение болезни не имеет симптомов. Суть метода заключается в молекулярно-генетической диагностике путем изучения полимеразно-цепной реакции, при которой возможно определение вируса в организме. Это помогает узнавать о присутствии вирусной РНК на ранней стадии еще до того, как появятся признаки ковид или сформируются антитела.
Содержание статьи
- Виды лабораторной диагностики
- Подготовка к тесту на коронавирус методом ПЦР
- Особенности проведения и результаты ПЦР-диагностики
- Что еще способно повлиять на результаты тестирования ковида?
Виды лабораторной диагностики
Общее диагностирование в период массового распространения Covid желательно делать с целью оценки иммунитета и способности организма противостоять чужеродным клеткам и патогенам. Для чего необходимо проводить:
- общий клинический анализ крови. Обычно сдавать нужно из пальца;
- биохимический анализ крови. Результат позволит выявить развитие осложнений в виде функциональных нарушений органов и систем, декомпенсацию сопутствующих заболеваний;
- исследование уровня СРБ в сыворотке крови, благодаря которому получают информацию о тяжести течения ковид и распространенности воспалительного процесса, чтобы сделать дальнейший прогноз при возникновении пневмонии;
- сдавать ИФА сыворотки (иммуноферментный анализ) для обнаружения антител lgG/lgA, свидетельствующих о недавно перенесенном или текущем заболевании, чтобы выяснить стадию его развития. Брать кровь будут из локтевой вены.
В случае если во время пульсоксиметрии, назначенной для проверки содержания количества кислорода в крови (SpO2), прибор будет показывать сатурацию ниже 95%, то это означает, что пациент нуждается в респираторной поддержке. При имеющихся признаках острой дыхательной недостаточности будут брать материал на коагулограмму для определения времени свертываемости крови, характеризующего скорость и риск активного тромбообразования. Кровь придется сдавать тоже из вены.
Специфическое обследование на SARS-CoV-2 делают двумя способами: методом ПЦР и посредством экспресс-тестирования.
Подготовка к тесту на коронавирус методом ПЦР
От того, насколько правильно человек накануне будет подготовлен к тестированию, зависит достоверность его результата. Что для этого необходимо делать:
- воздержаться от употребления алкоголя не менее чем за двое суток до забора материала;
- минимум за 3-4 часа постараться не полоскать дезрастворами рот, откуда придется брать образец;
- перед тем, как сдавать мазок, не чистить зубы, не рассасывать таблетки с антисептическими свойствами;
- в день сдачи не капать в нос капли, не применять мази;
- до того, как будет браться биоматериал, не использовать какие-либо освежающие полость рта спреи и декоративную косметику для губ;
- часа за три отказаться от курения, приема пищи и воды;
- заранее освободить носовую полость от избыточной слизи (если она присутствует).
Делать данное тестирование лучше тем людям, кто не вступал в контакт с больными ковид на протяжении 14 дней и у кого отсутствуют признаки респираторной инфекции в виде слабости, кашля, одышки, гипертермии и пр. При себе следует иметь паспорт, СНИЛС, ИНН.
Обращаем ваше внимание, что специалисты клиник берут мазки с использованием обязательных средств индивидуальной защиты.
Сеть медицинских центров «CityMed» проводит любые виды тестирования в организациях, расположенных в г. Москве, или на дому у клиента. Если вы планируете сдавать анализы, то для получения более подробной информации или заключения договора можете связаться с оператором по указанному на сайте номеру колл-центра.
Особенности проведения и результаты ПЦР-диагностики
Как делают ПЦР-тест на ковид? Суть процедуры заключается в том, что для максимально точного результата мазок должен браться правильно: с поверхности небных дужек, миндалин, носовых ходов. Причем в последнем случае зонд вводится вращательными движениями достаточно глубоко с прижатием к стенкам слизистой. Если брать мазок из преддверия отверстий, то он может не показывать реальную картину, а демонстрировать только отрицательный результат.
Перед тем, как сдавать, предупредите медперсонал заранее об искривлении перегородки или проведенных оперативных вмешательствах на полости носа.
Что еще способно повлиять на результаты тестирования ковида?
Нарушение техники взятия мазка при случайном касании перчатки или других посторонних предметов приводит к загрязнению тампона. Контаминация может произойти за счет заражения реагентов, рабочих зон, лабораторного оборудования посторонними РНК и ДНК, что зачастую становится причиной ложноположительного результата ПЦР-теста.
Помимо вышеперечисленных технических проблем на искажение информативности влияют неправильные подготовка или самостоятельное взятие мазка пациентом.
Хотя это довольно несложная и безопасная процедура, но если делать ее некорректно, то тестирование будет ошибочным. Когда в доме проживает несколько членов семьи, брать нужно для каждого отдельный набор, включающий:
- Анкету пациента.
- Инструкцию, как и откуда следует брать мазок для выполнения теста на ковид.
- Транспортировочную упаковку.
- 3 одинаковых стерильных зонда для забора материала (два для каждого носового хода и один для ротоглотки).
- Шпатель для более удобного доступа к ротоглотке.
- Пробирку со средой (эппендорф).
- Зип-пакетик для пробирки.
- Термопакет для упаковки готового биоматериала.
По окончании манипуляции термопакеты отдаются на руки курьеру или их приносят в медицинскую организацию сами пациенты. Узнать результаты проверки теста можете любым удобным для вас способом: по телефону или электронной почте.
Обращаем ваше внимание, что СитиМед соблюдает все правила по забору, транспортировке и хранению биоматериала в соответствии с методическим рекомендациям МЗ Российской Федерации.
Популярные услуги
Похожие статьи
The US CDC’s COVID-19 laboratory test kit
COVID-19 testing involves analyzing samples to assess the current or past presence of SARS-CoV-2. The two main types of tests detect either the presence of the virus or antibodies produced in response to infection.[1][2] Molecular tests for viral presence through its molecular components are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Antibody tests (serology immunoassays) instead show whether someone once had the disease.[3] They are less useful for diagnosing current infections because antibodies may not develop for weeks after infection.[4] It is used to assess disease prevalence, which aids the estimation of the infection fatality rate.[5]
Individual jurisdictions have adopted varied testing protocols, including whom to test, how often to test, analysis protocols, sample collection and the uses of test results.[6][7][8] This variation has likely significantly impacted reported statistics, including case and test numbers, case fatality rates and case demographics.[9][10][11][12] Because SARS-CoV-2 transmission occurs days after exposure (and before onset of symptoms), there is an urgent need for frequent surveillance and rapid availability of results.[13]
Test analysis is often performed in automated, high-throughput, medical laboratories by medical laboratory scientists. Rapid self-tests and point-of-care testing are also available and can offer a faster and less expensive method to test for the virus although with a lower accuracy.[14][15]
Methods
Explanation of the underlying pathophysiology pertaining to diagnosis of COVID-19[16]
Positive viral tests indicate a current infection, while positive antibody tests indicate a prior infection.[17] Other techniques include a CT scan, checking for elevated body temperature, checking for low blood oxygen level, and detection by trained dogs.[18][19][20]
Detection of the virus
Detection of the virus is usually done either by looking for the virus’s inner RNA, or pieces of protein on the outside of the virus. Tests that look for the viral antigens (parts of the virus) are called antigen tests.
There are multiple types of tests that look for the virus by detecting the presence of the virus’s RNA. These are called nucleic acid or molecular tests, after molecular biology. As of 2021, the most common form of molecular test is the reverse transcription polymerase chain reaction (RT-PCR) test.[21] Other methods used in molecular tests include CRISPR, isothermal nucleic acid amplification, digital polymerase chain reaction, microarray analysis, and next-generation sequencing.[21]
Reverse transcription polymerase chain reaction (RT-PCR) test
Polymerase chain reaction (PCR) is a process that amplifies (replicates) a small, well-defined segment of DNA many hundreds of thousands of times, creating enough of it for analysis. Test samples are treated with certain chemicals[22][23] that allow DNA to be extracted. Reverse transcription converts RNA into DNA.
Reverse transcription polymerase chain reaction (RT-PCR) first uses reverse transcription to obtain DNA, followed by PCR to amplify that DNA, creating enough to be analyzed.[23] RT-PCR can thereby detect SARS-CoV-2, which contains only RNA. The RT-PCR process generally requires a few hours.[24] These tests are also referred to as molecular or genetic assays.[3]
Real-time PCR (qPCR)[25] provides advantages including automation, higher-throughput and more reliable instrumentation. It has become the preferred method.[26][27]
The combined technique has been described as real-time RT-PCR[28] or quantitative RT-PCR[29] and is sometimes abbreviated qRT-PCR,[30] rRT-PCR[31] or RT-qPCR,[32] although sometimes RT-PCR or PCR are used. The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines propose the term RT-qPCR,[25] but not all authors adhere to this.
Average sensitivity for rapid molecular tests depend on the brand. For ID NOW, the average sensitivity was 73.0% with an average specificity of 99.7%; for Xpert Xpress the average sensitivity was 100% with an average specificity of 97.2%.[33][34]
In a diagnostic test, sensitivity is a measure of how well a test can identify true positives and specificity is a measure of how well a test can identify true negatives. For all testing, both diagnostic and screening, there is usually a trade-off between sensitivity and specificity, such that higher sensitivities will mean lower specificities and vice versa.
Sensitivity and Specificity
A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.
Samples can be obtained by various methods, including a nasopharyngeal swab, sputum (coughed up material),[35] throat swabs,[36] deep airway material collected via suction catheter[36] or saliva.[37][38] Drosten et al. remarked that for 2003 SARS, «from a diagnostic point of view, it is important to note that nasal and throat swabs seem less suitable for diagnosis, since these materials contain considerably less viral RNA than sputum, and the virus may escape detection if only these materials are tested.»[39]
Sensitivity of clinical samples by RT-PCR is 63% for nasal swab, 32% for pharyngeal swab, 48% for feces, 72–75% for sputum, and 93–95% for bronchoalveolar lavage.[40]
The likelihood of detecting the virus depends on collection method and how much time has passed since infection. According to Drosten tests performed with throat swabs are reliable only in the first week. Thereafter the virus may abandon the throat and multiply in the lungs. In the second week, sputum or deep airways collection is preferred.[36]
Collecting saliva may be as effective as nasal and throat swabs,[37] although this is not certain.[41][38] Sampling saliva may reduce the risk for health care professionals by eliminating close physical interaction.[42] It is also more comfortable for the patient.[43] Quarantined people can collect their own samples.[42] A saliva test’s diagnostic value depends on sample site (deep throat, oral cavity, or salivary glands).[38] Some studies have found that saliva yielded greater sensitivity and consistency when compared with swab samples.[44][45][46]
On 15 August 2020, the US FDA granted an emergency use authorization for a saliva test developed at Yale University that gives results in hours.[47][48]
On 4 January 2021, the US FDA issued an alert about the risk of false results, particularly false negative results, with the Curative SARS-Cov-2 Assay real-time RT-PCR test.[49]
Viral burden measured in upper respiratory specimens declines after symptom onset.[50] Following recovery, many patients no longer have detectable viral RNA in upper respiratory specimens. Among those who do, RNA concentrations three days following recovery are generally below the range in which replication-competent virus has been reliably isolated.[51] No clear correlation has been described between length of illness and duration of post-recovery shedding of viral RNA in upper respiratory specimens.[52]
-
A PCR machine
Other molecular tests
Isothermal nucleic acid amplification tests also amplify the virus’s genome. They are faster than PCR because they do not involve repeated heating and cooling cycles. These tests typically detect DNA using fluorescent tags, which are read out with specialized machines.[citation needed]
CRISPR gene editing technology was modified to perform the detection: if the CRISPR enzyme attaches to the sequence, it colors a paper strip. The researchers expect the resulting test to be cheap and easy to use in point-of-care settings.[53][54] The test amplifies RNA directly, without the RNA-to-DNA conversion step of RT-PCR.[55]
Antigen tests
COVID-19 Antigen Rapid Test Kit; the timer is provided by the user.
Mucus from nose or throat in a test liquid is placed onto a COVID-19 rapid antigen diagnostic test device.
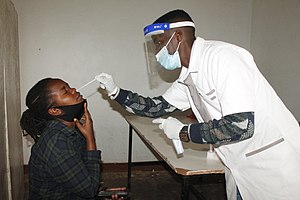
COVID-19 rapid testing in Rwanda
An antigen is the part of a pathogen that elicits an immune response. Antigen tests look for antigen proteins from the viral surface. In the case of a coronavirus, these are usually proteins from the surface spikes.[56] SARS-CoV-2 antigens can be detected before onset of COVID-19 symptoms (as soon as SARS-CoV-2 virus particles) with more rapid test results, but with less sensitivity than PCR tests for the virus.[57]
COVID-19 rapid antigen tests are lateral flow immunoassays that detect the presence of a specific viral antigen, which indicates current viral infection. Antigen tests produce results quickly (within approximately 15–30 minutes), and most can be used at the point-of-care or as self-tests. Self-tests are rapid tests that can be taken at home or anywhere, are easy to use, and produce rapid results.[58] Antigen tests can be performed on nasopharyngeal, nasal swab, or saliva specimens.[15]
Antigen tests that can identify SARS-CoV-2 offer a faster and less expensive method to test for the virus.[14] Antigen tests are generally less sensitive than real-time reverse transcription polymerase chain reaction (RT-PCR) and other nucleic acid amplification tests (NAATs).[15]
Antigen tests may be one way to scale up testing to much greater levels.[56] Isothermal nucleic acid amplification tests can process only one sample at a time per machine. RT-PCR tests are accurate but require too much time, energy and trained personnel to run the tests.[56] «There will never be the ability on a [PCR] test to do 300 million tests a day or to test everybody before they go to work or to school,» Deborah Birx, head of the White House Coronavirus Task Force, said on 17 April 2020. «But there might be with the antigen test.»[59]
Samples may be collected via nasopharyngeal swab, a swab of the anterior nares, or from saliva (obtained by various methods including lollipop tests for children).[60] The sample is then exposed to paper strips containing artificial antibodies designed to bind to coronavirus antigens. Antigens bind to the strips and give a visual readout. The process takes less than 30 minutes, can deliver results at point of care, and does not require expensive equipment or extensive training.[56]
Swabs of respiratory viruses often lack enough antigen material to be detectable.[61] This is especially true for asymptomatic patients who have little if any nasal discharge. Viral proteins are not amplified in an antigen test.[56][62] A Cochrane review based on 64 studies investigating the efficacy of 16 different antigen tests determined that they correctly identified COVID-19 infection in an average of 72% of people with symptoms, compared to 58% of people without symptoms.[63][needs update] Tests were most accurate (78%) when used in the first week after symptoms first developed, likely because people have the most virus in their system in the first days after they are infected.[63] While some scientists doubt whether an antigen test can be useful against COVID-19,[62] others have argued that antigen tests are highly sensitive when viral load is high and people are contagious, making them suitable for public health screening.[64][65] Routine antigen tests can quickly identify when asymptomatic people are contagious, while follow-up PCR can be used if confirmatory diagnosis is needed.[66]
Antibody tests
The body responds to a viral infection by producing antibodies that help neutralize the virus.[67] Blood tests (also called serology tests or serology immunoassays[3]) can detect the presence of such antibodies.[68] Antibody tests can be used to assess what fraction of a population has once been infected, which can then be used to calculate the disease’s mortality rate.[5] They can also be used to determine how much antibody is contained in a unit of convalescent plasma, for COVID-19 treatment, or to verify if a given vaccine generates an adequate immune response.[69]
SARS-CoV-2 antibodies’ potency and protective period have not been established.[5][70] Therefore, a positive antibody test may not imply immunity to a future infection. Further, whether mild or asymptomatic infections produce sufficient antibodies for a test to detect has not been established.[71][needs update] Antibodies for some diseases persist in the bloodstream for many years, while others fade away.[56]
The most notable antibodies are IgM and IgG. IgM antibodies are generally detectable several days after initial infection, although levels over the course of infection and beyond are not well characterized.[72] IgG antibodies generally become detectable 10–14 days after infection and normally peak around 28 days after infection.[73][74] This pattern of antibody development seen with other infections, often does not apply to SARS-CoV-2, however, with IgM sometimes occurring after IgG, together with IgG or not occurring at all.[75] Generally, however, median IgM detection occurs 5 days after symptom onset, whereas IgG is detected a median 14 days after symptom onset.[76] IgG levels significantly decline after two or three months.[77]
Genetic tests verify infection earlier than antibody tests. Only 30% of those with a positive genetic test produced a positive antibody test on day 7 of their infection.[71]
Antibody Test Types
Rapid diagnostic test (RDT)
RDTs typically use a small, portable, positive/negative lateral flow assay that can be executed at point of care. RDTs may process blood samples, saliva samples, or nasal swab fluids. RDTs produce colored lines to indicate positive or negative results.[78]
Enzyme-linked immunosorbent assay (ELISA)
ELISAs can be qualitative or quantitative and generally require a lab. These tests usually use whole blood, plasma, or serum samples. A plate is coated with a viral protein, such as a SARS-CoV-2 spike protein. Samples are incubated with the protein, allowing any antibodies to bind to it. The antibody-protein complex can then be detected with another wash of antibodies that produce a color/fluorescent readout.[78]
Neutralization assay
Neutralization assays assess whether sample antibodies prevent viral infection in test cells.[67] These tests sample blood, plasma or serum. The test cultures cells that allow viral reproduction (e.g., Vero E6 cells). By varying antibody concentrations, researchers can visualize and quantify how many test antibodies block virus replication.[78]
Chemiluminescent immunoassay
Chemiluminescent immunoassays are quantitative lab tests. They sample blood, plasma, or serum. Samples are mixed with a known viral protein, buffer reagents and specific, enzyme-labeled antibodies. The result is luminescent. A chemiluminescent microparticle immunoassay uses magnetic, protein-coated microparticles. Antibodies react to the viral protein, forming a complex. Secondary enzyme-labeled antibodies are added and bind to these complexes. The resulting chemical reaction produces light. The radiance is used to calculate the number of antibodies. This test can identify multiple types of antibodies, including IgG, IgM, and IgA.[78]
Neutralizing vis-à-vis binding antibodies
Most if not all large scale COVID-19 antibody testing looks for binding antibodies only and does not measure the more important neutralizing antibodies (NAb).[79][80][81] A NAb is an antibody that neutralizes the infectivity of a virus particle by blocking its attachment to or entry into a susceptible cell; enveloped viruses, like e.g. SARS-CoV-2, are neutralized by the blocking of steps in the replicative cycle up to and including membrane fusion.[82][67] A non-neutralizing antibody either does not bind to the crucial structures on the virus surface or binds but leaves the virus particle infectious; the antibody may still contribute to the destruction of virus particles or infected cells by the immune system.[83][67] It may even enhance infectivity by interacting with receptors on macrophages.[84] Since most COVID-19 antibody tests return a positive result if they find only binding antibodies, these tests cannot indicate that the subject has generated protective NAbs that protect against re-infection.[80][81]
It is expected that binding antibodies imply the presence of NAbs[81] and for many viral diseases total antibody responses correlate somewhat with NAb responses[85] but this is not established for COVID-19. A study of 175 recovered patients in China who experienced mild symptoms reported that 10 individuals had no detectable NAbs at discharge, or thereafter. How these patients recovered without the help of NAbs and whether they were at risk of re-infection was not addressed.[80] An additional source of uncertainty is that even if NAbs are present, viruses such as HIV can evade NAb responses.[79]
Studies have indicated that NAbs to the original SARS virus (the predecessor to the current SARS-CoV-2) can remain active for two years[86] and are gone after six years.[87] Nevertheless, memory cells including memory B cells and memory T cells[88] can last much longer and may have the ability to reduce reinfection severity.[87]
-
A point of care test in Peru. A blood droplet is collected by a pipette.
-
The rapid diagnostic test shows reactions of IgG and IgM antibodies.
Other tests
Sniff tests
Sudden loss of smell can be used to screen people on a daily basis for COVID-19. A study by the National Institutes of Health showed that those infected with SARS-CoV-2 could not smell a 25% mixture of ethanol and water.[89] Because various conditions can lead to the loss of the sense of smell, a sniff test would not be definitive but indicate the need for a PCR test. Because the loss of the sense of smell shows up before other symptoms, there has been a call for widespread sniff testing.[90] Health care bureaucracies have generally ignored sniff tests even though they are quick, easy and capable of being self-administered daily. This has led some medical journals to write editorials supporting the adoption of sniff testing.[91]
Imaging
Typical visible features on CT initially include bilateral multilobar ground-glass opacities with a peripheral or posterior distribution.[92] COVID-19 can be identified with higher precision using CT than with RT-PCR.[93]
Subpleural dominance, crazy paving, and consolidation may develop as the disease evolves.[92][94] Chest CT scans and chest x-rays are not recommended for diagnosing COVID-19. Radiologic findings in COVID-19 lack specificity.[92][95]
Chest X-rays, computed tomography scans and ultrasounds are all ways the coronavirus disease can be detected.
A chest x-ray is a portable lightweight machine. This machine is typically more available than polymerase chain reaction and computerized tomography scans. it only takes approximately 15 seconds per patient.[96] This makes chest-x ray readily accessible and inexpensive. It also has quick turnaround time and can be crucial to the clinical equipment in the detection of coronavirus disease.[97]
Computerized tomography scans involve looking at 3D images from various angles. This is not as available as chest x-ray, but still only takes about 15 minutes per patient.[96] Computerized tomography has been a known routine scanning for pneumonia diagnosis, therefore can also be used to diagnose coronavirus disease. Computerized tomography scans may help with ongoing illness monitoring throughout treatment. Patients who had low-grade symptoms and high body temperatures revealed significant lung indications on their chest computed tomography scans. They emphasized how important chest computerized tomography scans are for determining how serious the coronavirus disease infection is.[98]
Ultrasound can be another tool to detect coronavirus disease. An ultrasound is a type of imaging exam that produces images using sound waves. Unlike computerized tomography scans and x-rays, ultrasound does not use radiation. Moreover, it is inexpensive, simple to use, repeatable, and has several additional advantages. Using a hand-held mobile machine, ultrasound examinations can be performed in a variety of healthcare settings.[99]
There are some downsides to using imaging, however. The equipment needed for computed tomography scans is not available in most hospitals, making it not as effective as some other tools used for detection of the coronavirus disease.[96] One of the difficult tasks in a pandemic is manually inspecting each report, which takes numerous radiology professionals and time.[100] There were several problems with early studies of using chest computerized tomography scans for diagnosing coronavirus. Some of these problems included the disease severity characters being different in severe and hospitalized cases. The criteria for doing a chest computerized tomography scan were not defined. There was also no characterization of positive chest computerized tomography scans results. The computerized tomography scans findings were not the same as positive computerized tomography scans findings of coronavirus.[101] In a typical clinical setting, chest imaging is not advised for routine screening of COVID-19. Patients with asymptomatic to mild symptoms are not recommended to be tested via chest computerized tomography scans. However, it is still crucial to use, particularly when determining complications or disease progression. Chest imaging also is not always the first route to take with patients who have high risk factors for COVID. High risk patients that had mild symptoms, chest imaging findings were limited. Although a computerized tomography scan is a strong tool in the diagnosis of COVID-19, it is insufficient to identify COVID-19 alone due to the poor specificity and the difficulties that radiologists may experience in distinguishing COVID-19 from other viral pneumonia on chest computerized tomography scans.[98]
Article body
Serology (CoLab score) tests
The standard blood test (quick scan) taken at the emergency room measures different values. By use of the blood quick scan the CoLab score is calculated with a developed algorithm based on how the coronavirus causes changes in the blood. The software is intended for use in emergency rooms to quickly rule out the presence of the disease in incoming patients. A not negative result is followed by a PCR (polymerase chain reaction) or LAMP (loop-mediated isothermal amplification) test.[102]
Breath tests
The breath test by a Coronavirus breathalyzer is a pre-screening test for people who have no or mild symptoms of COVID-19. A not negative result is followed by a PCR or LAMP test.[citation needed]
Animals
In May 2021, Reuters reported that Dutch researchers at Wageningen University had shown that trained bees could detect the virus in infected samples in seconds and this could benefit countries where test facilities are in short supply.[103] A two-month study by the Necker-Cochin hospital Paris in conjunction with the French national veterinary school reported in May 2021 that dogs were more reliable than current lateral flow tests.[104]
Researchers in Paris in March 2022 reported in a preprint not yet peer-reviewed that trained dogs were very effective for rapidly detecting the presence of SARS-Cov2 in people, whether displaying symptoms or not. The dogs were presented with sweat samples to smell from 335 people, of whom 78 with symptoms and 31 without tested positive by PCR. The dogs detected 97% of the symptomatic and 100% of the asymptomatic infections. They were 91% accurate at identifying volunteers who were not infected, and 94% accurate at ruling out the infection in people without symptoms. The authors said «Canine testing is non-invasive and provides immediate and reliable results.Further studies will be focused on direct sniffing by dogs to evaluate sniffer dogs for mass pre-test in airports, harbors, railways stations, cultural activities or sporting events.»[105][106]
Functional assays
Tollotest is a molecular test that detects the activity of a SARS-CoV2 protease, which is a biomarker for active infection.[107]
History
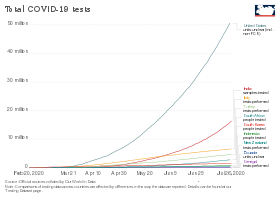
Timeline of total number of tests in different countries[108]
In January 2020, scientists from China published the first genetic sequences of SARS-CoV-2 via the GISAID initiative, a program that had handled mostly genetic sequence data from animal-borne influenzas.[109][110] Researchers around the world used that data to build molecular tests for the virus. Antigen- and antibody-based tests were developed later.[citation needed]
Even once the first tests were created, the supply was limited. As a result, no countries had reliable data on the prevalence of the virus early in the pandemic.[111] The WHO and other experts called for ramping up testing as the best way to slow the spread of the virus.[112][113] Shortages of reagent and other testing supplies became a bottleneck for mass testing in the EU, the UK and the US.[114][115][116] Early tests also encountered problems with reliability.[117][118]
Testing protocols
Drive-through testing
In drive-through testing, the person undergoing testing remains in a vehicle while a healthcare professional approaches the vehicle and obtains a sample, all while taking appropriate precautions such as wearing personal protective equipment (PPE).[119][120] Drive-through centers helped South Korea accelerate its testing program.[121]
Home collection
A Randox PCR home test kit in the UK, showing the swab, and multi-layer packaging to deliver it to the lab
In Hong Kong test subjects can stay home and receive a specimen tube. They spit into it, return it and later get the result.[122]
Pooled testing
Pooled testing can improve turnaround time, by combining a number of samples to be tested together. If the pool result is negative, all samples are negative. If the test result is positive, samples will need to be individually tested.[69]
In Israel, researchers at Technion and Rambam Hospital developed a method for testing samples from 64 patients simultaneously, by pooling the samples and only testing further if the combined sample was positive.[123][124][125] Pool testing was then adopted in Israel, Germany, Ghana[126][127][128] South Korea,[129] Nebraska,[130] China[131] and the Indian states of Uttar Pradesh,[132] West Bengal,[133] Punjab,[134] Chhattisgarh[135] and Maharashtra.[136]
Open source, multiplexed designs released by Origami Assays can test as many as 1122 patient samples using only 93 assays.[137] These balanced designs can be run in small laboratories without robotic liquid handlers.
Multi-tiered testing
One study proposed a rapid immune response assay as a screening test, with a confirmatory nucleic acid test for diagnosis, followed by a rapid antibody test to determine course of action and assess population exposure/herd immunity.[138]
Required volume
Required testing levels are a function of disease spread. The more the cases, the more tests are needed to manage the outbreak. COVID-19 tends to grow exponentially at the beginning of an outbreak, meaning that the number of required tests initially also grows exponentially. If properly targeted testing grows more rapidly than cases, it can be contained.[citation needed]
WHO recommends increasing testing until fewer than 10% are positive in any given jurisdiction.[139]
United States
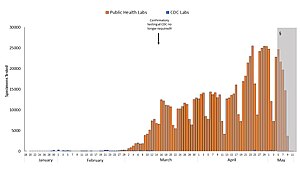
Number of tests done per day in the US, as of April 2020.
Blue: CDC lab
Orange: Public health lab
Gray: Data incomplete due to reporting lag
Not shown: Testing at private labs; total exceeded 100,000 per day by 27 March.[140]
Economist Paul Romer reported that the US has the technical capacity to scale up to 20 million tests per day, which is his estimate of the scale needed to fully remobilize the economy.[141] The Edmond J. Safra Center for Ethics estimated on 4 April 2020 that this capacity could be available by late July 2020.[142] Romer pointed to single-molecule real-time sequencing equipment from Pacific Biosciences[141][143] and to the Ion Torrent Next-Generation Sequencing equipment from ThermoFisher Scientific.[141][144] According to Romer, «Recent research papers suggest that any one of these has the potential to scale up to millions of tests per day.» This plan requires removing regulatory hurdles. Romer estimated that $100 billion would cover the costs.[141]
Romer also claimed that high test accuracy is not required if tests are administered frequently enough. He ran model simulations in which 7% of the population is tested every day using a test with a 20% false negative rate and a 1% false positive rate. The average person would be tested roughly every two weeks. Those who tested positive would go into quarantine. Romer’s simulation indicated that the fraction of the population that is infected at any given time (known as the attack rate) peaks reaches roughly 8% in about thirty days before gradually declining, in most runs reaching zero at 500 days, with cumulative prevalence remaining below 20%.[145]
Snapshot mass-testing
A study found that, despite possibly suboptimal implementation, the snapshot mass-testing approach conducted by Slovakia by which ~80% of its population was tested for COVID-19 within a weekend at the end of October 2020 was highly efficacious, decreasing observed prevalence by 58% within one week and by 70% compared to a hypothetical scenario of no snapshot mass-testing.[146][147] The significant reduction resulted from a set of complementary lockdown and quarantine measures whereby citizens who tested positive were quarantined synchronously the weeks afterwards.[148]
Surveillance and screening of populations
As of August 2020, the WHO recognizes wastewater surveillance of SARS-CoV-2 as a potentially useful source of information on the prevalence and temporal trends of COVID-19 in communities, while highlighting that gaps in research such as viral shedding characteristics should be addressed.[149] Such aggregative testing may have detected early cases.[150] Studies show that wastewater-based epidemiology has the potential for an early warning system and monitoring for COVID-19 infections.[151][152][153][154][155] This may prove particularly useful once large shares of regional populations are vaccinated or recovered and do not need to conduct rapid tests while in some cases being infectious nevertheless.[156]
Available tests
A temporary drive-in testing site for COVID-19 set up with tents in a parking lot
Countries around the world developed tests independently and in partnership with others.
Nucleic acid tests
Tests are available that look for viral DNA using either polymerase chain reaction (PCR) or loop-mediated isothermal amplification (LAMP) technology.
Tests developed in China, France, Germany, Hong Kong, Japan, the United Kingdom, and the US targeted different parts of the viral genome. WHO adopted the German system for manufacturing kits sent to low-income countries without the resources to develop their own.[citation needed]
PowerChek Coronavirus looks for the «E» gene shared by all beta coronaviruses, and the RdRp gene specific to SARS-CoV-2.[157]
Nucleic acid testing conducted using an Abbott Laboratories ID Now device
Abbott Laboratories’ ID Now nucleic acid test uses isothermal amplification technology.[158] The assay amplifies a unique region of the virus’s RdRp gene; the resulting copies are then detected with «fluorescently-labeled molecular beacons».[159] The test kit uses the company’s «toaster-size» ID Now device, which is widely deployed in the US.[160] The device can be used in laboratories or in point of care settings, and provides results in 13 minutes or less.[159]
Primerdesign offers its Genesig Real-Time PCR test system. Roche Molecular Systems offers the Cobas 6800/8800 systems; they are offered among others by the United Nations.[citation needed]
Antigen tests
Innova SARS-CoV-2 Antigen Rapid Qualitative Lateral Flow Test kit showing a negative result. This device has been subject to accuracy concerns and a recall in the United States.
Antigen tests are readily available worldwide and have been approved by several health regulators.
Quidel’s «Sofia 2 SARS Antigen FIA»[66][161] is a lateral flow test that uses monoclonal antibodies to detect the virus’s nucleocapsid (N) protein.[162] The result is read out by the company’s Sofia 2 device using immunofluorescence.[162] The test is simpler and cheaper but less accurate than nucleic acid tests. It can be deployed in laboratories or at point of care and gives results in 15 minutes.[161] A false negative result occurs if the sample’s antigen level is positive but below the test’s detection limit, requiring confirmation with a nucleic acid test.[162]
The Innova SARS-CoV-2 Antigen Rapid Qualitative Test was never approved for use in the United States, but was being sold by the company anyway. The FDA inspected Innova facilities in California in March and April 2021, and found inadequate quality assurance of tests manufactured in China.[163] On 23 April 2021, the company issued a recall. The FDA warned consumers to return or destroy the devices because the rate of false positives and false negatives found in clinical trials were higher than the rate claimed by the packaging.[164] Over 1 billion tests from the company have been distributed in the UK, with £3 billion in funding as part of Operation Moonshot, and the MHRK has authorized exceptional use until at least 28 August 2021.[163] Concerned experts pointed out that accuracy dropped significantly when screening was conducted by the public instead of by a medical professional, and that the test was not designed to screen asymptomatic people.[163] A 2020 study found 79% of positive cases were found when used by laboratory scientists, but only 58% when used by the general public and 40% when used for city-wide screening in Liverpool.[165]
Serology (antibody) tests
Antibodies are usually detectable 14 days after the onset of the infection. Multiple jurisdictions survey their populations using these tests.[166][167] The test requires a blood sample.
Private US labs including Quest Diagnostics and LabCorp offer antibody testing upon request.[168]
Certain antibody tests are available in several European countries and also in the US.[169][170]
Roche offers a selective ELISA serology test.[171]
A summary review in BMJ has noted that while some «serological tests … might be cheaper and easier to implement at the point of care [than RT-PCR]», and such testing can identify previously infected individuals, «caution is warranted … using serological tests for … epidemiological surveillance». The review called for higher quality studies assessing accuracy with reference to a standard of «RT-PCR performed on at least two consecutive specimens, and, when feasible, includ[ing] viral cultures.»[172][173] CEBM researchers have called for in-hospital ‘case definition’ to record «CT lung findings and associated blood tests»[174] and for the WHO to produce a «protocol to standardise the use and interpretation of PCR» with continuous re-calibration.[175]
Accuracy
| Samples source | Positive rate |
|---|---|
| Bronchoalveolar lavage fluid specimens | 93% (14/15) |
| Sputum | 72% (75/104) |
| Nasal swabs | 63% (5/8) |
| Fibrobronchoscope brush biopsy | 46% (6/13) |
| Pharyngeal swabs | 32% (126/398) |
| Feces | 29% (44/153) |
| Blood | 1% (3/307) |
Accuracy is measured in terms of specificity and selectivity. Test errors can be false positives (the test is positive, but the virus is not present) or false negatives, (the test is negative, but the virus is present).[177]
Sensitivity and specificity
Sensitivity indicates whether the test accurately identifies whether the virus is present. Each test requires a minimum level of viral load in order to produce a positive result. A 90% sensitive test will correctly identify 90% of infections, missing the other 10% (a false negative). Even relatively high sensitivity rates can produce high rates of false negatives in populations with low incidence rates.[177]
In a diagnostic test, sensitivity is a measure of how well a test can identify true positives and specificity is a measure of how well a test can identify true negatives. For all testing, both diagnostic and screening, there is usually a trade-off between sensitivity and specificity, such that higher sensitivities will mean lower specificities and vice versa.
Sensitivity and Specificity
A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.[citation needed]
Low-specificity tests have a low positive predictive value (PPV) when prevalence is low. For example, suppose incidence is 5%. Testing 100 people at random using a test that has a specificity of 95% would yield on average 5 people who are actually negative who would incorrectly test positive. Since 5% of the subjects actually are positive, another five would also test positive correctly, totaling 10 positive results. Thus, the PPV is 50%,[178] an outcome no different from a coin toss. In this situation, assuming that the result of a second test is independent of the first test, retesting those with a first positive result increases the PPV to 94.5%, meaning that only 4.5% of the second tests would return the incorrect result, on average less than 1 incorrect result.[179]
Causes of test error
The time course of infection affects the accuracy of some tests. Samples may be collected before the virus has had a chance to establish itself or after the body has begun to eliminate it. A May 2020 review of PCR-RT testing found that the median probability of a false-negative result decreased from 100% on day 1, to 67% on day 4. On the day of symptom onset, the probability was 38%, which decreased to 20% 3 days later.[180][needs update]
PCR-based test
Detection of SARS-CoV-2 by nasal swab over six weeks in patients who experienced mild to moderate illness
RT-PCR is the most commonly-used diagnostic test.[181] PCR tests by nasopharyngeal swab have a sensitivity of 73%, but systematic analysis of specificity has not been determined due to the lack of PCR studies with a control group.[182]
In one study sensitivity was highest at week one (100%), followed by 89.3%, 66.1%, 32.1%, 5.4% and zero by week six since symptom onset.[183][failed verification][184]
Sensitivity is also a function of the number of PCR cycles, as well as time and temperature between sample collection and analysis.[185] A cycle threshold of 20 cycles would be adequate to detect SARS-Cov-2 in a highly infective person.[185] Cycle thresholds above 34 are increasingly likely to give false positives outside of high biosafety level facilities.[185]
On July 16, 2020, Dr. Anthony Fauci of the US CDC indicated that positive results obtained from RT-PCR tests run at more than 35 cycles were almost always «just dead nucleotides».[186] On August 29, 2020, the New York Times reported that, «In three sets of testing data that include cycle thresholds, compiled by officials in Massachusetts, New York and Nevada … most tests set the limit at 40 [cycles], a few at 37» and that the CDC was examining the use of cycle threshold measures «for policy decisions,»[187] On July 21, 2021, the CDC, in their «Real-Time RT-PCR Diagnostic Pan: Instructions for Use», indicated tests results should be determined at 40 cycles.[188]
A Dutch CDC-led laboratory investigation compared 7 PCR kits.[189] Test kits made by BGI, R-Biopharm AG, BGI, KH Medical and Seegene showed high sensitivity.[190]
High sensitivity kits are recommended to assess people without symptoms, while lower sensitivity tests are adequate when diagnosing symptomatic patients.[189]
The University of Oxford’s Centre for Evidence-Based Medicine (CEBM) has pointed to mounting evidence[191][192] that «a good proportion of ‘new’ mild cases and people re-testing positives via RT-PCR after quarantine or discharge from hospital are not infectious, but are simply clearing harmless virus particles which their immune system has efficiently dealt with», and have called for «an international effort to standardize and periodically calibrate testing».[174] On 7 September, the UK government issued «guidance for procedures to be implemented in laboratories to provide assurance of positive SARS-CoV-2 RNA results during periods of low prevalence, when there is a reduction in the predictive value of positive test results».[193]
On 4 January 2021, the US FDA issued an alert about the risk of false results, particularly false negative results, with the Curative SARS-Cov-2 Assay real-time RT-PCR test.[49]
Isothermal nucleic amplification test
One study reported that the ID Now COVID-19 test showed sensitivity of 85.2%. Abbott responded that the issue could have been caused by analysis delays.[194] Another study rejected the test in their clinical setting because of this low sensitivity.[195]
Confirmatory testing
The WHO recommends countries that do not have testing capacity and national laboratories with limited experience on COVID-19 send their first five positives and the first ten negative COVID-19 samples to one of the 16 WHO reference laboratories for confirmatory testing.[196][197] Out of the sixteen reference laboratories, seven are in Asia, five in Europe, two in Africa, one in North America and one in Australia.[198]
National or regional responses
Iceland
Iceland managed the pandemic with aggressive contact tracing, inbound travel restrictions, testing, and quarantining, but with less aggressive lock-downs.[199]
India
Italy
Researchers tested the entire population of Vo’, the site of Italy’s first COVID-19 death. They tested about 3,400 people twice, at an interval of ten days. About half the people testing positive had no symptoms. All discovered cases were quarantined. Along with restricting travel to the commune, new infections were eliminated.[200]
Japan
Unlike other Asian countries, Japan did not experience a pandemic of SARS or MERS, so the country’s PCR testing system was not well developed.[201][202] Japan preferentially tested patients with severe illness and their close contacts at the beginning. Japan’s Novel Coronavirus Expert Meeting chose cluster measures to identify infections clusters.[201][202] The Expert Meeting analyzed the outbreak from Wuhan and identified conditions leading to clusters (closed spaces, crowded spaces and close-contact), and asked people to avoid them.[202][203]
In January, contact tracers took action shortly after the first infection was found. Only administrative tests were carried out at first, until insurance began covering PCR tests on 6 March. Private companies began to test, and the test system gradually expanded.[201][204]
On 3 April, those with positive tests were legally permitted to recuperate at home or in a hotel if they had asymptomatic or mild illness, ending the hospital bed shortage.[205] The first wave (from China) was contained,[206] but a second wave (caused by returnees from Europe and the US) in mid-March led to spreading infection in April.[202] On 7 April, Japan declared a state of emergency (less strict than a lockdown, because it did not block cities or restrict outings).[202][205][207] On 13 May, antigen test kits became covered by insurance, and were combined with a PCR test for diagnosis.[208][209]
Japan’s PCR test count per capita remained far smaller than in some other countries even though its positive test rate was lower. Excess mortality was observed in March.[203][failed verification][207][failed verification][210] The Expert Meeting stated, «The Japanese health care system originally carries out pneumonia surveillance, allowing it to detect most of the severely ill patients who develop pneumonia. There are a large number of CT scanners in Japan and they have spread to small hospitals all over the country, so pneumonia patients are rarely missed. In that sense, it meets the same standards as other countries that mainly carry out PCR tests.»[203][210] The group recommended using CT scans data and doctor’s findings for diagnosis.[211][212] On the Diamond Princess cruise ship, many people who initially tested negative later tested positive. Half of coronavirus-positives there who remained mild or asymptomatic had pneumonia findings on CT scans and their CT image showed a frosted glass shadow that is characteristic of infection.[211][213]
As of 18 July, Japan’s daily PCR testing capacity was about 32,000, more than three times the 10,000 cases as of April. When the antigen test is added to it, the number is about 58,000. The number of tests per 1,000 people in the United States is about 27 times that of Japan, the UK is 20 times, Italy is 8 times, and South Korea is twice (as of 26 July).[214][215][216]
The number of those infected with coronavirus and inpatients has increased in July, but the number of serious cases has not increased. This is thought to be due to the proper testing of those infected in July compared to those in April. In April, the number of tests could not catch up with the increase in the number of infected people, and the test standards were strict, so the test positive rate exceeded 30% at the peak. It means that there were quite a few cases where the those infected was not PCR tested. It is thought that the severe case was preferentially tested though there were a lot of mild cases and asymptomatic carriers mainly in the young during the first wave. In other words, it became possible to grasp the actual situation of infection much better than before by strengthening the testing system.[217] At the end of July, accommodation facilities for mild and asymptomatic carriers became full, and the authorities requested hospitals to prepare beds for the mild. However, it became difficult to treat patients with other illnesses and to maintain the ICU system including the staff due to the occupation of hospital beds by patients with mild symptoms.[218][219][220]
Russia
On 27 April 2020, Russia tested 3 million people and had 183,000 positive results.[221] On 28 April Anna Popova, head of Federal Service for Surveillance in Healthcare (Roszdravnadzor) stated that 506 laboratories were testing; that 45% of those who tested positive had no symptoms; that 5% of patients had a severe form; and 40% of infections were from family members. Illness improved from six days to one day after symptoms appeared. Antibody testing was carried out on 3,200 Moscow doctors, finding 20% immunity.[222]
Singapore
With contact tracing, inbound travel restrictions, testing, and quarantining, Singapore arrested the initial spread without complete lockdown.[223]
Slovakia
In late October 2020 Slovakia tested 3.62 million people in a weekend, from a population of 5.4m, representing 67% of the total (or 82% of the adult population), 38,359 tested positive, representing 1.06% of those tested. The government considered the mass test would significantly assist in controlling the virus and avoid a lockdown and may repeat the exercise at a later date.[224]
South Korea
South Korea’s broad testing approach helped reduce spread. Testing capacity, largely in private sector labs, was built up over several years by the South Korean government in the early 2000s.[225]
The government exploited the resident registration number (RRN) system. Authorities mobilized young men who were eligible for military service as social service agents, security and public health doctors. Public health doctors were mainly dispatched to public health centers and life treatment centers where mildly ill patients were accommodated. They performed PCR tests and managed mild patients. Social service agents worked in pharmacies to fill staff shortages. Korea’s 10k PCR tests per million residents was the world’s highest as of 13 April rising to 20k by mid-June. Twenty-seven Korean companies exported test kits worth $48.6 million in March, and were asked to provide test kits or humanitarian assistance by more than 120 countries. Korean authorities set up a treatment center to isolate and manage patients with asymptomatic and minor illnesses in one facility in order to vacate hospital beds for the more severely ill.
Centers were sited mainly at national facilities and corporate training centers. The failure of Korea’s MERS quarantine in May 2015 left Korea more prepared for COVID-19 than countries that did not face that pandemic. Then President Park Geun-hye allowed Korean CDC-approved private sector testing for infectious diseases in 2016. Korea already had a system for isolating, testing and treating infectious disease patients separately from others. Patients with respiratory illness but no epidemiological relevance were treated at the National Hospital, and those with epidemiological relevance were treated at selected clinics.[226][227][228][229][230][231][232][233][234]
Korea established a large scale drive-through/walk-through» test testing program. However, the most common method was «mobile examination». In Daegu City, 54% of samples were collected by 23 March in home or hospital. Collecting samples door-to-door of avoided the risk of travel by possibly infected patients, but required additional staff. Korea solved the problem by drafting more than 2,700 public insurance doctors.[226][230][229]
The government disclosed personal information to the public via KCDC without patient consent. The authorities used digital surveillance to trace possible spread.[227][230][231][233][234][235][236][237][238][239][excessive citations]
Taiwan
Health insurance IDs and national identification card numbers were used to trace contacts.[240][241][242][243]
United Arab Emirates
In January 2021, the COVID-19 testing results of the UAE came under scrutiny, as Denmark suspended the Emirati flights for five days. The European nation said that it barred the flights from the UAE due to growing suspicion of irregularities in the testing process being followed in the Gulf nation. Denmark’s Minister of Transport, Benny Engelbrecht said that they were taking time to ensure that the negative tests of travelers from the Emirates were a real screening carried out appropriately.[244]
United States
New York State
New York State’s control measures consisted of PCR tests, stay-at-home measures and strengthening the healthcare system. On 29 February before its first case, the state allowed testing at the Wordsworth Center. They managed to convince the CDC to approve tests at state laboratories and the FDA to approve a test kit. As of 13 March the state was conducting more than 1,000 daily tests, growing to 10,000/day on 19 March. In April, the number exceeded 20,000. Many people queued at hospitals to get tested. On 21 March New York City health officials directed medical providers to test only those entering the hospital, for lack of PPE.[233][245][246][247][248][excessive citations]
USS Theodore Roosevelt
Following an outbreak, 94% of the 4,800 aircraft carrier crew were tested. Roughly 60 percent of the 600-plus sailors who tested positive were asymptomatic.[249] Five infected sailors who completed quarantine subsequently developed flu-like symptoms and again tested positive.[250]
Nevada
In 2020, Nevada received a donation of 250,000 Covid testing kits, which were a product of China’s leading genetics company, BGI Group. A UAE-based firm owned by Tahnoun bin Zayed Al Nahyan, Group 42 partnered with the BGI Group to supply the testing kits to Nevada. However, the US Department of Homeland Security and the State Department raised a warning for Nevada hospitals to not use the Chinese-made testing kits, as there were concerns around the involvement of the Chinese government, test accuracy and privacy of the patients.[251]
Delayed testing
A shortage of trained medical laboratory scientists, assay reagents, analyzers, transport medium, and PPE coupled with high demand had limited initially limited the availability of testing and led to significantly increased turnaround times.[citation needed]
Testing statistics by country
Testing strategies vary by country and over time,[252] with some countries testing very widely,[8] while others have at times focused narrowly on only testing the seriously ill.[6] The country that tests only people showing symptoms will have a higher figure for «Confirmed»/»tested» than the country that also tests others.[253] If two countries are alike in every respect, including which people they test, the one that tests more people will have a higher «Confirmed / population». Studies have also found that countries that test more, relative to the number of deaths, have lower estimated case fatality rates[9] and younger age distributions of cases.[11]
| Country or region | Date[a] | Tested | Units[b] | Confirmed (cases) |
Confirmed / tested, % |
Tested / population, % |
Confirmed / population, % |
Ref. |
|---|---|---|---|---|---|---|---|---|
| 17 Dec 2020 | 154,767 | samples | 49,621 | 32.1 | 0.40 | 0.13 | [254] | |
| 18 Feb 2021 | 428,654 | samples | 96,838 | 22.6 | 15.0 | 3.4 | [255] | |
| 2 Nov 2020 | 230,553 | samples | 58,574 | 25.4 | 0.53 | 0.13 | [256][257] | |
| 23 Feb 2022 | 300,307 | samples | 37,958 | 12.6 | 387 | 49.0 | [258] | |
| 2 Feb 2021 | 399,228 | samples | 20,981 | 5.3 | 1.3 | 0.067 | [259] | |
| 6 Mar 2021 | 15,268 | samples | 832 | 5.4 | 15.9 | 0.86 | [260] | |
| 16 Apr 2022 | 35,716,069 | samples | 9,060,495 | 25.4 | 78.3 | 20.0 | [261] | |
| 29 May 2022 | 3,099,602 | samples | 422,963 | 13.6 | 105 | 14.3 | [262] | |
| 9 Sep 2022 | 78,548,492 | samples | 10,112,229 | 12.9 | 313 | 40.3 | [263] | |
| 4 Jan 2023 | 204,725,396 | samples | 5,719,585 | 2.8 | 2,300 | 64.2 | [264] | |
| 11 May 2022 | 6,838,458 | samples | 792,638 | 11.6 | 69.1 | 8.0 | [265] | |
| 28 Nov 2022 | 259,366 | samples | 37,483 | 14.5 | 67.3 | 9.7 | [266] | |
| 3 Dec 2022 | 10,578,766 | samples | 696,614 | 6.6 | 674 | 44.4 | [267] | |
| 24 Jul 2021 | 7,417,714 | samples | 1,151,644 | 15.5 | 4.5 | 0.70 | [268] | |
| 14 Oct 2022 | 770,100 | samples | 103,014 | 13.4 | 268 | 35.9 | [269] | |
| 9 May 2022 | 13,217,569 | samples | 982,809 | 7.4 | 139 | 10.4 | [270] | |
| 21 Dec 2022 | 36,317,596 | samples | 4,668,248 | 12.9 | 315 | 40.5 | [271] | |
| 8 Jun 2022 | 572,900 | samples | 60,694 | 10.6 | 140 | 14.9 | [272][273] | |
| 4 May 2021 | 595,112 | samples | 7,884 | 1.3 | 5.1 | 0.067 | [274] | |
| 28 Feb 2022 | 1,736,168 | samples | 12,702 | 0.73 | 234 | 1.71 | [275] | |
| 5 Jun 2022 | 4,358,669 | cases | 910,228 | 20.9 | 38.1 | 8.0 | [276] | |
| 27 Sep 2022 | 1,872,934 | samples | 399,887 | 21.4 | 54.7 | 11.7 | [277] | |
| 11 Jan 2022 | 2,026,898 | 232,432 | 11.5 | 89.9 | 10.3 | [278][279] | ||
| 19 Feb 2021 | 23,561,497 | samples | 10,081,676 | 42.8 | 11.2 | 4.8 | [280][281] | |
| 2 Aug 2021 | 153,804 | samples | 338 | 0.22 | 33.5 | 0.074 | [282] | |
| 25 Dec 2022 | 10,888,076 | samples | 1,291,288 | 11.9 | 157 | 18.6 | [283] | |
| 4 Mar 2021 | 158,777 | samples | 12,123 | 7.6 | 0.76 | 0.058 | [256][284] | |
| 5 Jan 2021 | 90,019 | 884 | 0.98 | 0.76 | 0.0074 | [285] | ||
| 1 Aug 2021 | 1,812,706 | 77,914 | 4.3 | 11.2 | 0.48 | [286] | ||
| 18 Feb 2021 | 942,685 | samples | 32,681 | 3.5 | 3.6 | 0.12 | [256] | |
| 26 Nov 2022 | 66,343,123 | samples | 4,423,053 | 6.7 | 175 | 11.7 | [287] | |
| 2 Mar 2021 | 99,027 | samples | 4,020 | 4.1 | 0.72 | 0.029 | [256][288] | |
| 23 Dec 2022 | 47,538,151 | samples | 5,001,737 | 10.5 | 249 | 26.2 | [289] | |
| 31 Jul 2020 | 160,000,000 | cases | 87,655 | 0.055 | 11.1 | 0.0061 | [290][291] | |
| 24 Nov 2022 | 36,875,818 | samples | 6,314,769 | 17.1 | 76.4 | 13.1 | [292][293] | |
| 2 Nov 2021 | 2,575,363 | samples | 561,054 | 21.8 | 51.5 | 11.2 | [294] | |
| 25 Dec 2022 | 5,415,197 | cases | 1,261,997 | 23.3 | 133 | 31.0 | [295] | |
| 25 Dec 2022 | 14,268,594 | samples | 1,111,887 | 7.8 | 126 | 9.8 | [296][297] | |
| 25 Dec 2022 | 27,494,341 | samples | 631,111 | 2.3 | 3,185 | 73.1 | [298] | |
| 23 Dec 2022 | 22,502,031 | samples | 4,577,186 | 20.3 | 210 | 42.8 | [299] | |
| 22 Dec 2022 | 67,509,781 | samples | 3,381,011 | 5.0 | 1,159 | 58.0 | [300][301] | |
| 28 Apr 2022 | 305,941 | 15,631 | 5.1 | 33.2 | 1.7 | [302] | ||
| 20 Jun 2022 | 209,803 | cases | 14,821 | 7.1 | 293 | 20.7 | [303] | |
| 22 Jul 2022 | 3,574,665 | samples | 626,030 | 17.5 | 32.9 | 5.8 | [304] | |
| 28 Feb 2021 | 124,838 | 25,961 | 20.8 | 0.14 | 0.029 | [256][305] | ||
| 23 Jul 2021 | 1,627,189 | samples | 480,720 | 29.5 | 9.5 | 2.8 | [306] | |
| 23 Jul 2021 | 3,137,519 | samples | 283,947 | 9.1 | 3.1 | 0.28 | [256][307] | |
| 18 Mar 2022 | 1,847,861 | samples | 161,052 | 8.7 | 28.5 | 2.5 | [308] | |
| 12 Dec 2022 | 397,874 | 17,089 | 4.3 | 30.4 | 1.3 | [309] | ||
| 20 Dec 2022 | 3,607,122 | samples | 611,350 | 16.9 | 272 | 46.0 | [310] | |
| 8 Dec 2021 | 415,110 | 49,253 | 11.9 | 36.5 | 4.3 | [311] | ||
| 24 Jun 2021 | 2,981,185 | samples | 278,446 | 9.3 | 2.6 | 0.24 | [312] | |
| 27 Feb 2022 | 774,000 | samples | 34,237 | 4.4 | 1,493 | 65.7 | [313] | |
| 24 Nov 2022 | 665,164 | samples | 68,375 | 10.3 | 74.2 | 7.6 | [314] | |
| 14 Jan 2022 | 9,042,453 | samples | 371,135 | 4.1 | 163 | 6.7 | [315] | |
| 15 May 2022 | 272,417,258 | samples | 29,183,646 | 10.7 | 417 | 44.7 | [316] | |
| 23 Jul 2021 | 958,807 | samples | 25,325 | 2.6 | 3.1 | 0.082 | [317] | |
| 15 Feb 2021 | 43,217 | samples | 4,469 | 10.3 | 2.0 | 0.21 | [318] | |
| 3 Nov 2021 | 4,888,787 | samples | 732,965 | 15.0 | 132 | 19.7 | [319] | |
| 7 Jul 2021 | 65,247,345 | samples | 3,733,519 | 5.7 | 77.8 | 4.5 | [320][321] | |
| 3 Jul 2021 | 1,305,749 | samples | 96,708 | 7.4 | 4.2 | 0.31 | [322] | |
| 18 Dec 2022 | 101,576,831 | samples | 5,548,487 | 5.5 | 943 | 51.5 | [323] | |
| 30 Jan 2022 | 164,573 | samples | 10,662 | 6.5 | 293 | 19.0 | [324] | |
| 11 May 2021 | 28,684 | 161 | 0.56 | 25.7 | 0.14 | [325] | ||
| 17 Dec 2022 | 6,463,092 | samples | 1,184,754 | 18.3 | 37.4 | 6.9 | [326] | |
| 21 Jul 2021 | 494,898 | samples | 24,878 | 5.0 | 3.8 | 0.19 | [256][327] | |
| 7 Jul 2022 | 145,231 | 8,400 | 5.8 | 7.7 | 0.45 | [328] | ||
| 15 Jun 2022 | 648,569 | cases | 66,129 | 10.2 | 82.5 | 8.4 | [329] | |
| 26 Nov 2022 | 223,475 | cases | 33,874 | 15.2 | 2.0 | 0.30 | [330] | |
| 26 Nov 2021 | 1,133,782 | samples | 377,859 | 33.3 | 11.8 | 3.9 | [331] | |
| 10 May 2022 | 11,394,556 | samples | 1,909,948 | 16.8 | 118 | 19.8 | [332] | |
| 9 Aug 2022 | 1,988,652 | samples | 203,162 | 10.2 | 546 | 55.8 | [333] | |
| 8 Jul 2022 | 866,177,937 | samples | 43,585,554 | 5.0 | 63 | 31.7 | [334][335] | |
| 9 Jan 2023 | 73,691,812 | cases | 6,723,812 | 9.1 | 27.3 | 2.5 | [336][336] | |
| 31 May 2022 | 52,269,202 | samples | 7,232,268 | 13.8 | 62.8 | 8.7 | [337] | |
| 3 Aug 2022 | 19,090,652 | samples | 2,448,484 | 12.8 | 47.5 | 6.1 | [338] | |
| 13 Dec 2022 | 12,881,518 | samples | 1,684,717 | 13.1 | 262 | 34.2 | [339] | |
| 17 Jan 2022 | 41,373,364 | samples | 1,792,137 | 4.3 | 451 | 19.5 | [340] | |
| 29 Dec 2022 | 262,558,741 | samples | 25,143,705 | 9.6 | 435 | 41.7 | [341] | |
| 3 Mar 2021 | 429,177 | samples | 33,285 | 7.8 | 1.6 | 0.13 | [342] | |
| 30 Sep 2022 | 1,184,973 | samples | 151,931 | 12.8 | 43.5 | 5.6 | [343] | |
| 1 Mar 2021 | 8,487,288 | 432,773 | 5.1 | 6.7 | 0.34 | [344] | ||
| 6 Jun 2021 | 7,407,053 | samples | 739,847 | 10.0 | 69.5 | 6.9 | [345] | |
| 28 May 2021 | 11,575,012 | samples | 385,144 | 3.3 | 62.1 | 2.1 | [346] | |
| 5 Mar 2021 | 1,322,806 | samples | 107,729 | 8.1 | 2.8 | 0.23 | [347] | |
| 31 May 2021 | 611,357 | cases | 107,410 | 17.6 | 33.8 | 5.9 | [348] | |
| 9 Mar 2022 | 7,754,247 | samples | 624,573 | 8.1 | 181 | 14.6 | [349] | |
| 10 Feb 2021 | 695,415 | samples | 85,253 | 12.3 | 10.7 | 1.3 | [350] | |
| 1 Mar 2021 | 114,030 | cases | 45 | 0.039 | 1.6 | 0.00063 | [351] | |
| 5 Sep 2021 | 3,630,095 | samples | 144,518 | 4.0 | 189 | 7.5 | [352] | |
| 14 Jun 2021 | 4,599,186 | samples | 542,649 | 11.8 | 67.4 | 8.0 | [353] | |
| 30 Mar 2022 | 431,221 | 32,910 | 7.6 | 21.5 | 1.6 | [354] | ||
| 17 Jul 2021 | 128,246 | 5,396 | 4.2 | 2.5 | 0.11 | [355] | ||
| 14 Apr 2022 | 2,578,215 | samples | 501,862 | 19.5 | 37.6 | 7.3 | [256][356] | |
| 20 Dec 2022 | 8,992,468 | samples | 1,160,878 | 12.9 | 322 | 41.5 | [357][358] | |
| 12 May 2022 | 4,248,188 | samples | 244,182 | 5.7 | 679 | 39.0 | [359] | |
| 19 Feb 2021 | 119,608 | cases | 19,831 | 16.6 | 0.46 | 0.076 | [360] | |
| 29 Nov 2022 | 624,784 | samples | 88,086 | 14.1 | 3.3 | 0.46 | [361] | |
| 7 Sep 2021 | 23,705,425 | cases | 1,880,734 | 7.9 | 72.3 | 5.7 | [362] | |
| 13 Mar 2022 | 2,216,560 | samples | 174,658 | 7.9 | 398 | 31.3 | [363][364] | |
| 7 Jul 2021 | 322,504 | samples | 14,449 | 4.5 | 1.6 | 0.071 | [256][365] | |
| 8 Sep 2021 | 1,211,456 | samples | 36,606 | 3.0 | 245 | 7.4 | [366] | |
| 16 Apr 2021 | 268,093 | 18,103 | 6.8 | 6.1 | 0.41 | [367] | ||
| 22 Nov 2020 | 289,552 | samples | 494 | 0.17 | 22.9 | 0.039 | [368] | |
| 15 Oct 2021 | 10,503,678 | cases | 3,749,860 | 35.7 | 8.2 | 2.9 | [369] | |
| 20 Apr 2022 | 3,213,594 | samples | 516,864 | 16.1 | 122 | 19.6 | [370] | |
| 10 Jul 2021 | 3,354,200 | cases | 136,053 | 4.1 | 100 | 4.1 | [371] | |
| 10 May 2021 | 394,388 | samples | 98,449 | 25.0 | 62.5 | 15.6 | [372][373] | |
| 17 Dec 2022 | 14,082,633 | cases | 1,270,820 | 9.0 | 38.2 | 3.4 | [374] | |
| 22 Jul 2021 | 688,570 | samples | 105,866 | 15.4 | 2.2 | 0.34 | [375] | |
| 16 Sep 2021 | 4,047,680 | samples | 440,741 | 10.9 | 7.4 | 0.81 | [376] | |
| 4 Jul 2022 | 1,062,663 | samples | 166,229 | 15.6 | 38.7 | 6.1 | [377] | |
| 26 Jul 2022 | 5,804,358 | samples | 984,475 | 17.0 | 20.7 | 3.5 | [378] | |
| 6 Jul 2021 | 14,526,293 | cases | 1,692,834 | 11.7 | 83.4 | 9.7 | [379] | |
| 3 Sep 2021 | 41,962 | samples | 136 | 0.32 | 15.7 | 0.050 | [380] | |
| 18 Dec 2022 | 7,690,738 | samples | 2,027,981 | 26.4 | 154 | 40.7 | [381][382] | |
| 22 Feb 2021 | 79,321 | cases | 4,740 | 6.0 | 0.35 | 0.021 | [383] | |
| 28 Feb 2021 | 1,544,008 | samples | 155,657 | 10.1 | 0.75 | 0.076 | [384] | |
| 25 Nov 2020 | 16,914 | cases | 0 | 0 | 0.066 | 0 | [385] | |
| 1 Jul 2021 | 881,870 | samples | 155,689 | 17.7 | 42.5 | 7.5 | [386][387] | |
| 12 Jul 2022 | 7,096,998 | samples | 103,034 | 1.5 | 2,177 | 31.6 | [388] | |
| 20 Jan 2022 | 9,811,888 | samples | 554,778 | 5.7 | 183 | 10.3 | [389] | |
| 28 Oct 2020 | 509,959 | samples | 114,434 | 22.4 | 11.0 | 2.5 | [390] | |
| 5 Mar 2021 | 9,173,593 | samples | 588,728 | 6.4 | 4.2 | 0.27 | [391] | |
| 5 Feb 2022 | 3,078,533 | samples | 574,105 | 18.6 | 60.9 | 11.4 | [392] | |
| 17 Dec 2022 | 7,361,620 | samples | 1,020,961 | 13.9 | 176 | 24.4 | [393] | |
| 17 Feb 2021 | 47,490 | cases | 961 | 2.0 | 0.53 | 0.011 | [394] | |
| 27 Mar 2022 | 2,609,819 | samples | 647,950 | 24.8 | 36.6 | 9.1 | [395] | |
| 17 Nov 2022 | 36,073,768 | samples | 4,177,786 | 11.6 | 109.9 | 12.7 | [396] | |
| 19 Dec 2022 | 33,903,886 | samples | 4,057,129 | 12.0 | 33.6 | 4.0 | [397][398] | |
| 27 Apr 2022 | 36,064,311 | samples | 5,993,861 | 16.6 | 94.0 | 15.6 | [399] | |
| 5 Jan 2022 | 27,515,490 | samples | 1,499,976 | 5.5 | 268 | 14.6 | [400] | |
| 11 Nov 2022 | 4,061,988 | cases | 473,440 | 11.7 | 141 | 16.4 | [401] | |
| 29 Jan 2021 | 5,405,393 | samples | 724,250 | 13.4 | 27.9 | 3.7 | [402] | |
| 6 Jun 2022 | 295,542,733 | samples | 18,358,459 | 6.2 | 201 | 12.5 | [403][404] | |
| 6 Oct 2021 | 2,885,812 | samples | 98,209 | 3.4 | 22.3 | 0.76 | [405] | |
| 26 Aug 2021 | 30,231 | cases | 995 | 3.3 | 57.6 | 1.9 | [406] | |
| 7 Oct 2022 | 212,132 | samples | 29,550 | 13.9 | 116.6 | 16.2 | [407] | |
| 10 Dec 2022 | 112,841 | cases | 9,500 | 8.4 | 102.4 | 8.6 | [408] | |
| 1 Jan 2023 | 189,365 | samples | 23,176 | 12.2 | 553 | 67.7 | [409] | |
| 26 Apr 2022 | 41,849,069 | samples | 753,632 | 1.8 | 120 | 2.2 | [410] | |
| 12 Jul 2021 | 624,502 | samples | 46,509 | 7.4 | 3.9 | 0.29 | [411] | |
| 24 Dec 2022 | 11,817,342 | cases | 2,440,248 | 20.6 | 170 | 35.0 | [412] | |
| 3 Aug 2021 | 16,206,203 | samples | 65,315 | 0.40 | 284 | 1.1 | [413][414] | |
| 26 Dec 2022 | 7,371,522 | samples | 1,858,747 | 25.2 | 135 | 34.1 | [415] | |
| 26 Dec 2022 | 2,803,682 | samples | 1,301,004 | 46.4 | 134 | 62.1 | [416] | |
| 24 May 2021 | 11,378,282 | cases | 1,637,848 | 14.4 | 19.2 | 2.8 | [417][418] | |
| 1 Mar 2021 | 6,592,010 | samples | 90,029 | 1.4 | 12.7 | 0.17 | [419] | |
| 26 May 2021 | 164,472 | 10,688 | 6.5 | 1.3 | 0.084 | [420] | ||
| 1 Jul 2021 | 54,128,524 | samples | 3,821,305 | 7.1 | 116 | 8.2 | [421][422] | |
| 30 Mar 2021 | 2,384,745 | samples | 93,128 | 3.9 | 10.9 | 0.43 | [423][424] | |
| 7 Jan 2021 | 158,804 | samples | 23,316 | 14.7 | 0.36 | 0.053 | [256] | |
| 24 May 2021 | 9,996,795 | samples | 1,074,751 | 10.8 | 96.8 | 10.4 | [425][426] | |
| 7 Nov 2022 | 23,283,909 | samples | 4,276,836 | 18.4 | 270 | 49.7 | [427] | |
| 18 Dec 2022 | 28,928,047 | samples | 8,588,414 | 29.69 | 122.6 | 36.385 | [428] | |
| 18 Nov 2020 | 3,880 | 509 | 13.1 | 0.0065 | 0.00085 | [256] | ||
| 4 Mar 2021 | 1,579,597 | cases | 26,162 | 1.7 | 2.3 | 0.038 | [429] | |
| 10 Dec 2022 | 802,777 | 39,337 | 4.9 | 9.3 | 0.46 | [430] | ||
| 3 Jan 2022 | 512,730 | cases | 92,997 | 18.1 | 37.6 | 6.8 | [431] | |
| 23 Aug 2021 | 2,893,625 | samples | 703,732 | 24.3 | 24.5 | 6.0 | [432] | |
| 2 Jul 2021 | 61,236,294 | samples | 5,435,831 | 8.9 | 73.6 | 6.5 | [433] | |
| 11 Feb 2021 | 852,444 | samples | 39,979 | 4.7 | 1.9 | 0.087 | [434] | |
| 24 Nov 2021 | 15,648,456 | samples | 3,367,461 | 21.5 | 37.2 | 8.0 | [435] | |
| 4 Jan 2023 | 198,046,693 | samples | 1,047,290 | 0.53 | 2,063 | 10.9 | [436] | |
| 19 May 2022 | 522,526,476 | samples | 22,232,377 | 4.3 | 774 | 32.9 | [437] | |
| 29 Jul 2022 | 929,349,291 | samples | 90,749,469 | 9.8 | 281 | 27.4 | [438][439] | |
| 16 Apr 2022 | 6,089,116 | samples | 895,592 | 14.7 | 175 | 25.8 | [440] | |
| 7 Sep 2020 | 2,630,000 | samples | 43,975 | 1.7 | 7.7 | 0.13 | [441] | |
| 30 Mar 2021 | 3,179,074 | samples | 159,149 | 5.0 | 11.0 | 0.55 | [442] | |
| 28 Aug 2022 | 45,772,571 | samples | 11,403,302 | 24.9 | 46.4 | 11.6 | [443] | |
| 10 Mar 2022 | 3,301,860 | samples | 314,850 | 9.5 | 19.0 | 1.8 | [444] | |
| 15 Oct 2022 | 2,529,087 | samples | 257,893 | 10.2 | 17.0 | 1.7 | [256][445] | |
|
See also
- 2002–2004 SARS outbreak
- Coronavirus breathalyzer
- Coronavirus disease 2019
- COVID-19 misinformation § PCR testing
- COVID-19 pandemic
- Philippine government response to the COVID-19 pandemic § COVID-19 testing controversy
References
This article incorporates public domain material from Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19. Centers for Disease Control and Prevention. Retrieved 5 May 2020.
- ^ «Coronavirus Disease 2019 (COVID-19)». U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Archived from the original on 14 March 2020. Retrieved 9 June 2020.
- ^ Kobokovich A, West R, Gronvall G. «Global Progress on COVID-19 Serology-Based Testing». Johns Hopkins Center for Health Security. Archived from the original on 9 June 2020. Retrieved 9 June 2020.
- ^ a b c Kubina R, Dziedzic A (June 2020). «Molecular and Serological Tests for COVID-19 a Comparative Review of SARS-CoV-2 Coronavirus Laboratory and Point-of-Care Diagnostics». Diagnostics. 10 (6): 434. doi:10.3390/diagnostics10060434. PMC 7345211. PMID 32604919.
- ^ «Test for Past Infection». U.S. Centers for Disease Control and Prevention (CDC). 2020. Archived from the original on 16 May 2020. Retrieved 19 May 2020.
Antibody blood tests, also called antibody tests, check your blood by looking for antibodies, which show if you had a previous infection with the virus. Depending on when someone was infected and the timing of the test, the test may not find antibodies in someone with a current COVID-19 infection.
- ^ a b c Abbasi J (May 2020). «The Promise and Peril of Antibody Testing for COVID-19». JAMA. 323 (19): 1881–1883. doi:10.1001/jama.2020.6170. PMID 32301958. Archived from the original on 20 April 2020. Retrieved 20 April 2020.
- ^ a b Brotschi M (7 March 2020). «Bund sucht nicht mehr alle Corona-Infizierten» [The federal government is no longer looking for all those infected with corona]. Der Bund (in German). ISSN 0774-6156. Archived from the original on 29 March 2020. Retrieved 9 June 2020.
- ^ Van Beusekom M (24 March 2020). «Italian doctors note high COVID-19 death rate, urge action». CIDRAP News. Archived from the original on 9 June 2020. Retrieved 9 June 2020.
- ^ a b Otmani M (22 March 2020). «COVID-19: First results of the voluntary screening in Iceland». Nordic Life Science. Archived from the original on 29 March 2020. Retrieved 9 June 2020.
- ^ a b Ward D (April 2020). «Sampling bias: explaining wide variations in COVID-19 case fatality rates». Preprint. Bern, Switzerland: WardEnvironment. doi:10.13140/RG.2.2.24953.62564/1.
- ^ Henriques M (2 April 2020). «Coronavirus: Why death and mortality rates differ». BBC News. Archived from the original on 2 April 2020. Retrieved 9 June 2020.
- ^ a b Ward D (May 2020). Sampling Bias: Explaining Variations in Age Distributions of COVID-19 Cases. Technical Report (Report). WardEnvironment. doi:10.13140/RG.2.2.27321.19047/2.
- ^ «Why More Younger People Are Testing Positive for COVID-19». Time. Archived from the original on 26 February 2021. Retrieved 18 August 2020.
- ^ Mina MJ, Parker R, Larremore DB (November 2020). «Rethinking Covid-19 Test Sensitivity — A Strategy for Containment». The New England Journal of Medicine. 383 (22): e120. doi:10.1056/NEJMp2025631. PMID 32997903. S2CID 222158786.
- ^ a b «Antigen-detection in the diagnosis of SARS-CoV-2 infection». www.who.int. Retrieved 12 July 2022.
- ^ a b c CDC (11 February 2020). «Guidance for Antigen Testing for SARS-CoV-2 for Healthcare Providers Testing Individuals in the Community». Centers for Disease Control and Prevention. Retrieved 12 July 2022.
- ^ «Siouxsie Wiles & Toby Morris: What we don’t know about Covid-19». The Spinoff. 6 May 2020. Archived from the original on 22 August 2020. Retrieved 6 May 2020.
- ^ «Testing for COVID-19». U.S. Centers for Disease Control and Prevention (CDC). 20 May 2020. Archived from the original on 19 May 2020. Retrieved 20 May 2020.
Two kinds of tests are available for COVID-19: viral tests and antibody tests.
- ^ Tanner T (23 September 2020). «Finland deploys coronavirus-sniffing dogs at main airport». Associated Press. Helsinki. Archived from the original on 27 October 2020. Retrieved 28 October 2020.
- ^ Jones RT, Guest C, Lindsay SW, Kleinschmidt I, Bradley J, Dewhirst S, et al. (December 2020). «Could bio-detection dogs be used to limit the spread of COVID-19 by travellers?». Journal of Travel Medicine. 27 (8). doi:10.1093/jtm/taaa131. PMC 7454791. PMID 32789466.
- ^ Jendrny P, Schulz C, Twele F, Meller S, von Köckritz-Blickwede M, Osterhaus AD, et al. (July 2020). «Scent dog identification of samples from COVID-19 patients — a pilot study». BMC Infectious Diseases. 20 (1): 536. doi:10.1186/s12879-020-05281-3. PMC 7376324. PMID 32703188.
- ^ a b Habibzadeh P, Mofatteh M, Silawi M, Ghavami S, Faghihi MA (September 2021). «Molecular diagnostic assays for COVID-19: an overview». Critical Reviews in Clinical Laboratory Sciences. 58 (6): 385–398. doi:10.1080/10408363.2021.1884640. PMC 7898297. PMID 33595397.
- ^ «RNA Extraction». AssayGenie. Archived from the original on 6 May 2020. Retrieved 7 May 2020.
- ^ a b «How is the COVID-19 Virus Detected using Real Time RT-PCR?». IAEA. 27 March 2020. Archived from the original on 1 May 2020. Retrieved 5 May 2020.
- ^ «Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe». GlobeNewswire News Room. 30 January 2020. Archived from the original on 31 January 2020. Retrieved 1 February 2020.
- ^ a b Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (April 2009). «The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments». Clinical Chemistry. 55 (4): 611–622. doi:10.1373/clinchem.2008.112797. PMID 19246619.
- ^ «Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis» (PDF). Clinical Science. 23 September 2005. Archived (PDF) from the original on 24 November 2020. Retrieved 5 May 2020.
- ^ «The Basics: RT-PCR». ThermoFisher Scientific. Archived from the original on 14 April 2020. Retrieved 5 May 2020.
- ^ Kang XP, Jiang T, Li YQ, Lin F, Liu H, Chang GH, et al. (June 2010). «A duplex real-time RT-PCR assay for detecting H5N1 avian influenza virus and pandemic H1N1 influenza virus». Virology Journal. 7: 113. doi:10.1186/1743-422X-7-113. PMC 2892456. PMID 20515509.
- ^ Joyce C (2002). Quantitative RT-PCR. A review of current methodologies. Methods Mol. Biol. Vol. 193. pp. 83–92. doi:10.1385/1-59259-283-X:083. ISBN 978-1-59259-283-8. PMID 12325527.
- ^ Varkonyi-Gasic E, Hellens RP (2010). «qRT-PCR of Small RNAs». Plant Epigenetics. Methods in Molecular Biology. Vol. 631. pp. 109–22. doi:10.1007/978-1-60761-646-7_10. ISBN 978-1-60761-645-0. PMID 20204872.
- ^ «Accelerated Emergency Use Authorization (Eua) Summary Covid-19 Rt-Pcr Test (Laboratory Corporation of America)». FDA. Archived from the original on 16 January 2021. Retrieved 3 April 2020.
- ^ Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M (April 2010). «A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines». Methods. 50 (4): S1–S5. doi:10.1016/j.ymeth.2010.01.005. PMID 20215014.
- ^ Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. (March 2021). «Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection». The Cochrane Database of Systematic Reviews. 3 (4): CD013705. doi:10.1002/14651858.CD013705.pub2. PMC 8078597. PMID 33760236.
- ^ Dinnes J, Sharma P, Berhane S, van Wyk SS, Nyaaba N, Domen J, et al. (July 2022). «Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection». The Cochrane Database of Systematic Reviews. 2022 (7): CD013705. doi:10.1002/14651858.CD013705.pub3. PMC 9305720. PMID 35866452.
- ^ «Real-Time RT-PCR Panel for Detection 2019-nCoV». U.S. Centers for Disease Control and Prevention (CDC). 29 January 2020. Archived from the original on 30 January 2020. Retrieved 1 February 2020.
- ^ a b c Drosten C (26 March 2020). «Coronavirus-Update Folge 22» [Coronavirus update episode 22] (PDF). NDR. Archived (PDF) from the original on 31 March 2020. Retrieved 2 April 2020.
- ^ a b «Here’s where things stand on COVID-19 tests in the U.S.» Science News. ScienceNews. 17 April 2020. Archived from the original on 28 April 2020. Retrieved 6 May 2020.
- ^ a b c Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q (April 2020). «Saliva: potential diagnostic value and transmission of 2019-nCoV». International Journal of Oral Science. 12 (1): 11. doi:10.1038/s41368-020-0080-z. PMC 7162686. PMID 32300101.
- ^ Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. (May 2003). «Identification of a novel coronavirus in patients with severe acute respiratory syndrome». The New England Journal of Medicine. 348 (20): 1967–1976. doi:10.1056/NEJMoa030747. PMID 12690091.
- ^ Ghoshal U, Vasanth S, Tejan N (June 2020). «A guide to laboratory diagnosis of Corona Virus Disease-19 for the gastroenterologists». Indian Journal of Gastroenterology. 39 (3): 236–242. doi:10.1007/s12664-020-01082-3. PMC 7462729. PMID 32875524.
- ^ «COVID-19 saliva tests: What is the benefit?». Mayo Clinic. 16 April 2020. Archived from the original on 1 May 2020. Retrieved 6 May 2020.
- ^ a b «New Rutgers Saliva Test for Coronavirus Gets FDA Approval». Rutgers.edu. 13 April 2020. Archived from the original on 30 April 2020. Retrieved 1 May 2020.
- ^ «FDA authorizes Covid-19 saliva test for emergency use». CNN. 14 April 2020. Archived from the original on 27 April 2020. Retrieved 1 May 2020.
- ^ Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, et al. (September 2020). «Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2». The New England Journal of Medicine. 383 (13): 1283–1286. doi:10.1056/NEJMc2016359. PMC 7484747. PMID 32857487. S2CID 221358482.
- ^ Service RF (August 2020). «Spit shines for easier coronavirus testing». Science. 369 (6507): 1041–1042. Bibcode:2020Sci…369.1041S. doi:10.1126/science.369.6507.1041. PMID 32855317. S2CID 221358939.
- ^ «Yale University School of Public Health finds saliva samples promising alternative to nasopharyngeal swab». Merck Manual. 29 April 2020. Archived from the original on 28 May 2020. Retrieved 6 April 2020.
- ^ «FDA gives emergency approval to ‘game changer’ COVID-19 saliva test». The Washington Times. Archived from the original on 16 August 2020. Retrieved 15 August 2020.
- ^ «Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization to Yale School of Public Health for SalivaDirect, Which Uses a New Method of Saliva Sample Processing». U.S. Food and Drug Administration (FDA) (Press release). 15 August 2020. Archived from the original on 16 August 2020. Retrieved 6 November 2020.
- ^ a b
One or more of the preceding sentences incorporates text from this source, which is in the public domain: «Risk of False Results with the Curative SARS-Cov-2 Test for COVID-19». U.S. Food and Drug Administration (FDA). 4 January 2021. Archived from the original on 4 January 2021. Retrieved 4 January 2021.
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- COVID-19 Investigation Team (June 2020). «Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States». Nature Medicine. 26 (6): 861–868. doi:10.1038/s41591-020-0877-5. PMID 32327757.
- Young BE, Ong SW, Kalimuddin S, Low JG, Tan SY, Loh J, et al. (April 2020). «Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore». JAMA. 323 (15): 1488–1494. doi:10.1001/jama.2020.3204. PMC 7054855. PMID 32125362.
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. (March 2020). «SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients». The New England Journal of Medicine. 382 (12): 1177–1179. doi:10.1056/NEJMc2001737. PMC 7121626. PMID 32074444.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. (May 2020). «Virological assessment of hospitalized patients with COVID-2019». Nature. 581 (7809): 465–469. Bibcode:2020Natur.581..465W. doi:10.1038/s41586-020-2196-x. PMID 32235945.
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Young et al. (2020)
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- COVID-19 Investigation Team (June 2020). «Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States». Nature Medicine. 26 (6): 861–868. doi:10.1038/s41591-020-0877-5. PMID 32327757.
- ^ Zimmer C (5 May 2020). «With Crispr, a Possible Quick Test for the Coronavirus». The New York Times. ISSN 0362-4331. Archived from the original on 14 May 2020. Retrieved 14 May 2020.
- ^ «STOPCovid». stopcovid.science. Archived from the original on 10 June 2020. Retrieved 14 June 2020.
- ^ Joung J, Ladha A, Saito M, Segel M, Bruneau R, Huang MW, et al. (May 2020). «Point-of-care testing for COVID-19 using SHERLOCK diagnostics». medRxiv: 2020.05.04.20091231. doi:10.1101/2020.05.04.20091231. PMC 7273289. PMID 32511521. Archived from the original on 16 May 2021. Retrieved 2 July 2021.
- ^ a b c d e f «Developing Antibodies and Antigens for COVID-19 Diagnostics». Technology Networks. 6 April 2020. Archived from the original on 30 April 2020. Retrieved 30 April 2020.
- ^ Guglielmi G (September 2020). «Fast coronavirus tests: what they can and can’t do». Nature. 585 (7826): 496–498. Bibcode:2020Natur.585..496G. doi:10.1038/d41586-020-02661-2. PMID 32939084. S2CID 221768935.
- ^ CDC (11 February 2020). «COVID-19 and Your Health». Centers for Disease Control and Prevention. Retrieved 12 July 2022.
- ^ «Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing». whitehouse.gov. 17 April 2020. Archived from the original on 20 January 2021. Retrieved 30 April 2020 – via National Archives.
- ^ Müllender F (11 March 2021). «Grundschulen – Corona-Pool-Tests gelten als kindgerecht, unkompliziert und sicher» (in German). Deutschlandfunk. Archived from the original on 24 July 2021. Retrieved 5 June 2021.
- ^ «NIH launches competition to speed COVID-19 diagnostics». AAAS. 29 April 2020. Archived from the original on 1 May 2020. Retrieved 1 May 2020.
- ^ a b «What to know about the three main types of coronavirus tests». CNN. 29 April 2020. Archived from the original on 10 May 2020. Retrieved 30 April 2020.
- ^ a b Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. (Cochrane COVID-19 Diagnostic Test Accuracy Group) (March 2021). «Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection». The Cochrane Database of Systematic Reviews. 3 (3): CD013705. doi:10.1002/14651858.CD013705.pub2. PMC 8078597. PMID 33760236.
- ^ «Rapid Tests». Rapid Tests. Archived from the original on 31 May 2021. Retrieved 2 July 2021.
- ^ Shaw J (3 August 2020). «Failing the Coronavirus-Testing Test». Harvard Magazine. Archived from the original on 30 June 2021. Retrieved 2 July 2021.
- ^ a b Office of the Commissioner (9 May 2020). «Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients». FDA. Archived from the original on 29 May 2021. Retrieved 2 July 2021.
- ^ a b c d Klasse PJ (9 September 2014). «Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives». Advances in Biology. Hindawi Limited. 2014: 1–24. doi:10.1155/2014/157895. PMC 4835181. PMID 27099867.
- ^ «The next frontier in coronavirus testing: Identifying the full scope of the pandemic, not just individual infections». STAT. 27 March 2020. Archived from the original on 29 June 2020. Retrieved 30 April 2020.
- ^ a b Tang EW, Bobenchik AM, Lu S (September 2020). «Testing for SARS-CoV-2 (COVID-19): A General Review». Rhode Island Medical Journal. 103 (8): 20–23. PMID 32900007.
- ^ «What Immunity to COVID-19 Really Means». Scientific American. 10 April 2020. Archived from the original on 28 April 2020.
- ^ a b Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al. (June 2020). «Antibody tests for identification of current and past infection with SARS-CoV-2». The Cochrane Database of Systematic Reviews. 2020 (6): CD013652. doi:10.1002/14651858.CD013652. PMC 7387103. PMID 32584464. S2CID 220061130.
- ^ «Cellex Emergency Use Authorization». FDA. 1 April 2020. Archived from the original on 9 April 2020. Retrieved 10 April 2020.
- ^ «Will an Antibody Test Allow Us to Go Back to School or Work?». The New York Times. 10 April 2020. Archived from the original on 15 April 2020. Retrieved 15 April 2020.
- ^ «Mount Sinai Emergency Use Authorization». FDA. 15 April 2020. Retrieved 18 April 2020.
- ^ Bauer G (January 2021). «The variability of the serological response to SARS-corona virus-2: Potential resolution of ambiguity through determination of avidity (functional affinity)». Journal of Medical Virology. 93 (1): 311–322. doi:10.1002/jmv.26262. PMC 7361859. PMID 32633840.
- ^ Ravi N, Cortade DL, Ng E, Wang SX (October 2020). «Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape». Biosensors & Bioelectronics. 165: 112454. doi:10.1016/j.bios.2020.112454. PMC 7368663. PMID 32729549.
- ^ Goudouris ES (2020). «Laboratory diagnosis of COVID-19». Jornal de Pediatria. 97 (1): 7–12. doi:10.1016/j.jped.2020.08.001. PMC 7456621. PMID 32882235.
- ^ a b c d «Global Progress on COVID-19 Serology-Based Testing». Johns Hopkins Center for Health Security. Archived from the original on 14 June 2020. Retrieved 14 June 2020.
- ^ a b Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, et al. (September 2020). «A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction». Nature Biotechnology. 38 (9): 1073–1078. doi:10.1038/s41587-020-0631-z. PMID 32704169. S2CID 220720953.
- ^ a b c Mallapaty S (April 2020). «Will antibody tests for the coronavirus really change everything?». Nature. 580 (7805): 571–572. Bibcode:2020Natur.580..571M. doi:10.1038/d41586-020-01115-z. PMID 32313159. S2CID 216048544. Archived from the original on 24 June 2020. Retrieved 20 April 2020.
- ^ a b c «Q&A on COVID-19 Antibody Tests». factcheck.org. 27 April 2020. Archived from the original on 27 April 2020. Retrieved 28 April 2020.
- ^ «Neutralising antibody». Biology-Online. 2008. Archived from the original on 8 July 2018. Retrieved 4 July 2009.
- ^ Schmaljohn AL (July 2013). «Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts». Current HIV Research. 11 (5): 345–353. doi:10.2174/1570162×113116660057. PMID 24191933.
- ^ Rhorer J, Ambrose CS, Dickinson S, Hamilton H, Oleka NA, Malinoski FJ, Wittes J (February 2009). «Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials». Vaccine. Virology Blog. 27 (7): 1101–1110. doi:10.1016/j.vaccine.2008.11.093. PMID 19095024. Archived from the original on 23 April 2020. Retrieved 29 April 2020.
- ^ «expert reaction to announcement by Roche of its new serology test for COVID-19 antibodies». Science Media Centre. 17 April 2020. Archived from the original on 30 April 2020. Retrieved 28 April 2020.
- ^ Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH (September 2007). «Disappearance of antibodies to SARS-associated coronavirus after recovery». The New England Journal of Medicine. NEJM. 357 (11): 1162–1163. doi:10.1056/NEJMc070348. PMID 17855683.
- ^ a b «Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study» (PDF). Journal of Immunology. 19 April 2011. Archived (PDF) from the original on 1 May 2020. Retrieved 1 May 2020.
- ^ Leslie M (May 2020). «T cells found in coronavirus patients ‘bode well’ for long-term immunity». Science. 368 (6493): 809–810. Bibcode:2020Sci…368..809L. doi:10.1126/science.368.6493.809. PMID 32439770. S2CID 218834495.
- ^ Calvo-Henriquez C, Maldonado-Alvarado B, Chiesa-Estomba C, Rivero-Fernández I, Sanz-Rodriguez M, Villarreal IM, et al. (October 2020). «Ethyl alcohol threshold test: a fast, reliable and affordable olfactory Assessment tool for COVID-19 patients». European Archives of Oto-Rhino-Laryngology. 277 (10): 2783–2792. doi:10.1007/s00405-020-06131-3. PMC 7312102. PMID 32583183.
- ^ Hayes J, Exten C, State P (24 December 2020). «At-home DIY smell tests could catch Covid-19 cases». CNN Health. The Conversation. Retrieved 7 September 2021.
- ^ Menni C, Sudre CH, Steves CJ, Ourselin S, Spector TD (November 2020). «Widespread smell testing for COVID-19 has limited application — Authors’ reply». Lancet. 396 (10263): 1630–1631. doi:10.1016/S0140-6736(20)32316-3. PMC 7832202. PMID 33157000.
- ^ a b c Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (July 2020). «Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients». AJR. American Journal of Roentgenology. 215 (1): 87–93. doi:10.2214/AJR.20.23034. PMID 32174129.
Known features of COVID-19 on initial CT include bilateral multilobar ground-glass opacification (GGO) with a peripheral or posterior distribution, mainly in the lower lobes and less frequently within the right middle lobe.
- ^ Manigandan S, Wu MT, Ponnusamy VK, Raghavendra VB, Pugazhendhi A, Brindhadevi K (November 2020). «A systematic review on recent trends in transmission, diagnosis, prevention and imaging features of COVID-19». Process Biochemistry. 98: 233–240. doi:10.1016/j.procbio.2020.08.016. PMC 7439988. PMID 32843849.
- ^ Lee EY, Ng MY, Khong PL (April 2020). «COVID-19 pneumonia: what has CT taught us?». The Lancet. Infectious Diseases. 20 (4): 384–385. doi:10.1016/S1473-3099(20)30134-1. PMC 7128449. PMID 32105641.
- ^ «ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection». American College of Radiology. 22 March 2020. Archived from the original on 13 May 2020. Retrieved 20 May 2020.
- ^ a b c Tabik S, Gomez-Rios A, Martin-Rodriguez JL, Sevillano-Garcia I, Rey-Area M, Charte D, et al. (December 2020). «COVIDGR Dataset and COVID-SDNet Methodology for Predicting COVID-19 Based on Chest X-Ray Images». IEEE Journal of Biomedical and Health Informatics. 24 (12): 3595–3605. doi:10.1109/JBHI.2020.3037127. PMID 33170789. S2CID 219179286.
- ^ Tay YX, Kothan S, Kada S, Cai S, Lai CW (May 2021). «Challenges and optimization strategies in medical imaging service delivery during COVID-19». World Journal of Radiology. 13 (5): 102–121. doi:10.4329/wjr.v13.i5.102. PMC 8188837. PMID 34141091.
- ^ a b Alsharif W, Qurashi A (May 2021). «Effectiveness of COVID-19 diagnosis and management tools: A review». Radiography. 27 (2): 682–687. doi:10.1016/j.radi.2020.09.010. PMC 7505601. PMID 33008761.
- ^ Inui S, Gonoi W, Kurokawa R, Nakai Y, Watanabe Y, Sakurai K, et al. (November 2021). «The role of chest imaging in the diagnosis, management, and monitoring of coronavirus disease 2019 (COVID-19)». Insights into Imaging. 12 (1): 155. doi:10.1186/s13244-021-01096-1. PMC 8561360. PMID 34727257.
- ^ Panwar H, Gupta PK, Siddiqui MK, Morales-Menendez R, Singh V (September 2020). «Application of deep learning for fast detection of COVID-19 in X-Rays using nCOVnet». Chaos, Solitons, and Fractals. 138: 109944. Bibcode:2020CSF…13809944P. doi:10.1016/j.chaos.2020.109944. PMC 7254021. PMID 32536759.
- ^ Inui S, Gonoi W, Kurokawa R, Nakai Y, Watanabe Y, Sakurai K, et al. (November 2021). «The role of chest imaging in the diagnosis, management, and monitoring of coronavirus disease 2019 (COVID-19)». Insights into Imaging. 12 (1): 155. doi:10.1186/s13244-021-01096-1. PMC 8561360. PMID 34727257.
- ^ «Dutch corona blood test from Eindhoven goes international». 19 April 2021. Archived from the original on 27 April 2021. Retrieved 2 July 2021.
- ^ Biesemans B. «Bees in the Netherlands trained to detect COVID-19 infections». Reuters. Archived from the original on 30 June 2021. Retrieved 2 July 2021.
- ^ Henley J (20 May 2021). «Dogs can better detect Covid in humans than lateral flow tests, finds study». The Guardian. Archived from the original on 29 June 2021.
- ^ Grandjean D, Elie C, Gallet C, Julien C, Roger V, Desquilbet L, et al. (8 March 2022). «Diagnostic accuracy of non-invasive detection of SARS-CoV-2 infection by canine olfaction». PLOS ONE. Cold Spring Harbor Laboratory. 17 (6): e0268382. doi:10.1101/2022.03.07.22271219. PMC 9159600. PMID 35648737. S2CID 247291441.
- ^ «Dogs Sniff Out Coronavirus With High Accuracy». Medscape. Reuters. 10 March 2022.
- ^ «Todos Medical Announces Positive Data in Hospitalized and Outpatient Setting for TolloTest, a Novel SARS-CoV-2 3CL Protease Biomarker Assay». Yahoo.
- ^ Roser M, Ritchie H, Ortiz-Ospina E, Hasell J (4 March 2020). «Coronavirus Disease (COVID-19) – Statistics and Research». Our World in Data. Archived from the original on 19 March 2020. Retrieved 2 July 2021 – via ourworldindata.org.
- ^ Schnirring L (11 January 2020). «China releases genetic data on new coronavirus, now deadly». CIDRAP. Archived from the original on 11 January 2020. Retrieved 12 January 2020.
- ^ Shu Y, McCauley J (March 2017). «GISAID: Global initiative on sharing all influenza data — from vision to reality». Euro Surveillance. 22 (13). doi:10.2807/1560-7917.ES.2017.22.13.30494. PMC 5388101. PMID 28382917.
- ^ Ioannidis JP (17 March 2020). «A fiasco in the making? As the coronavirus pandemic takes hold, we are making decisions without reliable data». STAT. Archived from the original on 5 April 2020. Retrieved 22 March 2020.
- ^ «‘Test, Test, Test’: WHO Chief’s Coronavirus Message to World». The New York Times. Reuters. 16 March 2020. Archived from the original on 20 March 2020. Retrieved 16 March 2020.
- ^ Farge E, Revill J (17 March 2020). «‘Test, test, test’: WHO chief’s coronavirus message to world». Reuters. Archived from the original on 3 November 2020. Retrieved 6 November 2020.
- ^ «Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK» (PDF). European Centre for Disease Prevention and Control. 25 March 2020. pp. 15–16. Archived (PDF) from the original on 26 March 2020. Retrieved 29 March 2020.
the current shortages of laboratory consumables and reagents affect diagnostic capacity and hamper the epidemic response at the national and local levels. The laboratories have experienced delayed or missing deliveries of swabbing material, plastic consumables, RNA extraction and RT-PCR reagents, and PPE. This is affecting laboratories in all EU/EEA countries.
- ^ Baird RP (24 March 2020). «Why Widespread Coronavirus Testing Isn’t Coming Anytime Soon». The New Yorker. Archived from the original on 28 March 2020. Retrieved 29 March 2020.
South Dakota, said that her state’s public-health laboratory—the only lab doing COVID-19 testing in the state—had so much trouble securing reagents that it was forced to temporarily stop testing altogether. also noted critical shortages of extraction kits, reagents, and test kits
- ^ Ossola A (25 March 2020). «Here are the coronavirus testing materials that are in short supply in the US». Quartz. Archived from the original on 26 March 2020. Retrieved 29 March 2020.
extract the virus’s genetic material—in this case, RNA—using a set of chemicals that usually come in pre-assembled kits. ‘The big shortage is extraction kits’ There are no easy replacements here: ‘These reagents that are used in extraction are fairly complex chemicals. They have to be very pure, and they have to be in pure solution’
- ^ Temple-Raston D (6 November 2020). «CDC Report: Officials Knew Coronavirus Test Was Flawed But Released It Anyway». NPR. Archived from the original on 11 June 2021. Retrieved 20 March 2021.
- ^ Armario C (7 October 2020). «Peru bet heavily on cheap COVID tests; it didn’t go well». Associated Press. Archived from the original on 14 January 2021. Retrieved 20 March 2021.
- ^ Kiger J (12 March 2020). «Mayo Clinic starts drive-thru testing for COVID-19». PostBulletin.com. Archived from the original on 12 March 2020. Retrieved 13 March 2020.
- ^ Hawkins AJ (11 March 2020). «Some states are offering drive-thru coronavirus testing». The Verge. Archived from the original on 11 March 2020. Retrieved 13 March 2020.
- ^ «South Korea’s Drive-Through Testing For Coronavirus Is Fast – And Free». npr. 11 March 2020. Archived from the original on 20 March 2020. Retrieved 16 March 2020.
- ^ Beaubien J (23 February 2020). «In Age of COVID-19, Hong Kong Innovates To Test And Quarantine Thousands». NPR. Archived from the original on 24 February 2020. Retrieved 26 February 2020.
- ^ «Pooling method allows dozens of COVID-19 tests to run simultaneously». medicalxpress.com. Archived from the original on 22 March 2020. Retrieved 24 March 2020.
- ^ «Israeli team has coronavirus test kit to test dozens of people at once». The Jerusalem Post | JPost.com. Archived from the original on 23 March 2020. Retrieved 24 March 2020.
- ^ Israel21c Staff (19 March 2020). «Israelis introduce method for accelerated COVID-19 testing». Israel21c. Archived from the original on 22 March 2020. Retrieved 24 March 2020.
- ^ «We ‘pool’ coronavirus samples to test 1,000s at a go; we’ve done 30,000 since Sunday – Noguchi». GhanaWeb. 22 April 2020. Archived from the original on 15 May 2020. Retrieved 22 April 2020.
- ^ «Pooling samples boosts Ghana’s COVID-19 testing». WHO Africa. 31 July 2020. Archived from the original on 5 August 2020. Retrieved 31 July 2020.
- ^ «Pooling samples boosts Ghana’s COVID-19 testing». World Health Organization. 30 July 2020. Archived from the original on 21 August 2020. Retrieved 30 July 2020.
- ^ «[Coronavirus] Verified ‘sample pooling’ introduced to prevent herd infection in S. Korea». ajudaily.com. 9 April 2020. Archived from the original on 10 April 2020. Retrieved 19 April 2020.
- ^ «Gov. Ricketts provides update on coronavirus testing». KMTV. 24 March 2020. Archived from the original on 20 April 2020. Retrieved 19 April 2020.
- ^ Lanese N (28 May 2020). «Wuhan tested millions of people for COVID-19 in just days. Could US cities do the same?». livescience.com. Archived from the original on 28 June 2020. Retrieved 28 June 2020.
- ^ «Latest coronavirus update: UP to begin ‘pool testing’ of Covid suspects». Free Press Journal. Archived from the original on 17 April 2020. Retrieved 19 April 2020.
- ^ Yengkhom S. «West Bengal to start pool testing of samples in low-risk zones». The Times of India. Archived from the original on 20 April 2020. Retrieved 19 April 2020.
- ^ «Punjab launches pool testing». Archived from the original on 4 May 2020. Retrieved 19 April 2020.
- ^ «‘Chhattisgarh to adopt pool sample testing’: Health minister TS Singh Deo on Covid-19″. Hindustan Times. 15 April 2020. Archived from the original on 19 April 2020. Retrieved 19 April 2020.
- ^ «Maharashtra to go for pool testing to defeat coronavirus». Deccan Herald. 12 April 2020. Archived from the original on 15 April 2020. Retrieved 19 April 2020.
- ^ «Origami Assays». Origami Assays. 2 April 2020. Archived from the original on 5 April 2020. Retrieved 7 April 2020.
- ^ Pulia MS, O’Brien TP, Hou PC, Schuman A, Sambursky R (August 2020). «Multi-tiered screening and diagnosis strategy for COVID-19: a model for sustainable testing capacity in response to pandemic». Annals of Medicine. 52 (5): 207–214. doi:10.1080/07853890.2020.1763449. PMC 7877955. PMID 32370561. S2CID 218519851.
- ^ «Which States Are Doing Enough Testing? This Benchmark Helps Settle The Debate». NPR.org. 22 April 2020. Archived from the original on 11 May 2020. Retrieved 11 May 2020.
- ^ Lee TB (2 April 2020). «America’s COVID-19 testing has stalled, and that’s a big problem». Ars Technica. Archived from the original on 14 June 2020. Retrieved 5 April 2020.
- ^ a b c d Romer P. «Roadmap to responsibly reopen America» (PDF). Archived (PDF) from the original on 11 May 2020. Retrieved 11 May 2020.
- ^ «ROADMAP TO PANDEMIC RESILIENCE» (PDF). Edmond J. Safra Center for Ethics. 20 April 2020. Archived (PDF) from the original on 20 May 2020. Retrieved 19 May 2020.
- ^ «Certified Service Providers». Pacific Biosciences. Archived from the original on 10 June 2020. Retrieved 18 May 2020.
- ^ «Service Provider Program – US». www.thermofisher.com. ThermoFisher Scientific. Archived from the original on 10 June 2020. Retrieved 18 May 2020.
- ^ «Paul Romer». paulromer.net. Simulating Covid-19: Part 2. Archived from the original on 18 May 2020. Retrieved 19 May 2020.
- ^ Lewis T. «Slovakia Offers a Lesson in How Rapid Testing Can Fight COVID». Scientific American. Archived from the original on 19 April 2021. Retrieved 19 April 2021.
- ^ Pavelka M, Van-Zandvoort K, Abbott S, Sherratt K, Majdan M, Jarčuška P, et al. (May 2021). «The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia». Science. 372 (6542): 635–641. Bibcode:2021Sci…372..635P. doi:10.1126/science.abf9648. PMC 8139426. PMID 33758017.
- ^ «Slovakia’s mass Covid testing cut infection rate by 60%, researchers say». The Guardian. 7 December 2020. Archived from the original on 5 May 2021. Retrieved 30 April 2021.
- ^ Sharif S, Ikram A, et al. (24 June 2020). «Detection of SARs-CoV-2 in wastewater, using the existing environmental surveillance network: An epidemiological gateway to an early warning for COVID-19 in communities». medRxiv. doi:10.1101/2020.06.03.20121426. S2CID 219322544.
- ^ «Coronavirus traces found in March 2019 sewage sample, Spanish study shows». Reuters. 26 June 2020. Retrieved 28 July 2021.
- ^ Kreier F (May 2021). «The myriad ways sewage surveillance is helping fight COVID around the world». Nature. doi:10.1038/d41586-021-01234-1. PMID 33972790. S2CID 234360319.
- ^ Agrawal S, Orschler L, Lackner S (March 2021). «Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany». Scientific Reports. 11 (1): 5372. Bibcode:2021NatSR..11.5372A. doi:10.1038/s41598-021-84914-2. PMC 7940401. PMID 33686189.
- ^ Rooney CM, Moura IB, Wilcox MH (January 2021). «Tracking COVID-19 via sewage». Current Opinion in Gastroenterology. 37 (1): 4–8. doi:10.1097/MOG.0000000000000692. PMID 33074996. S2CID 224811450.
- ^ Larsen DA, Wigginton KR (October 2020). «Tracking COVID-19 with wastewater». Nature Biotechnology. 38 (10): 1151–1153. doi:10.1038/s41587-020-0690-1. PMC 7505213. PMID 32958959.
- ^ Michael-Kordatou I, Karaolia P, Fatta-Kassinos D (October 2020). «Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification». Journal of Environmental Chemical Engineering. 8 (5): 104306. doi:10.1016/j.jece.2020.104306. PMC 7384408. PMID 32834990.
- ^ Seeger C. «Abwasserbasierte EpidemiologieAbwassermonitoring als Frühwarnsystem für Pandemien» (PDF). Retrieved 28 July 2021.
- ^ «[New Product] COVID-19 Kit». kogene.co.kr. 27 February 2020. Archived from the original on 23 April 2020.
- ^ «Letter from FDA». FDA. 27 March 2020. Archived from the original on 28 March 2020. Retrieved 2 April 2020.
- ^ a b ID NOW COVID-19 Archived 16 January 2021 at the Wayback Machine, Instruction for Use, FDA
- ^ «The scramble for the rapid coronavirus tests everybody wants». The Washington Post. 1 April 2020. Archived from the original on 10 February 2021. Retrieved 2 July 2021.
- ^ a b «FDA issues emergency approval of new antigen test that is cheaper, faster and simpler». The Washington Post. 9 May 2020. Archived from the original on 26 January 2021. Retrieved 2 July 2021.
- ^ a b c Sofia 2 SARS Antigen FIA Archived 2 April 2021 at the Wayback Machine Instructions for Use, FDA.gov
- ^ a b c Peplow M (14 June 2021). «COVID-19 test used in UK mass screening program receives stinging rebuke from FDA». Archived from the original on 15 June 2021. Retrieved 2 July 2021.
- ^ FDA Division of Industry and Consumer Education (10 June 2021). «Stop Using Innova Medical Group SARS-CoV-2 Antigen Rapid Qualitative Test: FDA Safety Communication». FDA. Archived from the original on 2 July 2021. Retrieved 2 July 2021.
- ^ Mina MJ, Peto TE, García-Fiñana M, Semple MG, Buchan IE (April 2021). «Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19». Lancet. 397 (10283): 1425–1427. doi:10.1016/S0140-6736(21)00425-6. PMC 8049601. PMID 33609444.
- ^ «NIH Begins Study to Quantify Undetected Cases of Coronavirus Infection | NIH: National Institute of Allergy and Infectious Diseases». niaid.nih.gov. Archived from the original on 10 April 2020. Retrieved 11 April 2020.
- ^ Mandavilli A, Thomas K (10 April 2020). «Will an Antibody Test Allow Us to Go Back to School or Work?». The New York Times. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
- ^ «Quest Diagnostics Launches Consumer-Initiated COVID-19 Antibody Test Through QuestDirect™». Quest Diagnosics. 28 April 2020. Archived from the original on 17 May 2021. Retrieved 2 July 2021.
- ^ Fellmann F. (March 2020). (in German) «Jetzt beginnt die Suche nach den Genesenen» Archived 28 March 2020 at the Wayback Machine. Tages Anzeiger. Retrieved 28 March 2020.
- ^ Herrera T (27 October 2020). «What You Need to Know About the Covid-19 Antibody Test». The New York Times. Retrieved 18 July 2021.
- ^ «EUA Authorized Serology Test Performance». U.S. Food and Drug Administration (FDA). 7 May 2020. Archived from the original on 8 May 2020. Retrieved 8 May 2020.
- ^ Lisboa Bastos M, Tavaziva G, Abidi SK, Campbell JR, Haraoui LP, Johnston JC, et al. (July 2020). «Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis». BMJ. 370: m2516. doi:10.1136/bmj.m2516. PMC 7327913. PMID 32611558.
- ^ Spencer E, Henighan C (1 September 2020). «Overview of BMJ: Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis». CEBM. Archived from the original on 3 October 2020. Retrieved 24 September 2020.
- ^ a b Spencer E, Jefferson T, Brassey J, Heneghan C (11 September 2020). «When is Covid, Covid?». CEBM. Archived from the original on 19 September 2020. Retrieved 19 September 2020.
- ^ Jefferson T, Spencer E, Brassey J, Heneghan C (3 September 2020). «Viral cultures for COVID-19 infectivity assessment. Systematic review». medRxiv. doi:10.1101/2020.08.04.20167932. S2CID 220962177.
{{cite journal}}: CS1 maint: url-status (link) - ^ Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (May 2020). «Detection of SARS-CoV-2 in Different Types of Clinical Specimens». JAMA. 323 (18): 1843–1844. doi:10.1001/jama.2020.3786. PMC 7066521. PMID 32159775.
- ^ a b Ferran M (7 May 2020). «COVID-19 tests are far from perfect, but accuracy isn’t the biggest problem». Popular Science. Archived from the original on 11 May 2020. Retrieved 10 May 2020.
- ^ «Serological testing for SARS-CoV-2 antibodies». American Medical Association. 14 May 2020. Archived from the original on 28 May 2020. Retrieved 29 May 2020.
- ^ «Interim Guidelines for COVID-19 Antibody Testing». U.S. Centers for Disease Control and Prevention (CDC). 23 May 2020. Archived from the original on 29 May 2020. Retrieved 29 May 2020.
- ^ Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J (August 2020). «Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure». Annals of Internal Medicine. 173 (4): 262–267. doi:10.7326/M20-1495. PMC 7240870. PMID 32422057.
- ^ «RT-PCR Testing». www.idsociety.org. Archived from the original on 24 June 2021. Retrieved 16 February 2021.
- ^ Böger B, Fachi MM, Vilhena RO, Cobre AF, Tonin FS, Pontarolo R (January 2021). «Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19». American Journal of Infection Control. 49 (1): 21–29. doi:10.1016/j.ajic.2020.07.011. PMC 7350782. PMID 32659413.
- ^ «Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19». U.S. Centers for Disease Control and Prevention (CDC). 30 April 2020. Archived from the original on 6 June 2021. Retrieved 28 August 2021.
- ^ Xiao AT, Tong YX, Zhang S (November 2020). «Profile of RT-PCR for SARS-CoV-2: A Preliminary Study From 56 COVID-19 Patients». Clinical Infectious Diseases. 71 (16): 2249–2251. doi:10.1093/cid/ciaa460. PMC 7188124. PMID 32306036.
- ^ a b c Engelmann I, Alidjinou EK, Ogiez J, Pagneux Q, Miloudi S, Benhalima I, et al. (March 2021). «Preanalytical Issues and Cycle Threshold Values in SARS-CoV-2 Real-Time RT-PCR Testing: Should Test Results Include These?». ACS Omega. 6 (10): 6528–6536. doi:10.1021/acsomega.1c00166. PMC 7970463. PMID 33748564.
- ^ Fauci A (16 July 2020). «This Week in Virology». YouTube. 4:20.
- ^ Mandavilli A (29 August 2020). «Your Coronavirus Test Is Positive. Maybe It Shouldn’t Be». The New York Times. ISSN 0362-4331. Retrieved 30 August 2021.
- ^ US CDC (20 July 2021). «Real-Time RT-PCR Diagnostic Panel: Instructions for Use». Food and Drug Administration. p. 35. Retrieved 30 August 2021.
- ^ a b van Kasteren PB, van der Veer B, van den Brink S, Wijsman L, de Jonge J, van den Brandt A, et al. (July 2020). «Comparison of seven commercial RT-PCR diagnostic kits for COVID-19». Journal of Clinical Virology. 128: 104412. doi:10.1016/j.jcv.2020.104412. PMC 7206434. PMID 32416600.
- ^ «Chinese Covid-19 test kit outstrips alternatives in Dutch study». South China Morning Post. 20 May 2020. Archived from the original on 23 May 2020. Retrieved 23 May 2020.
- ^ Heneghan C, Jefferson T (1 September 2020). «Virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR». CEBM. Archived from the original on 18 June 2021. Retrieved 19 September 2020.
- ^ Lu J, Peng J, Xiong Q, Liu Z, Lin H, Tan X, et al. (September 2020). «Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR». EBioMedicine. 59: 102960. doi:10.1016/j.ebiom.2020.102960. PMC 7444471. PMID 32853988.
- ^ «SARS-CoV-2 RNA testing: assurance of positive results during periods of low prevalence». GOV.UK. Archived from the original on 6 May 2021. Retrieved 19 September 2020.
- ^ «Study Raises Questions About False Negatives From Quick COVID-19 Test». NPR. 21 April 2020. Archived from the original on 1 May 2020. Retrieved 1 May 2020.
- ^ Thomas K (13 May 2020). «Coronavirus Testing Used by the White House Could Miss Infections». The New York Times. ISSN 0362-4331. Archived from the original on 13 May 2020. Retrieved 14 May 2020.
- ^ «National laboratories». who.int. Archived from the original on 31 January 2020. Retrieved 2 March 2020.
- ^ «PHE novel coronavirus diagnostic test rolled out across UK». GOV.UK. Archived from the original on 7 February 2020. Retrieved 12 April 2020.
In addition to processing samples from suspected cases in this country, PHE is now working as a reference laboratory for WHO, testing samples from countries that do not have assured testing capabilities.
- ^ «Specimen referral for COVID-19 – operational details of WHO reference laboratories providing confirmatory testing for COVID-19» (PDF). World Health Organization. Archived (PDF) from the original on 5 March 2020. Retrieved 29 March 2020.
- ^ «COVID-19: First results of the voluntary screening in Iceland». Nordic Life Science. 27 March 2020. Archived from the original on 29 March 2020. Retrieved 5 April 2020.
- ^ «How an experiment helped one Italian town find ‘submerged infections,’ cut new COVID-19 cases to zero». Nationalpost. 19 March 2020. Retrieved 29 March 2020.
- ^ a b c «PCR拡充が必要 専門家会議が会見 (全文1)» [PCR expansion required Expert meeting (Full text 1)]. THE PAGE (in Japanese). Yahoo!ニュース. 5 May 2020. p. 5. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ a b c d e «「新型コロナウイルス感染拡大阻止 最前線からの報告» [Report from the front line to prevent the spread of new coronavirus infection]. NHK (in Japanese). 15 April 2020. Archived from the original on 19 April 2020. Retrieved 27 May 2020.
- ^ a b c «Did Japan Just Beat the Virus Without Lockdowns or Mass Testing?». Bloomberg.com. 23 May 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ «PCR拡充が必要 専門家会議が会見 (全文1)» [PCR expansion required Expert meeting (Full text 1)]. THE PAGE (in Japanese). Yahoo!ニュース. 5 May 2020. p. 3. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ a b «新型コロナウイルス 感染爆発をどう防ぐか» [How to prevent the outbreak of new coronavirus infection]. NHK (in Japanese). 8 April 2020. Archived from the original on 8 April 2020. Retrieved 27 May 2020.
- ^ «第1波は終息するも欧米からの帰国者経由の第2波が拡大» [The first wave is over, but the second wave is expanding via returnees from Europe and the United States]. 日経メディカル (Nikkei Medical) (in Japanese). 12 May 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ a b «専門家に聞く»新型コロナウイルス»との闘い方と対策» [Ask experts how to fight the «new coronavirus» and countermeasures]. NHK (in Japanese). 27 March 2020. Archived from the original on 8 April 2020. Retrieved 27 May 2020.
- ^ «新型コロナ抗原検査キット、13日から実用化 加藤厚労相が発表 PCRとの併用を想定» [New corona antigen test kit put into practical use from 13th. Minister of Health, Labor and Welfare Kato announced that it will be used in combination with PCR]. 毎日新聞 (Mainichi newspaper ) (in Japanese). 12 May 2020. Archived from the original on 27 May 2020. Retrieved 27 May 2020.
- ^ «コロナ抗原検査が使用可能に、陽性のみ確定診断» [Corona antigen test available, positive only definitive diagnosis]. 日経メディカル (Nikkei Medical) (in Japanese). 12 May 2020. Archived from the original on 21 May 2020. Retrieved 15 May 2020.
- ^ a b «PCR拡充が必要 専門家会議が会見 (全文1)» [PCR expansion required Expert meeting (Full text 1)]. THE PAGE (in Japanese). Yahoo!ニュース. 5 May 2020. p. 4. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ a b «クルーズ船112人治療で「院内感染」ゼロ!「自衛隊中央病院」はなぜ奇跡を起こせたのか» [No «nosocomial infection» with treatment of 112 cruise ships! Why did «Self-Defense Forces Central Hospital» cause a miracle?]. 週刊新潮 (Shukan Shincho) (in Japanese). 30 April 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ «「PCR検査数少ないが、死亡者数・率低い」専門家会議» [«The number of PCR tests is small, but the number of deaths and rate is low» Expert meeting]. m3.com (in Japanese). 5 May 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ «調査報告クルーズ船 ウイルス対策のカギは?» [Survey Report What is the key to anti-virus measures for cruise ships?]. NHK (in Japanese). 7 May 2020. Archived from the original on 12 May 2020. Retrieved 24 May 2020.
- ^ «新型コロナウイルス感染症の現在の状況と厚生労働省の対応について(令和2年7月20日版)» [Current status of new coronavirus infection and response by the Ministry of Health, Labor and Welfare (Reiwa 20 July, 2nd edition)] (in Japanese). 厚生労働省. 20 July 2000. Archived from the original on 4 August 2020. Retrieved 1 August 2020.
- ^ «PCR検査能力、4月の3倍 それでも受けにくいわけは» [PCR test capacity, 3 times that of April]. Asahi Shimbun (in Japanese). 28 July 2020. Archived from the original on 31 July 2020. Retrieved 1 August 2020.
- ^ «日本のコロナ検査能力、米英の1割どまり» [Japan’s corona inspection ability, only 10% of the US and UK] (in Japanese). The Nikkei. 21 July 2020. Archived from the original on 31 July 2020. Retrieved 1 August 2020.
- ^ «新型コロナが弱毒化しているという根拠はない» [There is no evidence that the new corona is attenuated] (in Japanese). Yahoo!ニュース. 26 July 2020. Archived from the original on 27 July 2020. Retrieved 1 August 2020.
- ^ «軽症者施設、23都府県で不足 コロナ第2波推計» [Facility for mildly ill people, Insufficient in 23 prefectures Corona second wave estimation] (in Japanese). The Nikkei. 21 July 2020. Archived from the original on 31 July 2020. Retrieved 1 August 2020.
- ^ «患者急増、埋まりつつあるベッド 増床要請に頭抱える病院…スタッフは?一般患者は?経営は?» [The number of patients is increasing rapidly, and the beds are being filled up. Hospitals are having a request to increase the floor space … Staff? General patients? Management?]. Mainichi Shimbun (in Japanese). 22 July 2020. Archived from the original on 29 July 2020. Retrieved 1 August 2020.
- ^ «軽症患者ICUを圧迫 クラスターはほぼ終息 新型コロナで兵庫県対策協» [Squeezing ICU for mildly ill patients The cluster is almost over With the new corona] (in Japanese). 神戸新聞. 25 March 2020. Archived from the original on 22 October 2020. Retrieved 1 August 2020.
- ^ «Over 3 mln COVID-19 tests conducted in Russia». TASS. 27 April 2020. Archived from the original on 11 May 2020. Retrieved 29 April 2020.
- ^ «Popova said explosive growth in incidence was not allowed due to measures taken». TASS. 28 April 2020. Archived from the original on 29 August 2020. Retrieved 29 April 2020.
- ^ «COVID-19 outbreak: Petition to close schools in Singapore garners 7,700 signatures to date». msn.com. Archived from the original on 29 March 2020. Retrieved 29 March 2020.
- ^ «More than 3.6 million people tested during the weekend». The Slovak Spectator. 1 November 2020. Archived from the original on 2 January 2020. Retrieved 2 July 2021.
- ^ Kuhn A (12 March 2020). «Experts Credit South Korea’s Extensive Testing For Curbing Coronavirus Spread». NPR.org. Archived from the original on 16 March 2020. Retrieved 28 June 2020.
- ^ a b «日本が韓国の新型コロナウイルス対策から学べること──(1)検査体制» [What Japan can learn from Korea’s measures against the new coronavirus ── (1) Inspection system]. Newsweek Japan (in Japanese). 2 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ a b «日本が韓国の新型コロナウイルス対策から学べること──(3)情報公開» [What Japan can learn from Korea’s measures against the new coronavirus ── (3) Information disclosure]. Newsweek Japan (in Japanese). 21 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «日本が韓国の新型コロナウイルス対策から学べること──(4)軽症者の隔離・管理対策:「生活治療センター」» [What Japan can learn from Korea’s measures against the new coronavirus ── (4) Isolation and management measures for mildly ill people: «Life Treatment Center»]. Newsweek Japan (in Japanese). 11 May 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ a b «韓国のコロナ対策を称える日本に欠ける視点» [Japan’s lack of perspective to praise South Korea’s measures against corona]. Newsweek Japan (in Japanese). 2 May 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ a b c «韓国式大量検査は徴兵制の賜物…新型コロナが揺さぶる「自由」の価値» [Korean-style mass inspection is a gift of conscription … The value of «freedom» that the new corona shakes] (in Japanese). FNNプライム. 14 April 2020. Archived from the original on 27 April 2020. Retrieved 5 June 2020.
- ^ a b «韓国における新型コロナウィルス防疫事情(韓国)» [New Coronavirus Epidemic Prevention Circumstances in South Korea (Korea)] (in Japanese). 日本商工会議所. 10 May 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «韓国製PCR検査キットが新型コロナから世界を救う日» [The day when the Korean PCR test kit saves the world from the new corona]. Newsweek Japan (in Japanese). 14 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ a b c «新型ウイルス»パンデミック» 医療崩壊を防ぐには» [New virus «pandemic» How to prevent medical collapse]. NHK (in Japanese). 9 April 2020. Archived from the original on 19 April 2020. Retrieved 2 June 2020.
- ^ a b «IT活用でコロナ追跡 韓国、感染者の経路公開» [Corona tracking by utilizing IT South Korea, route disclosure of infected people]. Mainichi Shimbun (in Japanese). 16 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «コロナ対策で浮かび上がる「監視社会」韓国 個人情報をここまでさらしてよいのか» [«Surveillance society» that emerges from corona measures Can South Korea expose personal information to this extent?]. Tokyo Shimbun (in Japanese). 1 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «新型コロナ:「感染追跡」デジタル監視とプライバシーの新しい日常» [New Corona: «Infection Tracking» New Everyday Life in Digital Surveillance and Privacy] (in Japanese). Yahoo!ニュース. 26 March 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «韓国、コロナ隔離者に監視腕輪 「人権侵害」の声» [South Korea, Corona quarantine voice of surveillance bracelet «human rights violations»] (in Japanese). The Nikkei. 17 April 2020. Archived from the original on 29 May 2020. Retrieved 29 May 2020.
- ^ «South Korea is watching quarantined citizens with a smartphone app». MIT Technology Review. 6 March 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «Coronavirus privacy: Are South Korea’s alerts too revealing?». BBC. 5 March 2020. Archived from the original on 6 June 2020. Retrieved 5 June 2020.
- ^ «台湾がコロナ「優等生」になった理由。閣僚に医師出身、デジタル化の一方で強まる監視» [The reason why Taiwan became a corona «honor student». A doctor from a minister, increasing surveillance while digitizing]. Business Insider (in Japanese). 1 May 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ^ «台湾の新型コロナ対策が「善戦」しているワケ» [The reason why Taiwan’s new corona measures are «good fight»]. Wedge Infinity (in Japanese). 28 February 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ^ «台湾が新型コロナの感染拡大を抑制できている理由» [Why Taiwan is able to curb the spread of the new corona]. Wedge Infinity (in Japanese). 28 February 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ^ «新型コロナ対応の「優等生」は「台湾・韓国・ドイツ」» [Why Taiwan is able to curb the spread of the new corona …] (in Japanese). 日経ビジネス (Nikkei Business). 21 April 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ^ «Covid-19: Denmark suspends flights from the Emirates». Le Figaro. Archived from the original on 2 January 2020. Retrieved 22 January 2021.
- ^ «COVID-19 Public Policies #2 ニューヨークはいかにして検査数を増やしたのか» [COVID-19 Public Policies #2 How New York increased the number of inspections]. Office of the City of Yokohama Representative to the Americas (in Japanese). 14 May 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ^ «Coronavirus New York: health officials provide limits on testing patients for COVID-19». Eyewitness News. 21 March 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ^ «マスクも防護服も足りない! ニューヨークの病院で看護師が新型コロナウイルスに感染、死亡» [Not enough masks and protective clothing! A nurse is infected with a new coronavirus and dies at a hospital in New York]. Business Insider Japan (in Japanese). 27 March 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ^ «NY州感染者数、全米2位に 感染爆発で2週間封じ込め作戦へ» [The number of infected people in New York ranks second in the United States.]. Yahoo!ニュース (in Japanese). 11 March 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ^ «Coronavirus clue? Most cases aboard U.S. aircraft carrier are symptom-free». Reuters. 16 April 2020. Archived from the original on 11 December 2020. Retrieved 2 July 2021.
- ^ «Sailors on sidelined USS Theodore Roosevelt get virus for second time». NBC News. Archived from the original on 21 May 2020. Retrieved 21 May 2020.
- ^ «US warned Nevada not to use Chinese COVID tests from UAE». The Associated Press. Retrieved 15 October 2020.
- ^ «Special Report: Italy and South Korea virus outbreaks reveal disparity in deaths and tactics». Reuters. 13 March 2020. Archived from the original on 22 April 2020. Retrieved 22 June 2020.
- ^ «Want to know how many people have the coronavirus? Test randomly». The Conversation. 13 April 2020. Archived from the original on 9 May 2020. Retrieved 7 May 2020.
- ^ «M&E – Health Information System General Directorate – National Diseases Surveillance and Response». MoPH Data Warehouse – Dashboard. 17 December 2020.
- ^ «COVID19/ Ministria e Shëndetësisë: 736 të vaksinuar, 3935 testime, 991 të shëruar, 1112 raste të reja dhe 17 humbje jete në 24 orët e fundit». Ministria e Shëndetësisë dhe Mbrojtjes Sociale [Ministry of Health and Social Protection] (in Albanian). 18 February 2021.
- ^ a b c d e f g h i j k l «Coronavirus Disease 2019 (COVID-19)». Africa CDC.
- ^ «Documentation: Rapport de Situation Sur L’Epidemie de Coronavirus COVID-19». Ministère de la Santé de la Population et de la Réforme Hospitalière [Ministry of Health, Population and Hospital Reform] (in French). 2 November 2020.
- ^ «COVID-19 Dashboard». Government of Andorra. 1 March 2022.
- ^ «COVID-19: Angola Com 58 Novas Infecções e 44 Recuperados». Agência Angola Press (in Portuguese). 4 March 2021.
- ^ «COVID-19 Antigua & Barbuda Dashboard». Official Facebook page of the Ministry of Health & The Environment, Antigua and Barbuda. 6 March 2021.
- ^ «Sala de Situaciόn Coronavirus online» (PDF). Argentina.gob.ar (in Spanish). 16 April 2022.
- ^ Կորոնավիրուսային հիվանդություն (COVID-19). Հիվանդությունների վերահսկման և կանխարգելման ազգային կենտրոն [National Center for Disease Control and Prevention] (in Armenian). 30 May 2022.
- ^ «Coronavirus (COVID-19) current situation and case numbers». Department of Health. 10 September 2022.
- ^ «Coronavirus». AGES Dashboard COVID19 (in German). 5 January 2023.
- ^ «Azərbaycanda Carı Vəzıyyət». Azərbaycan Respublikasının Nazirlər Kabineti [Cabinet of Ministers of the Republic of Azerbaijan] (in Azerbaijani). 11 May 2022.
- ^ «News and Press Releases: COVID-19 Report Update». Government of the Bahamas. 29 November 2022.
- ^ الموقع الرسمي للمستجدات الصحية، مملكة الب9رين. وزارة الصحة [Ministry of Health] (in Arabic). 3 December 2022.
- ^ «Bangladesh Covid-19 Update». Institute of Epidemiology, Disease Control and Research. 24 July 2021.
- ^ «COVID-19 Update». Barbados Government Information Service. 15 October 2022.
- ^ Официальный Минздрав. Официальный канал Министерства здравоохранения Республики Беларусь [Telegram channel of the Ministry of Health of the Republic of Belarus] (in Russian). 9 May 2022.
- ^ «Epistat COVID19 Belgian Dashboard». Sciensano. 22 December 2022.
- ^ «COVID-19 Update». Facebook account of the Ministry of Health and Wellness Belize. 1 November 2021.
- ^ «Coronavirus (COVID-19) By the Numbers». Statistical Institute of Belize. 9 June 2022.
- ^ «Informations coronavirus (covid-19)». Gouvernement de la République du Bénin [Government of the Republic of Benin] (in French). 5 May 2021.
- ^ «National Situational Update on COVID-19». Ministry of Health. 28 February 2022.
- ^ «Reporte COVID-19 en Bolivia». Ministerio de Salud [Ministry of Health] (in Spanish). 5 June 2022.
- ^ «Službene informacije o koronavirusu u BiH». Ministarstvo civilnih poslova Bosne i Hercegovine [Ministry of Civil Affairs of Bosnia and Herzegovina] (in Bosnian). 28 September 2022.
- ^ «COVID-19 Botswana Dashboard». Government of Botswana. 11 January 2022.
- ^ «BW government on Facebook». Government of Botswana. 3 December 2020.
- ^ «COVID-19 Testes». Ministério da Saúde [Ministry of Health] (in Portuguese). 19 February 2021.
- ^ «Coronavírus Brasil». Ministério da Saúde [Ministry of Health] (in Portuguese). 19 February 2021.
- ^ «Press Release on the Current Situation of the COVID-19 Infection in Brunei Darussalam». Ministry of Health Brunei Darussalam. 2 August 2021.
- ^ COVID-19: Единен информационен портал. COVID-19: Единен информационен портал [COVID-19: United information portal] (in Bulgarian). 26 December 2022.
- ^ «Communiqué Coronavirus (COVID-19) au Burkina Faso». Facebook account of the Service d’Information du Gouvernement (SIG) [Government Information Service] (in French). 5 March 2021.
- ^ «Update on COVID-19». Facebook account of the Ministère de la Santé Publique Burundi [Ministry of Public Health Burundi] (in French). 5 January 2021.
- ^ បច្ចុប្បន្នភាពនៃជំងឺកូរ៉ូណាថ្មី COVID-19 នៅប្រទេសកម្ពុជា. Communicable Disease Control Department, Ministry of Health (Cambodia) (in Khmer). 1 August 2021.
- ^ «Coronavirus disease (COVID-19): Outbreak update». Government of Canada. Retrieved 5 December 2022.
- ^ «Communiqué N*320 de la Coordination Nationale de Riposte Sanitaire». Official Facebook account of the Ministère de la Santé Publique du Tchad [Ministry of Public Health of Chad] (in French). 2 March 2021.
- ^ «Cifras Oficiales: COVID-19». Gobierno de Chile [Government of Chile] (in Spanish). 23 December 2022.
- ^ 我国核酸日检测能力达484万份. 中华人民共和国中央人民政府 [The Central People’s Government of the People’s Republic of China] (in Chinese). 6 August 2020.
- ^ «Aug 1: Daily briefing on novel coronavirus cases in China». National Health Commission of the People’s Republic of China. 1 August 2020.
- ^ «#COVID19 en Colombia 28-01-2021». Instituto Nacional de Salud de Colombia [Colombia’s National Institute of Health] (in Spanish). 17 January 2021.
- ^ «#ReporteCOVID19». Cuenta Oficial del Ministerio de Salud y Protección Social de Colombia [Official Account of Health and Social Protection Ministry of Columbia] (in Spanish). 24 November 2022.
- ^ «Situación Nacional COVID-19». Geovisión; Ministerio de Salud, Costa Rica [Ministry of Health, Costa Rica] (in Spanish). 2 November 2021.
- ^ «xxx novih slučajeva u protekla 24 sata». Koronavirus.hr (in Croatian). 26 December 2022.
- ^ «Covid19CubaData». Covid19CubaData (in Spanish). 21 July 2021.
- ^ «Coronavirus en Cuba». Ministerio de Salud Pública [Ministry of Public Health] (in Spanish). 26 December 2022.
- ^ Η εξάπλωση της COVID-19 στην Κύπρο. Πανεπιστήμιο Κύπρου [University of Cyprus] (in Greek). 30 December 2022.
- ^ «Přehled situace v ČR: COVID-19». Ministerstvo zdravotnictví České republiky [The Ministry of Health of the Czech Republic] (in Czech). 24 December 2022.
- ^ «Tal og overvågning over coronavirus/COVID-19 – Sundhedsstyrelsen». Sundhedsstyrelsen [The National Board of Health] (in Danish). 23 December 2022.
- ^ «Statens Serum Institut COVID-19 – Danmark». State20 Serum Institut [The National Board of Health] (in Danish). 15 November 2022.
- ^ «Poit de Presse Sur La Situation COVID19 Par Le Secrétaire De La Santé Dr Meeke Mohamed Moussa». Official Facebook account of the Ministere de la Santé de Djibouti [Djibouti Ministry of Health] (in French). 28 April 2022.
- ^ «Commonwealth of Dominica Coronavirus [COVID-19] Report». Facebook account of the Ministry of Health, Wellness and New Health Investment. 21 June 2022.
- ^ «Boletin Especial 484 COVID 19». Dirección General de Epidemiología [General Directorate of Epidemiology] (in Spanish). 23 July 2022.
- ^ «Situation Épidémiologique en RDC». Stop Coronavirus COVID-19 RDC (in French). 28 February 2021.
- ^ «Situación Nacional Por COVID-19 Infografía N°400» (PDF). Ministerio de Salud Pública [Ministry of Public Health] (in Spanish). 23 July 2021.
- ^ «facebook.com/EgyMohpSpokes». Facebook page for the Egyptian Ministry of Health and Population (MOHP) spokesperson (in Arabic). 23 July 2021.
- ^ «Situación nacional COVID-19». Gobierno de El Salvador [Government of El Salvador] (in Spanish). 19 March 2022.
- ^ «Estadísticas COVID-19» [Ministry of Health and Social Welfare]. Ministerio de Sanidad y Bienestar Social (in Spanish). Equatorial Guinea. 13 December 2022.
- ^ «Koroonakaart». Koroonakaart. 20 December 2022.
- ^ «COVID-19 Eswatini Dashboard». 8 December 2021.
- ^ የኢትዮጵያ የተቀናጀ የኮቪድ-19 መቆጣጠሪያ ስርዓት. covid19.et (in Amharic). 24 July 2021.
- ^ «Corona í Føroyum». Føroya Landsstýri [The Government of the Faroe Islands]. 27 February 2022.
- ^ «COVID-19 Update». Ministry o10 Health & Medical Services. Fiji. 24 November 2022.
- ^ «Confirmed coronavirus cases (COVID-19) in Finland». Terveyden ja hyvinvoinnin laitos (ArcGIS) [National Institute for Health and Welfare (ArcGIS)]. 14 January 2022.
- ^ «info coronavirus covid-19-carte et donnes covid 19 en france». Gouvernement.fr (in French). 15 May 2022.
- ^ «Situation Épidémiologique au Gabon». Info Covid19 Gabon (in French). 23 July 2021.
- ^ «The Gambia COVID-19 Outbreak Situational Report» (PDF). Ministry of Health. 15 February 2021.
- ^ COVID-19 სტატისტიკური მონაცემები. დაავადებათა კონტროლისა და საზოგადოებრივი ჯანმრთელობის ეროვნული ცენტრი [National Center for Disease Control and Public Health] (in Georgian). 3 November 2021.
- ^ «Robert Koch-Institut: COVID-19-Dashboard». Robert Koch-Institut [Robert Koch Institute]. 7 July 2021.
- ^ «Tabellen zu Testzahlen, Testkapazitäten und Probenrückstau pro Woche» (XLSX). Robert Koch-Institut [Robert Koch Institute]. 7 July 2021.
- ^ «Situation Update, COVID-19 Outbreak in Ghana». Ghana Health Service. 3 July 2021.
- ^ Ημερήσια έκθεση επιδημιολογικής επιτήρησης λοίμωξης από το νέο κορωνοϊό (COVID-19). Εθνικός Οργανισμός Δημόσιας Υγείας [National Public Health Organization] (in Greek). 20 December 2022.
- ^ «Coronavirus i Grønland». Naalakkersuisut [Government of Greenland] (in Danish). 30 January 2022.
- ^ «COVID-19 Update | Grenada Dashboard». Ministry of Health Grenada (Facebook). 11 May 2021.
- ^ «Situación de COVID-19 en Guatemala». Ministerio de Salud Pública y Asistencia Social [Ministry of Public Health and Social Assistance] (in Spanish). 18 December 2022.
- ^ «Republique de Guinee COVID-19 Décompte des cas». Official Twitter account of the Agence Nationale de Sécurité Sanitaire [National Agency for Health Security] (in French). 23 July 2021.
- ^ «Situação Epidemiológica Da Covid-19 Na Guiné-Bissau». Official Facebook page of the Alto Comissariado para o Covid-19 [High Commissioner for Covid-19] (in Portuguese). 8 July 2022.
- ^ «Guyana COVID-19 Dashboard». Ministry of Health. 16 June 2022.
- ^ «Surveillance de la COVID-19, Haiti, 2020-2021». Ministère de la Santé Publique et de la Population [Ministry of Public Health and Population] (in French). 7 December 2022.
- ^ «Estadística Nacional de Coronavirus COVID-19». Biblio3eca Virtual en Salud de Honduras [Virtual Health Library of Honduras] (in Spanish). 26 November 2021.
- ^ «Tájékoztató oldal a koronavírusról». Tájékoztató oldal a koronavírusról [Coronavirus Information Page] (in Hungarian). Cabinet Office of the Prime Minister. 11 May 2022.
- ^ «COVID-19 in Iceland – Statistics». Covid.is. 9 August 2022.
- ^ «SARS-CoV-2 (COVID-19) Testing: Status Update». Indian Council of Medical Research. Retrieved 19 September 2021.
- ^ «Ministry of Health and Family Welfare». Ministry of Health and Family Welfare. Retrieved 1 October 2021.
- ^ a b «Peta Sebaran». COVID-19 Handling and National Economic Recovery Committee. Retrieved 9 January 2023.
- ^ «Health Ministry’s Updates on COVID-19». Government of the Islamic Republic of Iran. 1 June 2022.
- ^ «الموقف الوبائي اليومي لجائحة كورونا في العراق ليوم السبت الموافق ٥ كانون الاول ٢٠٢٠». وزارة الصحة العراقية (Facebook) [Iraqi Ministry of Health (Facebook)] (in Arabic). 3 August 2022.
- ^ «Ireland’s COVID-19 Data Hub». gov.ie. 15 December 2022.
- ^ קורונה – לוח בקרה. נגיף הקורונה [Coronavirus] (in Hebrew). Ministry of Health. 17 January 2022.
- ^ «29 Dicembre 2022 – Aggiornamento casi Covid-19» (PDF). Dipartimento della Protezione Civile (GitHub) [Civil Protection Department (GitHub)] (in Italian). 30 December 2022.
- ^ «Point de la situation de la COVID-19 au 3/03/2021». Official Facebook channel of Le Ministère de la Santé et de l’Hygiène Publique [Ministry of Health and Public Hygiene, Ivory Coast] (in French). 3 March 2021.
- ^ «COVID-19 Clinical Management Summary». Ministry of Health & Wellness. 3 October 2022.
- ^ 新型コロナウイルス感染症の現在の状況と厚生労働省の対応について(令和3年3月1日版). 厚生労働省 [The Ministry of Health, Labour and Welfare] (in Japanese). 1 March 2021.
- ^ «corona.moh.gov.jo/en». Jordan Ministry of Health. 6 June 2021.
- ^ Данные по COVID-19 в Казахстане. Национальный центр общественного здравоохранения Министерства здравоохранения Республики Казахстан [National Center of Public Health of the Ministry of Healthcare of the Republic of Kazakhstan] (in Russian). 29 May 2021.
- ^ «twitter.com/MOH_Kenya». Official Twitter Account of the Ministry of Health Kenya. 5 March 2021.
- ^ «facebook.com/IKSHPK». Official Facebook account of the Instituti Kombëtar i Shëndetësisë Publike të Kosovës [National Institute of Public Health of Kosova] (in Albanian). 31 May 2021.
- ^ «twitter.com/KUWAIT_MOH». Kuwait Ministry of Health (Twitter). 9 March 2022.
- ^
За сутки проведено 3436 ПЦР-исследований на коронавирус. Insta official (in Kyrgyz). 10 February 2021. - ^ «ຄະນະສະເພາະກິດ COVID-19». COVID-19 Task Force (in Lao). 1 March 2021.
- ^ «Covid-19 infekcijas izplatība Latvijā». Slimību profilakses un kontroles centrs (ArcGIS) [Center for Disease Prevention and Control (ArcGIS)] (in Latvian). 5 September 2021.
- ^ آخر اﻹحصاءات. فيروس كورونا: COVID-19 [Coronavirus: COVID-19] (in Arabic). Ministry of Information. 14 June 2021.
- ^ «COVID-19 Statistics». Official Twitter account of the National COVID-19 Secretariat (NACOSEC). 31 March 2022.
- ^ «#LiBCOVID19 Case Update». Official Facebook account of the National Public Health Institute of Liberia-NPHIL. 19 July 2021.
- ^ اليومي للوضع الوبائي المحلي لفيروس كورونا المستجد ليوم الأحد 28 فبراير 2021. Official Facebook account of the National Centre for Disease Control (NCDC) — Libya (in Arabic). 16 April 2022.
- ^ «Koronavirusas (COVID-19)». Lietuvos Respublikos sveikatos apsaugos ministerija [Ministry of Health of the Republic of Lithuania] (in Lithuanian). 21 December 2022.
- ^ «Korona Stop». Korona Stop. 16 May 2021.
- ^ «Coronavirus – Rapport Journalier» (PDF). La plate-forme de données luxembourgeoise [The luxembourgish data platform] (in French). Government of Luxembourg. 13 May 2022.
- ^ «COVID-19: Fivoaran’ny antontan’isa teto Madagasikara ny 13 Febroary ka hatramin’ny 19 Febroary 2021». Facebook account of the Ministère de la Santé Publique Madagascar [Ministry of Public Health Madagascar] (in French and Malagasy). 22 February 2021.
- ^ «COVID-19 Daily info update». Facebook page of the Ministry Of Health — Malawi. 29 November 2022.
- ^ «Situasi Terkini». Kementerian Kesihatan Malaysia [Ministry of Health Malaysia] (in Malay). 7 September 2021.
- ^ «COVID-19 Case Updates». Health Protection Agency (Twitter). 13 March 2022.
- ^ «COVID-19 Local Updates». Ministry of Health. 29 January 2021.
- ^ «Communique N°364 du Ministere de la Sante et du Développement Social Sur Le Suivi des Actions de Prevention et de Riposte Face a la Maladie a Coronvirus». Ministère de la Santé et du Développement Social du Mali [Ministry of Health and Social Development of Mali] (in French). 7 July 2021.
- ^ «COVID-19 Malta». Times of Malta (ArcGIS). 8 September 2021.
- ^ «المعطيات العامة للحالة الوبائية». Official Facebook page of the Ministère de la santé /وزارة الصحة [Ministry of Health] (in Arabic). Mauritania. 17 April 2021.
- ^ «Covid-19 : Communiqués». Republic of Mauritius. 23 October 2020.
- ^ «Covid-19 México». Gobierno de México [Government of Mexico] (in Spanish). 15 October 2021.
- ^ «Comunicate». Ministerul Sănătății Muncii și Protecției Sociale [Ministry of Health, Labour and Social Protection] (in Romanian). Moldova. 21 April 2022.
- ^ Нөхцөл байдлын мэдээ COVID-19. Эрүүл Мэндийн Яам [Ministry of Health] (in Mongolian). 10 July 2021.
- ^ «Uživo: COVID-19». Institut za javno zdravlje Crne Gore [Institute of Public Health of Montenegro] (in Montenegrin). 28 July 2020.
- ^ «Novosti». Institut za javno zdravlje Crne Gore [Institute of Public Health of Montenegro] (in Montenegrin). 11 May 2021.
- ^ مرض فيروس كورونا المستجد: الرصد الصحي بالمغرب. البوابة الرسمية لفيروس كورونا بالمغرب [The official portal of coronavirus in Morocco] (in Arabic). 18 December 2022.
- ^ «Boletim diário COVID-19 Nº379». Ministério da Saúde [Ministry of Health] (in Portuguese). 22 July 2021.
- ^ «Coronavirus Disease 2019 (COVID-19) Surveillance Dashboard (Myanmar)». Ministry of Health and Sports (in Burmese). 16 September 2021.
- ^ «COVID-19 update». Official Facebook account of the Ministry of Health and Social Services-Namibia. 5 July 2022.
- ^ «COVID-19 Dashboard». Ministry of Health and Population (Nepal). Retrieved 26 July 2022.
- ^ «Epidemiologische situatie van COVID-19 in Nederland» (PDF). Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment] (in Dutch). 6 July 2021.
- ^ «Info coronavirus Covid-19». Gouvernement de la Nouvelle-Calédonie [Government of New Caledonia] (in French). 4 September 2021.
- ^ «COVID-19: Testing data». Ministry of Health. 19 December 2022.
- ^ «COVID-19: Current cases». Ministry of Health. 19 December 2022.
- ^ «#Covid19Niger Bilan du 22/02/2021». Facebook account of the Ministère de la Santé Publique [Ministry of Public Health] (in French). 22 February 2021.
- ^ «Coronavirus COVID-19 Microsite». Nigeria Centre for Disease Control. 28 February 2021.
- ^ КНДР ввела максимальный уровень карантина. KBS World Radio (in Russian). 2 December 2020.
- ^ Регистрирани 237 Нови Случаи На Ковид 19 – Вкупно Дијагностицирани 84024, Оӡдравени 460 Пациенти – Починати 8 Лица. Министерство за здравство [Ministry of Health] (in Macedonian). 1 July 2021.
- ^ Во последните 24 часа. Министерство за здравство [Ministry of Health] (in Macedonian). 27 June 2021.
- ^ «COVID-19 Genel Durum». Kuzey Kıbrıs Türk Cumhuriyeti Sağlık Bakanlığı [Turkish Republic of Northern Cyprus Ministry of Health] (in Turkish). 13 July 2022.
- ^ «Dags- og ukerapporter om koronavirussykdom (covid-19)». Folkehelseinstituttet [Norwegian Institute of Public Health] (in Norwegian). 20 January 2022.
- ^ «Oman conducts over 500,000 COVID-19 tests since the start of pandemic». The Arabian Stories. 28 October 2020.
- ^ «Pakistan Cases Details». COVID-19 Health Advisory Platform. Ministry of National Health Services Regulations and Coordination. 5 March 2021.
- ^ فايروس كورونا (COVID-19) في فلسطين. فايروس كورونا (COVID-19) في فلسطين [Coronavirus (COVID-19) in Palestine] (in Arabic). 5 February 2022.
- ^ «Compartimos la actualización de datos sobre #COVID19 en nuestro país. Parte 1». Cuenta Oficial de Twitter del Ministerio de Salud de Panama [Official Twitter Account of the Ministry of Health Panama] (in Spanish). 20 December 2022.
- ^ «Official COVID-19 Info Website». Papua New Guinea Joint Agency Task Force, National Control Centre for COVID-19. 20 February 2021.
- ^ «Reportes – COVID19» (in Spanish). Ministe132 280rio de Salud Pública y Bienestar Social (Ministry of Public Health and Social Welfare). 28 March 2022.
- ^ «Sala Situacional». Covid-19 en ″el Perú [Covid-19 in Peru] (in Spanish). 19 November 2022.
- ^ «COVID-19 Tracker». Department of Health (Philippines). 19 December 2022.
- ^ «COVID-19 Tracker». Department of Health (Philippines). 16 April 2021.
- ^ «diagnostyka pod kątem koronawirusa». Official Twitter account of the Ministerstwo Zdrowia [Ministry of Health] (in Polish). 27 April 2022.
- ^ «Ponto de Situação Atual em Portugal». COVID-19 (in Portuguese). Ministry of Health. 5 January 2022.
- ^ «COVID19 Home». Ministry of Public Health. 12 November 2022.
- ^ «Buletin informativ». Ministerul Sănătăţii [Ministry of Health] (in Romanian). 29 January 2021.
- ^ Информационный бюллетень о ситуации и принимаемых мерах по недопущению распространения заболеваний, вызванных новым коронавирусом. Федеральная служба по надзору в сфере защиты прав потребителей и благополучия человека (Роспотребнадзор) [Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)] (in Russian). 7 June 2022.
- ^ стопкоронавирус. Оперативные данные [Stop Coronavirus] (in Russian). 4 June 2022.
- ^ «Amakuru Mashya | Update». Twitter account of the Ministry of Health-Rwanda. 6 October 2021.
- ^ «COVID-19 Updates». Government of St. Kitts and Nevis. 27 August 2021.
- ^ «Saint Lucia’s COVID-19 Dashboard». Ministry of Health and Wellness. 7 October 2022.
- ^ «COVID-19 Report». Ministry of Health, Wellness and the Environment (St. Vincent and the Grenadines). 12 December 2022.
- ^ «Aggiornamento Epidemia COVID-19». Istituto per la Sicurezza Sociale [Institute for Social Security] (in Italian). 2 January 2023.
- ^ «COVID 19 Dashboard: Saudi Arabia». Ministry of Health. 26 April 2022.
- ^ «Riposte à l’épidémie du nouveau coronavirus COVID-19, Sénégal» (PDF). Ministère de la Santé et l’Action sociale [Ministry of Health and Social Action] (in French). 12 July 2021.
- ^ «Coronavirus COVID-19». Ministry of Health of the Republic of Serbia. 25 December 2022.
- ^ «Updates on COVID-19 (Coronavirus Disease 2019) Local Situation». Ministry of Health. 3 August 2021.
- ^ «COVID-19 Situation Report». Ministry of Health. 2 March 2020.
- ^ «Covid-19 in graphs». korona.gov.sk. Office of the Deputy Prime Minister of the Slovak Republic for Investments and Informatization. 27 December 2022.
- ^ «Dnevno spremljanje okužb s SARS-CoV-2 (COVID-19)». Nacionalni inštitut za javno zdravje [National Institute of Public Health] (in Slovenian). 27 December 2022.
- ^ «COVID-19 South African coronavirus news and information». South African Government. 24 May 2021.
- ^ «COVID-19 statistics in South Africa». South Africa Health Twitter Account. 24 May 2021.
- ^ 코로나바이러스감염증-19(COVID-19). 코로나바이러스감염증-19(COVID-19) [Coronavirus infection-19 (COVID-19)] (in Korean). Ministry of Health and Welfare. 1 March 2021.
- ^ «Update on COVID-19 Response». Ministry of Health — South Sudan. 26 May 2021.
- ^ «La pandemia del coronavirus, en datos, mapas y gráficos». RTVE ( Radio y Televisión Española) [RTVE ( Spanish Radio and Television)] (in Spanish). 1 July 2021.
- ^ «Resumen de la situación — Pruebas de laboratorio». Ministerio de Sanidad, Consumo y Bienestar Social [Ministry of Health, Consumption and Social Welfare] (in Spanish). 5 July 2021.
- ^ «COVID-19 Situation Report». Health Promotion Bureau, Sri Lanka. 31 March 2021.
- ^ «COVID-19 : Live Situational Analysis Dashboard of Sri Lanka». Health Promotion Bureau, Sri Lanka. 31 March 2021.
- ^ «Veckorapport om covid-19, vecka 20» (PDF). folkhalsomyndigheten.se (in Swedish). Public Health Agency of Sweden. 28 May 2021. p. 18.
- ^ «Folkhalsomyndigheten Antal fall av Covid-19». folkhalsomyndigheten.se (in Swedish). Public Health Agency of Sweden. 1 February 2021.
- ^ «COVID-19 Switzerland». Federal Office of Public Health FOPH. 8 November 2022.
- ^ «Taiwan Centers for Disease Control». Taiwan Centers for Disease Control. 19 December 2022.
- ^ รายงานสถานการณ์โรคติดเชื้อไวรัสโคโรนา 2019 ฉบับที่ 426 วันที่ 4 มีนาคม 2564 (PDF). Department of Disease Control (in Thai). 4 March 2021.
- ^ «Coronavirus Au Togo». Government of Togo (in French). 11 December 2022.
- ^ «COVID-19 Update Trinidad and Tobago». Ministry of Health. 3 January 2022.
- ^ الأرقام الرئيسيّة المسجّلة بتاريخ 03 فيفري 2021 #كوفيد_19. Official Facebook account of the Ministére de la Santé وزارة الصحة [Ministry of Health, Tunisia] (in Arabic and French). 24 August 2021.
- ^ «Türkıye COVID-19 Hasta Tablosu». Türkiye Cumhuriyeti Sağlık Bakanlığı [Republic of Turkey Ministry of Health] (in Turkish). 2 July 2021.
- ^ «COVID-19 Daily Updates». Facebook page of the Ministry of Health — Uganda. 12 February 2021.
- ^ «COVID-19 pandemic in Ukraine». COVID-19 pandemic in Ukraine. Cabinet of Ministers of Ukraine. 24 November 2021.
- ^
«COVID-19 Updates – Ministry of Health and Prevention – UAE». Ministry of Health & Prevention. 5 January 2023. - ^ «Coronavirus (COVID-19) in the UK». GOV.UK. 19 May 2022.
- ^ «COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University». coronavirus.jhu.edu. 9 August 2021.
- ^ «COVID Data Tracker Weekly Review». Centers for Disease Control and Prevention. 30 July 2022. Retrieved 3 August 2022.
- ^ «Visualizador de casos coronavirus COVID-19 en Uruguay». Sistema Nacional de Emergencias [National Emergency System] (in Spanish). 16 April 2022.
- ^ Дневной прирост случаев COVID-19 продолжает увеличиваться. Gazeta.uz Газета.uz (in Russian). 11 September 2020.
- ^ «Día 353 de la lucha contra la COVID-19». COVID-19 Patria (in Spanish). 30 March 2021.
- ^ «COVID-19 in Viet Nam Situation Report 32». WHO. 30 August 2022. Retrieved 1 September 2022.
- ^ «Daily #COVID19 update». Official Twitter account of the Zambia National Public Health Institute. 10 March 2022.
- ^ «COVID-19 update». Official Twitter account of the Ministry of Health and Child Care (Zimbabwe). 16 October 2022.
Further reading
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. (January 2020). «Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR». Euro Surveillance. 25 (3). doi:10.2807/1560-7917.ES.2020.25.3.2000045. PMC 6988269. PMID 31992387.
- Guglielmi G (July 2020). «The explosion of new coronavirus tests that could help to end the pandemic». Nature. 583 (7817): 506–509. Bibcode:2020Natur.583..506G. doi:10.1038/d41586-020-02140-8. PMID 32681157.
- Kevadiya BD, Machhi J, Herskovitz J, Oleynikov MD, Blomberg WR, Bajwa N, et al. (May 2021). «Diagnostics for SARS-CoV-2 infections». Nature Materials. 20 (5): 593–605. Bibcode:2021NatMa..20..593K. doi:10.1038/s41563-020-00906-z. PMC 8264308. PMID 33589798. S2CID 231930978.
External links
- Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hasell J, MacDonald B, Giattino C, Appel C, Rodés-Guirao L, Roser M (13 July 2020). «Coronavirus (COVID-19) Testing». Our World in Data. – International testing statistics updated twice a week.
- «COVID-19 diagnostic tech tableau». BioCentury. Retrieved 22 June 2020.
- COVID-19 Testing (at least) – now Free for all? (CDC; US Congress; CSPAN video/6:00; 12 March 2020)
- «EUA Authorized Serology Test Performance». U.S. Food and Drug Administration (FDA). 25 May 2021.
- «Global Progress on COVID-19 Serology-Based Testing». Johns Hopkins Center for Health Security.
- «Testing FAQ». Johns Hopkins Coronavirus Resource Center.
- «New Rapid Testing Device for COVID-19 Immunity». Mantracourt Electronics & University of Exeter.
The US CDC’s COVID-19 laboratory test kit
COVID-19 testing involves analyzing samples to assess the current or past presence of SARS-CoV-2. The two main types of tests detect either the presence of the virus or antibodies produced in response to infection.[1][2] Molecular tests for viral presence through its molecular components are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Antibody tests (serology immunoassays) instead show whether someone once had the disease.[3] They are less useful for diagnosing current infections because antibodies may not develop for weeks after infection.[4] It is used to assess disease prevalence, which aids the estimation of the infection fatality rate.[5]
Individual jurisdictions have adopted varied testing protocols, including whom to test, how often to test, analysis protocols, sample collection and the uses of test results.[6][7][8] This variation has likely significantly impacted reported statistics, including case and test numbers, case fatality rates and case demographics.[9][10][11][12] Because SARS-CoV-2 transmission occurs days after exposure (and before onset of symptoms), there is an urgent need for frequent surveillance and rapid availability of results.[13]
Test analysis is often performed in automated, high-throughput, medical laboratories by medical laboratory scientists. Rapid self-tests and point-of-care testing are also available and can offer a faster and less expensive method to test for the virus although with a lower accuracy.[14][15]
Methods
Explanation of the underlying pathophysiology pertaining to diagnosis of COVID-19[16]
Positive viral tests indicate a current infection, while positive antibody tests indicate a prior infection.[17] Other techniques include a CT scan, checking for elevated body temperature, checking for low blood oxygen level, and detection by trained dogs.[18][19][20]
Detection of the virus
Detection of the virus is usually done either by looking for the virus’s inner RNA, or pieces of protein on the outside of the virus. Tests that look for the viral antigens (parts of the virus) are called antigen tests.
There are multiple types of tests that look for the virus by detecting the presence of the virus’s RNA. These are called nucleic acid or molecular tests, after molecular biology. As of 2021, the most common form of molecular test is the reverse transcription polymerase chain reaction (RT-PCR) test.[21] Other methods used in molecular tests include CRISPR, isothermal nucleic acid amplification, digital polymerase chain reaction, microarray analysis, and next-generation sequencing.[21]
Reverse transcription polymerase chain reaction (RT-PCR) test
Polymerase chain reaction (PCR) is a process that amplifies (replicates) a small, well-defined segment of DNA many hundreds of thousands of times, creating enough of it for analysis. Test samples are treated with certain chemicals[22][23] that allow DNA to be extracted. Reverse transcription converts RNA into DNA.
Reverse transcription polymerase chain reaction (RT-PCR) first uses reverse transcription to obtain DNA, followed by PCR to amplify that DNA, creating enough to be analyzed.[23] RT-PCR can thereby detect SARS-CoV-2, which contains only RNA. The RT-PCR process generally requires a few hours.[24] These tests are also referred to as molecular or genetic assays.[3]
Real-time PCR (qPCR)[25] provides advantages including automation, higher-throughput and more reliable instrumentation. It has become the preferred method.[26][27]
The combined technique has been described as real-time RT-PCR[28] or quantitative RT-PCR[29] and is sometimes abbreviated qRT-PCR,[30] rRT-PCR[31] or RT-qPCR,[32] although sometimes RT-PCR or PCR are used. The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines propose the term RT-qPCR,[25] but not all authors adhere to this.
Average sensitivity for rapid molecular tests depend on the brand. For ID NOW, the average sensitivity was 73.0% with an average specificity of 99.7%; for Xpert Xpress the average sensitivity was 100% with an average specificity of 97.2%.[33][34]
In a diagnostic test, sensitivity is a measure of how well a test can identify true positives and specificity is a measure of how well a test can identify true negatives. For all testing, both diagnostic and screening, there is usually a trade-off between sensitivity and specificity, such that higher sensitivities will mean lower specificities and vice versa.
Sensitivity and Specificity
A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.
Samples can be obtained by various methods, including a nasopharyngeal swab, sputum (coughed up material),[35] throat swabs,[36] deep airway material collected via suction catheter[36] or saliva.[37][38] Drosten et al. remarked that for 2003 SARS, «from a diagnostic point of view, it is important to note that nasal and throat swabs seem less suitable for diagnosis, since these materials contain considerably less viral RNA than sputum, and the virus may escape detection if only these materials are tested.»[39]
Sensitivity of clinical samples by RT-PCR is 63% for nasal swab, 32% for pharyngeal swab, 48% for feces, 72–75% for sputum, and 93–95% for bronchoalveolar lavage.[40]
The likelihood of detecting the virus depends on collection method and how much time has passed since infection. According to Drosten tests performed with throat swabs are reliable only in the first week. Thereafter the virus may abandon the throat and multiply in the lungs. In the second week, sputum or deep airways collection is preferred.[36]
Collecting saliva may be as effective as nasal and throat swabs,[37] although this is not certain.[41][38] Sampling saliva may reduce the risk for health care professionals by eliminating close physical interaction.[42] It is also more comfortable for the patient.[43] Quarantined people can collect their own samples.[42] A saliva test’s diagnostic value depends on sample site (deep throat, oral cavity, or salivary glands).[38] Some studies have found that saliva yielded greater sensitivity and consistency when compared with swab samples.[44][45][46]
On 15 August 2020, the US FDA granted an emergency use authorization for a saliva test developed at Yale University that gives results in hours.[47][48]
On 4 January 2021, the US FDA issued an alert about the risk of false results, particularly false negative results, with the Curative SARS-Cov-2 Assay real-time RT-PCR test.[49]
Viral burden measured in upper respiratory specimens declines after symptom onset.[50] Following recovery, many patients no longer have detectable viral RNA in upper respiratory specimens. Among those who do, RNA concentrations three days following recovery are generally below the range in which replication-competent virus has been reliably isolated.[51] No clear correlation has been described between length of illness and duration of post-recovery shedding of viral RNA in upper respiratory specimens.[52]
-
A PCR machine
Other molecular tests
Isothermal nucleic acid amplification tests also amplify the virus’s genome. They are faster than PCR because they do not involve repeated heating and cooling cycles. These tests typically detect DNA using fluorescent tags, which are read out with specialized machines.[citation needed]
CRISPR gene editing technology was modified to perform the detection: if the CRISPR enzyme attaches to the sequence, it colors a paper strip. The researchers expect the resulting test to be cheap and easy to use in point-of-care settings.[53][54] The test amplifies RNA directly, without the RNA-to-DNA conversion step of RT-PCR.[55]
Antigen tests
COVID-19 Antigen Rapid Test Kit; the timer is provided by the user.
Mucus from nose or throat in a test liquid is placed onto a COVID-19 rapid antigen diagnostic test device.
COVID-19 rapid testing in Rwanda
An antigen is the part of a pathogen that elicits an immune response. Antigen tests look for antigen proteins from the viral surface. In the case of a coronavirus, these are usually proteins from the surface spikes.[56] SARS-CoV-2 antigens can be detected before onset of COVID-19 symptoms (as soon as SARS-CoV-2 virus particles) with more rapid test results, but with less sensitivity than PCR tests for the virus.[57]
COVID-19 rapid antigen tests are lateral flow immunoassays that detect the presence of a specific viral antigen, which indicates current viral infection. Antigen tests produce results quickly (within approximately 15–30 minutes), and most can be used at the point-of-care or as self-tests. Self-tests are rapid tests that can be taken at home or anywhere, are easy to use, and produce rapid results.[58] Antigen tests can be performed on nasopharyngeal, nasal swab, or saliva specimens.[15]
Antigen tests that can identify SARS-CoV-2 offer a faster and less expensive method to test for the virus.[14] Antigen tests are generally less sensitive than real-time reverse transcription polymerase chain reaction (RT-PCR) and other nucleic acid amplification tests (NAATs).[15]
Antigen tests may be one way to scale up testing to much greater levels.[56] Isothermal nucleic acid amplification tests can process only one sample at a time per machine. RT-PCR tests are accurate but require too much time, energy and trained personnel to run the tests.[56] «There will never be the ability on a [PCR] test to do 300 million tests a day or to test everybody before they go to work or to school,» Deborah Birx, head of the White House Coronavirus Task Force, said on 17 April 2020. «But there might be with the antigen test.»[59]
Samples may be collected via nasopharyngeal swab, a swab of the anterior nares, or from saliva (obtained by various methods including lollipop tests for children).[60] The sample is then exposed to paper strips containing artificial antibodies designed to bind to coronavirus antigens. Antigens bind to the strips and give a visual readout. The process takes less than 30 minutes, can deliver results at point of care, and does not require expensive equipment or extensive training.[56]
Swabs of respiratory viruses often lack enough antigen material to be detectable.[61] This is especially true for asymptomatic patients who have little if any nasal discharge. Viral proteins are not amplified in an antigen test.[56][62] A Cochrane review based on 64 studies investigating the efficacy of 16 different antigen tests determined that they correctly identified COVID-19 infection in an average of 72% of people with symptoms, compared to 58% of people without symptoms.[63][needs update] Tests were most accurate (78%) when used in the first week after symptoms first developed, likely because people have the most virus in their system in the first days after they are infected.[63] While some scientists doubt whether an antigen test can be useful against COVID-19,[62] others have argued that antigen tests are highly sensitive when viral load is high and people are contagious, making them suitable for public health screening.[64][65] Routine antigen tests can quickly identify when asymptomatic people are contagious, while follow-up PCR can be used if confirmatory diagnosis is needed.[66]
Antibody tests
The body responds to a viral infection by producing antibodies that help neutralize the virus.[67] Blood tests (also called serology tests or serology immunoassays[3]) can detect the presence of such antibodies.[68] Antibody tests can be used to assess what fraction of a population has once been infected, which can then be used to calculate the disease’s mortality rate.[5] They can also be used to determine how much antibody is contained in a unit of convalescent plasma, for COVID-19 treatment, or to verify if a given vaccine generates an adequate immune response.[69]
SARS-CoV-2 antibodies’ potency and protective period have not been established.[5][70] Therefore, a positive antibody test may not imply immunity to a future infection. Further, whether mild or asymptomatic infections produce sufficient antibodies for a test to detect has not been established.[71][needs update] Antibodies for some diseases persist in the bloodstream for many years, while others fade away.[56]
The most notable antibodies are IgM and IgG. IgM antibodies are generally detectable several days after initial infection, although levels over the course of infection and beyond are not well characterized.[72] IgG antibodies generally become detectable 10–14 days after infection and normally peak around 28 days after infection.[73][74] This pattern of antibody development seen with other infections, often does not apply to SARS-CoV-2, however, with IgM sometimes occurring after IgG, together with IgG or not occurring at all.[75] Generally, however, median IgM detection occurs 5 days after symptom onset, whereas IgG is detected a median 14 days after symptom onset.[76] IgG levels significantly decline after two or three months.[77]
Genetic tests verify infection earlier than antibody tests. Only 30% of those with a positive genetic test produced a positive antibody test on day 7 of their infection.[71]
Antibody Test Types
Rapid diagnostic test (RDT)
RDTs typically use a small, portable, positive/negative lateral flow assay that can be executed at point of care. RDTs may process blood samples, saliva samples, or nasal swab fluids. RDTs produce colored lines to indicate positive or negative results.[78]
Enzyme-linked immunosorbent assay (ELISA)
ELISAs can be qualitative or quantitative and generally require a lab. These tests usually use whole blood, plasma, or serum samples. A plate is coated with a viral protein, such as a SARS-CoV-2 spike protein. Samples are incubated with the protein, allowing any antibodies to bind to it. The antibody-protein complex can then be detected with another wash of antibodies that produce a color/fluorescent readout.[78]
Neutralization assay
Neutralization assays assess whether sample antibodies prevent viral infection in test cells.[67] These tests sample blood, plasma or serum. The test cultures cells that allow viral reproduction (e.g., Vero E6 cells). By varying antibody concentrations, researchers can visualize and quantify how many test antibodies block virus replication.[78]
Chemiluminescent immunoassay
Chemiluminescent immunoassays are quantitative lab tests. They sample blood, plasma, or serum. Samples are mixed with a known viral protein, buffer reagents and specific, enzyme-labeled antibodies. The result is luminescent. A chemiluminescent microparticle immunoassay uses magnetic, protein-coated microparticles. Antibodies react to the viral protein, forming a complex. Secondary enzyme-labeled antibodies are added and bind to these complexes. The resulting chemical reaction produces light. The radiance is used to calculate the number of antibodies. This test can identify multiple types of antibodies, including IgG, IgM, and IgA.[78]
Neutralizing vis-à-vis binding antibodies
Most if not all large scale COVID-19 antibody testing looks for binding antibodies only and does not measure the more important neutralizing antibodies (NAb).[79][80][81] A NAb is an antibody that neutralizes the infectivity of a virus particle by blocking its attachment to or entry into a susceptible cell; enveloped viruses, like e.g. SARS-CoV-2, are neutralized by the blocking of steps in the replicative cycle up to and including membrane fusion.[82][67] A non-neutralizing antibody either does not bind to the crucial structures on the virus surface or binds but leaves the virus particle infectious; the antibody may still contribute to the destruction of virus particles or infected cells by the immune system.[83][67] It may even enhance infectivity by interacting with receptors on macrophages.[84] Since most COVID-19 antibody tests return a positive result if they find only binding antibodies, these tests cannot indicate that the subject has generated protective NAbs that protect against re-infection.[80][81]
It is expected that binding antibodies imply the presence of NAbs[81] and for many viral diseases total antibody responses correlate somewhat with NAb responses[85] but this is not established for COVID-19. A study of 175 recovered patients in China who experienced mild symptoms reported that 10 individuals had no detectable NAbs at discharge, or thereafter. How these patients recovered without the help of NAbs and whether they were at risk of re-infection was not addressed.[80] An additional source of uncertainty is that even if NAbs are present, viruses such as HIV can evade NAb responses.[79]
Studies have indicated that NAbs to the original SARS virus (the predecessor to the current SARS-CoV-2) can remain active for two years[86] and are gone after six years.[87] Nevertheless, memory cells including memory B cells and memory T cells[88] can last much longer and may have the ability to reduce reinfection severity.[87]
-
A point of care test in Peru. A blood droplet is collected by a pipette.
-
The rapid diagnostic test shows reactions of IgG and IgM antibodies.
Other tests
Sniff tests
Sudden loss of smell can be used to screen people on a daily basis for COVID-19. A study by the National Institutes of Health showed that those infected with SARS-CoV-2 could not smell a 25% mixture of ethanol and water.[89] Because various conditions can lead to the loss of the sense of smell, a sniff test would not be definitive but indicate the need for a PCR test. Because the loss of the sense of smell shows up before other symptoms, there has been a call for widespread sniff testing.[90] Health care bureaucracies have generally ignored sniff tests even though they are quick, easy and capable of being self-administered daily. This has led some medical journals to write editorials supporting the adoption of sniff testing.[91]
Imaging
Typical visible features on CT initially include bilateral multilobar ground-glass opacities with a peripheral or posterior distribution.[92] COVID-19 can be identified with higher precision using CT than with RT-PCR.[93]
Subpleural dominance, crazy paving, and consolidation may develop as the disease evolves.[92][94] Chest CT scans and chest x-rays are not recommended for diagnosing COVID-19. Radiologic findings in COVID-19 lack specificity.[92][95]
Chest X-rays, computed tomography scans and ultrasounds are all ways the coronavirus disease can be detected.
A chest x-ray is a portable lightweight machine. This machine is typically more available than polymerase chain reaction and computerized tomography scans. it only takes approximately 15 seconds per patient.[96] This makes chest-x ray readily accessible and inexpensive. It also has quick turnaround time and can be crucial to the clinical equipment in the detection of coronavirus disease.[97]
Computerized tomography scans involve looking at 3D images from various angles. This is not as available as chest x-ray, but still only takes about 15 minutes per patient.[96] Computerized tomography has been a known routine scanning for pneumonia diagnosis, therefore can also be used to diagnose coronavirus disease. Computerized tomography scans may help with ongoing illness monitoring throughout treatment. Patients who had low-grade symptoms and high body temperatures revealed significant lung indications on their chest computed tomography scans. They emphasized how important chest computerized tomography scans are for determining how serious the coronavirus disease infection is.[98]
Ultrasound can be another tool to detect coronavirus disease. An ultrasound is a type of imaging exam that produces images using sound waves. Unlike computerized tomography scans and x-rays, ultrasound does not use radiation. Moreover, it is inexpensive, simple to use, repeatable, and has several additional advantages. Using a hand-held mobile machine, ultrasound examinations can be performed in a variety of healthcare settings.[99]
There are some downsides to using imaging, however. The equipment needed for computed tomography scans is not available in most hospitals, making it not as effective as some other tools used for detection of the coronavirus disease.[96] One of the difficult tasks in a pandemic is manually inspecting each report, which takes numerous radiology professionals and time.[100] There were several problems with early studies of using chest computerized tomography scans for diagnosing coronavirus. Some of these problems included the disease severity characters being different in severe and hospitalized cases. The criteria for doing a chest computerized tomography scan were not defined. There was also no characterization of positive chest computerized tomography scans results. The computerized tomography scans findings were not the same as positive computerized tomography scans findings of coronavirus.[101] In a typical clinical setting, chest imaging is not advised for routine screening of COVID-19. Patients with asymptomatic to mild symptoms are not recommended to be tested via chest computerized tomography scans. However, it is still crucial to use, particularly when determining complications or disease progression. Chest imaging also is not always the first route to take with patients who have high risk factors for COVID. High risk patients that had mild symptoms, chest imaging findings were limited. Although a computerized tomography scan is a strong tool in the diagnosis of COVID-19, it is insufficient to identify COVID-19 alone due to the poor specificity and the difficulties that radiologists may experience in distinguishing COVID-19 from other viral pneumonia on chest computerized tomography scans.[98]
Article body
Serology (CoLab score) tests
The standard blood test (quick scan) taken at the emergency room measures different values. By use of the blood quick scan the CoLab score is calculated with a developed algorithm based on how the coronavirus causes changes in the blood. The software is intended for use in emergency rooms to quickly rule out the presence of the disease in incoming patients. A not negative result is followed by a PCR (polymerase chain reaction) or LAMP (loop-mediated isothermal amplification) test.[102]
Breath tests
The breath test by a Coronavirus breathalyzer is a pre-screening test for people who have no or mild symptoms of COVID-19. A not negative result is followed by a PCR or LAMP test.[citation needed]
Animals
In May 2021, Reuters reported that Dutch researchers at Wageningen University had shown that trained bees could detect the virus in infected samples in seconds and this could benefit countries where test facilities are in short supply.[103] A two-month study by the Necker-Cochin hospital Paris in conjunction with the French national veterinary school reported in May 2021 that dogs were more reliable than current lateral flow tests.[104]
Researchers in Paris in March 2022 reported in a preprint not yet peer-reviewed that trained dogs were very effective for rapidly detecting the presence of SARS-Cov2 in people, whether displaying symptoms or not. The dogs were presented with sweat samples to smell from 335 people, of whom 78 with symptoms and 31 without tested positive by PCR. The dogs detected 97% of the symptomatic and 100% of the asymptomatic infections. They were 91% accurate at identifying volunteers who were not infected, and 94% accurate at ruling out the infection in people without symptoms. The authors said «Canine testing is non-invasive and provides immediate and reliable results.Further studies will be focused on direct sniffing by dogs to evaluate sniffer dogs for mass pre-test in airports, harbors, railways stations, cultural activities or sporting events.»[105][106]
Functional assays
Tollotest is a molecular test that detects the activity of a SARS-CoV2 protease, which is a biomarker for active infection.[107]
History
Timeline of total number of tests in different countries[108]
In January 2020, scientists from China published the first genetic sequences of SARS-CoV-2 via the GISAID initiative, a program that had handled mostly genetic sequence data from animal-borne influenzas.[109][110] Researchers around the world used that data to build molecular tests for the virus. Antigen- and antibody-based tests were developed later.[citation needed]
Even once the first tests were created, the supply was limited. As a result, no countries had reliable data on the prevalence of the virus early in the pandemic.[111] The WHO and other experts called for ramping up testing as the best way to slow the spread of the virus.[112][113] Shortages of reagent and other testing supplies became a bottleneck for mass testing in the EU, the UK and the US.[114][115][116] Early tests also encountered problems with reliability.[117][118]
Testing protocols
Drive-through testing
In drive-through testing, the person undergoing testing remains in a vehicle while a healthcare professional approaches the vehicle and obtains a sample, all while taking appropriate precautions such as wearing personal protective equipment (PPE).[119][120] Drive-through centers helped South Korea accelerate its testing program.[121]
Home collection
A Randox PCR home test kit in the UK, showing the swab, and multi-layer packaging to deliver it to the lab
In Hong Kong test subjects can stay home and receive a specimen tube. They spit into it, return it and later get the result.[122]
Pooled testing
Pooled testing can improve turnaround time, by combining a number of samples to be tested together. If the pool result is negative, all samples are negative. If the test result is positive, samples will need to be individually tested.[69]
In Israel, researchers at Technion and Rambam Hospital developed a method for testing samples from 64 patients simultaneously, by pooling the samples and only testing further if the combined sample was positive.[123][124][125] Pool testing was then adopted in Israel, Germany, Ghana[126][127][128] South Korea,[129] Nebraska,[130] China[131] and the Indian states of Uttar Pradesh,[132] West Bengal,[133] Punjab,[134] Chhattisgarh[135] and Maharashtra.[136]
Open source, multiplexed designs released by Origami Assays can test as many as 1122 patient samples using only 93 assays.[137] These balanced designs can be run in small laboratories without robotic liquid handlers.
Multi-tiered testing
One study proposed a rapid immune response assay as a screening test, with a confirmatory nucleic acid test for diagnosis, followed by a rapid antibody test to determine course of action and assess population exposure/herd immunity.[138]
Required volume
Required testing levels are a function of disease spread. The more the cases, the more tests are needed to manage the outbreak. COVID-19 tends to grow exponentially at the beginning of an outbreak, meaning that the number of required tests initially also grows exponentially. If properly targeted testing grows more rapidly than cases, it can be contained.[citation needed]
WHO recommends increasing testing until fewer than 10% are positive in any given jurisdiction.[139]
United States
Number of tests done per day in the US, as of April 2020.
Blue: CDC lab
Orange: Public health lab
Gray: Data incomplete due to reporting lag
Not shown: Testing at private labs; total exceeded 100,000 per day by 27 March.[140]
Economist Paul Romer reported that the US has the technical capacity to scale up to 20 million tests per day, which is his estimate of the scale needed to fully remobilize the economy.[141] The Edmond J. Safra Center for Ethics estimated on 4 April 2020 that this capacity could be available by late July 2020.[142] Romer pointed to single-molecule real-time sequencing equipment from Pacific Biosciences[141][143] and to the Ion Torrent Next-Generation Sequencing equipment from ThermoFisher Scientific.[141][144] According to Romer, «Recent research papers suggest that any one of these has the potential to scale up to millions of tests per day.» This plan requires removing regulatory hurdles. Romer estimated that $100 billion would cover the costs.[141]
Romer also claimed that high test accuracy is not required if tests are administered frequently enough. He ran model simulations in which 7% of the population is tested every day using a test with a 20% false negative rate and a 1% false positive rate. The average person would be tested roughly every two weeks. Those who tested positive would go into quarantine. Romer’s simulation indicated that the fraction of the population that is infected at any given time (known as the attack rate) peaks reaches roughly 8% in about thirty days before gradually declining, in most runs reaching zero at 500 days, with cumulative prevalence remaining below 20%.[145]
Snapshot mass-testing
A study found that, despite possibly suboptimal implementation, the snapshot mass-testing approach conducted by Slovakia by which ~80% of its population was tested for COVID-19 within a weekend at the end of October 2020 was highly efficacious, decreasing observed prevalence by 58% within one week and by 70% compared to a hypothetical scenario of no snapshot mass-testing.[146][147] The significant reduction resulted from a set of complementary lockdown and quarantine measures whereby citizens who tested positive were quarantined synchronously the weeks afterwards.[148]
Surveillance and screening of populations
As of August 2020, the WHO recognizes wastewater surveillance of SARS-CoV-2 as a potentially useful source of information on the prevalence and temporal trends of COVID-19 in communities, while highlighting that gaps in research such as viral shedding characteristics should be addressed.[149] Such aggregative testing may have detected early cases.[150] Studies show that wastewater-based epidemiology has the potential for an early warning system and monitoring for COVID-19 infections.[151][152][153][154][155] This may prove particularly useful once large shares of regional populations are vaccinated or recovered and do not need to conduct rapid tests while in some cases being infectious nevertheless.[156]
Available tests
A temporary drive-in testing site for COVID-19 set up with tents in a parking lot
Countries around the world developed tests independently and in partnership with others.
Nucleic acid tests
Tests are available that look for viral DNA using either polymerase chain reaction (PCR) or loop-mediated isothermal amplification (LAMP) technology.
Tests developed in China, France, Germany, Hong Kong, Japan, the United Kingdom, and the US targeted different parts of the viral genome. WHO adopted the German system for manufacturing kits sent to low-income countries without the resources to develop their own.[citation needed]
PowerChek Coronavirus looks for the «E» gene shared by all beta coronaviruses, and the RdRp gene specific to SARS-CoV-2.[157]
Nucleic acid testing conducted using an Abbott Laboratories ID Now device
Abbott Laboratories’ ID Now nucleic acid test uses isothermal amplification technology.[158] The assay amplifies a unique region of the virus’s RdRp gene; the resulting copies are then detected with «fluorescently-labeled molecular beacons».[159] The test kit uses the company’s «toaster-size» ID Now device, which is widely deployed in the US.[160] The device can be used in laboratories or in point of care settings, and provides results in 13 minutes or less.[159]
Primerdesign offers its Genesig Real-Time PCR test system. Roche Molecular Systems offers the Cobas 6800/8800 systems; they are offered among others by the United Nations.[citation needed]
Antigen tests
Innova SARS-CoV-2 Antigen Rapid Qualitative Lateral Flow Test kit showing a negative result. This device has been subject to accuracy concerns and a recall in the United States.
Antigen tests are readily available worldwide and have been approved by several health regulators.
Quidel’s «Sofia 2 SARS Antigen FIA»[66][161] is a lateral flow test that uses monoclonal antibodies to detect the virus’s nucleocapsid (N) protein.[162] The result is read out by the company’s Sofia 2 device using immunofluorescence.[162] The test is simpler and cheaper but less accurate than nucleic acid tests. It can be deployed in laboratories or at point of care and gives results in 15 minutes.[161] A false negative result occurs if the sample’s antigen level is positive but below the test’s detection limit, requiring confirmation with a nucleic acid test.[162]
The Innova SARS-CoV-2 Antigen Rapid Qualitative Test was never approved for use in the United States, but was being sold by the company anyway. The FDA inspected Innova facilities in California in March and April 2021, and found inadequate quality assurance of tests manufactured in China.[163] On 23 April 2021, the company issued a recall. The FDA warned consumers to return or destroy the devices because the rate of false positives and false negatives found in clinical trials were higher than the rate claimed by the packaging.[164] Over 1 billion tests from the company have been distributed in the UK, with £3 billion in funding as part of Operation Moonshot, and the MHRK has authorized exceptional use until at least 28 August 2021.[163] Concerned experts pointed out that accuracy dropped significantly when screening was conducted by the public instead of by a medical professional, and that the test was not designed to screen asymptomatic people.[163] A 2020 study found 79% of positive cases were found when used by laboratory scientists, but only 58% when used by the general public and 40% when used for city-wide screening in Liverpool.[165]
Serology (antibody) tests
Antibodies are usually detectable 14 days after the onset of the infection. Multiple jurisdictions survey their populations using these tests.[166][167] The test requires a blood sample.
Private US labs including Quest Diagnostics and LabCorp offer antibody testing upon request.[168]
Certain antibody tests are available in several European countries and also in the US.[169][170]
Roche offers a selective ELISA serology test.[171]
A summary review in BMJ has noted that while some «serological tests … might be cheaper and easier to implement at the point of care [than RT-PCR]», and such testing can identify previously infected individuals, «caution is warranted … using serological tests for … epidemiological surveillance». The review called for higher quality studies assessing accuracy with reference to a standard of «RT-PCR performed on at least two consecutive specimens, and, when feasible, includ[ing] viral cultures.»[172][173] CEBM researchers have called for in-hospital ‘case definition’ to record «CT lung findings and associated blood tests»[174] and for the WHO to produce a «protocol to standardise the use and interpretation of PCR» with continuous re-calibration.[175]
Accuracy
| Samples source | Positive rate |
|---|---|
| Bronchoalveolar lavage fluid specimens | 93% (14/15) |
| Sputum | 72% (75/104) |
| Nasal swabs | 63% (5/8) |
| Fibrobronchoscope brush biopsy | 46% (6/13) |
| Pharyngeal swabs | 32% (126/398) |
| Feces | 29% (44/153) |
| Blood | 1% (3/307) |
Accuracy is measured in terms of specificity and selectivity. Test errors can be false positives (the test is positive, but the virus is not present) or false negatives, (the test is negative, but the virus is present).[177]
Sensitivity and specificity
Sensitivity indicates whether the test accurately identifies whether the virus is present. Each test requires a minimum level of viral load in order to produce a positive result. A 90% sensitive test will correctly identify 90% of infections, missing the other 10% (a false negative). Even relatively high sensitivity rates can produce high rates of false negatives in populations with low incidence rates.[177]
In a diagnostic test, sensitivity is a measure of how well a test can identify true positives and specificity is a measure of how well a test can identify true negatives. For all testing, both diagnostic and screening, there is usually a trade-off between sensitivity and specificity, such that higher sensitivities will mean lower specificities and vice versa.
Sensitivity and Specificity
A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.[citation needed]
Low-specificity tests have a low positive predictive value (PPV) when prevalence is low. For example, suppose incidence is 5%. Testing 100 people at random using a test that has a specificity of 95% would yield on average 5 people who are actually negative who would incorrectly test positive. Since 5% of the subjects actually are positive, another five would also test positive correctly, totaling 10 positive results. Thus, the PPV is 50%,[178] an outcome no different from a coin toss. In this situation, assuming that the result of a second test is independent of the first test, retesting those with a first positive result increases the PPV to 94.5%, meaning that only 4.5% of the second tests would return the incorrect result, on average less than 1 incorrect result.[179]
Causes of test error
The time course of infection affects the accuracy of some tests. Samples may be collected before the virus has had a chance to establish itself or after the body has begun to eliminate it. A May 2020 review of PCR-RT testing found that the median probability of a false-negative result decreased from 100% on day 1, to 67% on day 4. On the day of symptom onset, the probability was 38%, which decreased to 20% 3 days later.[180][needs update]
PCR-based test
Detection of SARS-CoV-2 by nasal swab over six weeks in patients who experienced mild to moderate illness
RT-PCR is the most commonly-used diagnostic test.[181] PCR tests by nasopharyngeal swab have a sensitivity of 73%, but systematic analysis of specificity has not been determined due to the lack of PCR studies with a control group.[182]
In one study sensitivity was highest at week one (100%), followed by 89.3%, 66.1%, 32.1%, 5.4% and zero by week six since symptom onset.[183][failed verification][184]
Sensitivity is also a function of the number of PCR cycles, as well as time and temperature between sample collection and analysis.[185] A cycle threshold of 20 cycles would be adequate to detect SARS-Cov-2 in a highly infective person.[185] Cycle thresholds above 34 are increasingly likely to give false positives outside of high biosafety level facilities.[185]
On July 16, 2020, Dr. Anthony Fauci of the US CDC indicated that positive results obtained from RT-PCR tests run at more than 35 cycles were almost always «just dead nucleotides».[186] On August 29, 2020, the New York Times reported that, «In three sets of testing data that include cycle thresholds, compiled by officials in Massachusetts, New York and Nevada … most tests set the limit at 40 [cycles], a few at 37» and that the CDC was examining the use of cycle threshold measures «for policy decisions,»[187] On July 21, 2021, the CDC, in their «Real-Time RT-PCR Diagnostic Pan: Instructions for Use», indicated tests results should be determined at 40 cycles.[188]
A Dutch CDC-led laboratory investigation compared 7 PCR kits.[189] Test kits made by BGI, R-Biopharm AG, BGI, KH Medical and Seegene showed high sensitivity.[190]
High sensitivity kits are recommended to assess people without symptoms, while lower sensitivity tests are adequate when diagnosing symptomatic patients.[189]
The University of Oxford’s Centre for Evidence-Based Medicine (CEBM) has pointed to mounting evidence[191][192] that «a good proportion of ‘new’ mild cases and people re-testing positives via RT-PCR after quarantine or discharge from hospital are not infectious, but are simply clearing harmless virus particles which their immune system has efficiently dealt with», and have called for «an international effort to standardize and periodically calibrate testing».[174] On 7 September, the UK government issued «guidance for procedures to be implemented in laboratories to provide assurance of positive SARS-CoV-2 RNA results during periods of low prevalence, when there is a reduction in the predictive value of positive test results».[193]
On 4 January 2021, the US FDA issued an alert about the risk of false results, particularly false negative results, with the Curative SARS-Cov-2 Assay real-time RT-PCR test.[49]
Isothermal nucleic amplification test
One study reported that the ID Now COVID-19 test showed sensitivity of 85.2%. Abbott responded that the issue could have been caused by analysis delays.[194] Another study rejected the test in their clinical setting because of this low sensitivity.[195]
Confirmatory testing
The WHO recommends countries that do not have testing capacity and national laboratories with limited experience on COVID-19 send their first five positives and the first ten negative COVID-19 samples to one of the 16 WHO reference laboratories for confirmatory testing.[196][197] Out of the sixteen reference laboratories, seven are in Asia, five in Europe, two in Africa, one in North America and one in Australia.[198]
National or regional responses
Iceland
Iceland managed the pandemic with aggressive contact tracing, inbound travel restrictions, testing, and quarantining, but with less aggressive lock-downs.[199]
India
Italy
Researchers tested the entire population of Vo’, the site of Italy’s first COVID-19 death. They tested about 3,400 people twice, at an interval of ten days. About half the people testing positive had no symptoms. All discovered cases were quarantined. Along with restricting travel to the commune, new infections were eliminated.[200]
Japan
Unlike other Asian countries, Japan did not experience a pandemic of SARS or MERS, so the country’s PCR testing system was not well developed.[201][202] Japan preferentially tested patients with severe illness and their close contacts at the beginning. Japan’s Novel Coronavirus Expert Meeting chose cluster measures to identify infections clusters.[201][202] The Expert Meeting analyzed the outbreak from Wuhan and identified conditions leading to clusters (closed spaces, crowded spaces and close-contact), and asked people to avoid them.[202][203]
In January, contact tracers took action shortly after the first infection was found. Only administrative tests were carried out at first, until insurance began covering PCR tests on 6 March. Private companies began to test, and the test system gradually expanded.[201][204]
On 3 April, those with positive tests were legally permitted to recuperate at home or in a hotel if they had asymptomatic or mild illness, ending the hospital bed shortage.[205] The first wave (from China) was contained,[206] but a second wave (caused by returnees from Europe and the US) in mid-March led to spreading infection in April.[202] On 7 April, Japan declared a state of emergency (less strict than a lockdown, because it did not block cities or restrict outings).[202][205][207] On 13 May, antigen test kits became covered by insurance, and were combined with a PCR test for diagnosis.[208][209]
Japan’s PCR test count per capita remained far smaller than in some other countries even though its positive test rate was lower. Excess mortality was observed in March.[203][failed verification][207][failed verification][210] The Expert Meeting stated, «The Japanese health care system originally carries out pneumonia surveillance, allowing it to detect most of the severely ill patients who develop pneumonia. There are a large number of CT scanners in Japan and they have spread to small hospitals all over the country, so pneumonia patients are rarely missed. In that sense, it meets the same standards as other countries that mainly carry out PCR tests.»[203][210] The group recommended using CT scans data and doctor’s findings for diagnosis.[211][212] On the Diamond Princess cruise ship, many people who initially tested negative later tested positive. Half of coronavirus-positives there who remained mild or asymptomatic had pneumonia findings on CT scans and their CT image showed a frosted glass shadow that is characteristic of infection.[211][213]
As of 18 July, Japan’s daily PCR testing capacity was about 32,000, more than three times the 10,000 cases as of April. When the antigen test is added to it, the number is about 58,000. The number of tests per 1,000 people in the United States is about 27 times that of Japan, the UK is 20 times, Italy is 8 times, and South Korea is twice (as of 26 July).[214][215][216]
The number of those infected with coronavirus and inpatients has increased in July, but the number of serious cases has not increased. This is thought to be due to the proper testing of those infected in July compared to those in April. In April, the number of tests could not catch up with the increase in the number of infected people, and the test standards were strict, so the test positive rate exceeded 30% at the peak. It means that there were quite a few cases where the those infected was not PCR tested. It is thought that the severe case was preferentially tested though there were a lot of mild cases and asymptomatic carriers mainly in the young during the first wave. In other words, it became possible to grasp the actual situation of infection much better than before by strengthening the testing system.[217] At the end of July, accommodation facilities for mild and asymptomatic carriers became full, and the authorities requested hospitals to prepare beds for the mild. However, it became difficult to treat patients with other illnesses and to maintain the ICU system including the staff due to the occupation of hospital beds by patients with mild symptoms.[218][219][220]
Russia
On 27 April 2020, Russia tested 3 million people and had 183,000 positive results.[221] On 28 April Anna Popova, head of Federal Service for Surveillance in Healthcare (Roszdravnadzor) stated that 506 laboratories were testing; that 45% of those who tested positive had no symptoms; that 5% of patients had a severe form; and 40% of infections were from family members. Illness improved from six days to one day after symptoms appeared. Antibody testing was carried out on 3,200 Moscow doctors, finding 20% immunity.[222]
Singapore
With contact tracing, inbound travel restrictions, testing, and quarantining, Singapore arrested the initial spread without complete lockdown.[223]
Slovakia
In late October 2020 Slovakia tested 3.62 million people in a weekend, from a population of 5.4m, representing 67% of the total (or 82% of the adult population), 38,359 tested positive, representing 1.06% of those tested. The government considered the mass test would significantly assist in controlling the virus and avoid a lockdown and may repeat the exercise at a later date.[224]
South Korea
South Korea’s broad testing approach helped reduce spread. Testing capacity, largely in private sector labs, was built up over several years by the South Korean government in the early 2000s.[225]
The government exploited the resident registration number (RRN) system. Authorities mobilized young men who were eligible for military service as social service agents, security and public health doctors. Public health doctors were mainly dispatched to public health centers and life treatment centers where mildly ill patients were accommodated. They performed PCR tests and managed mild patients. Social service agents worked in pharmacies to fill staff shortages. Korea’s 10k PCR tests per million residents was the world’s highest as of 13 April rising to 20k by mid-June. Twenty-seven Korean companies exported test kits worth $48.6 million in March, and were asked to provide test kits or humanitarian assistance by more than 120 countries. Korean authorities set up a treatment center to isolate and manage patients with asymptomatic and minor illnesses in one facility in order to vacate hospital beds for the more severely ill.
Centers were sited mainly at national facilities and corporate training centers. The failure of Korea’s MERS quarantine in May 2015 left Korea more prepared for COVID-19 than countries that did not face that pandemic. Then President Park Geun-hye allowed Korean CDC-approved private sector testing for infectious diseases in 2016. Korea already had a system for isolating, testing and treating infectious disease patients separately from others. Patients with respiratory illness but no epidemiological relevance were treated at the National Hospital, and those with epidemiological relevance were treated at selected clinics.[226][227][228][229][230][231][232][233][234]
Korea established a large scale drive-through/walk-through» test testing program. However, the most common method was «mobile examination». In Daegu City, 54% of samples were collected by 23 March in home or hospital. Collecting samples door-to-door of avoided the risk of travel by possibly infected patients, but required additional staff. Korea solved the problem by drafting more than 2,700 public insurance doctors.[226][230][229]
The government disclosed personal information to the public via KCDC without patient consent. The authorities used digital surveillance to trace possible spread.[227][230][231][233][234][235][236][237][238][239][excessive citations]
Taiwan
Health insurance IDs and national identification card numbers were used to trace contacts.[240][241][242][243]
United Arab Emirates
In January 2021, the COVID-19 testing results of the UAE came under scrutiny, as Denmark suspended the Emirati flights for five days. The European nation said that it barred the flights from the UAE due to growing suspicion of irregularities in the testing process being followed in the Gulf nation. Denmark’s Minister of Transport, Benny Engelbrecht said that they were taking time to ensure that the negative tests of travelers from the Emirates were a real screening carried out appropriately.[244]
United States
New York State
New York State’s control measures consisted of PCR tests, stay-at-home measures and strengthening the healthcare system. On 29 February before its first case, the state allowed testing at the Wordsworth Center. They managed to convince the CDC to approve tests at state laboratories and the FDA to approve a test kit. As of 13 March the state was conducting more than 1,000 daily tests, growing to 10,000/day on 19 March. In April, the number exceeded 20,000. Many people queued at hospitals to get tested. On 21 March New York City health officials directed medical providers to test only those entering the hospital, for lack of PPE.[233][245][246][247][248][excessive citations]
USS Theodore Roosevelt
Following an outbreak, 94% of the 4,800 aircraft carrier crew were tested. Roughly 60 percent of the 600-plus sailors who tested positive were asymptomatic.[249] Five infected sailors who completed quarantine subsequently developed flu-like symptoms and again tested positive.[250]
Nevada
In 2020, Nevada received a donation of 250,000 Covid testing kits, which were a product of China’s leading genetics company, BGI Group. A UAE-based firm owned by Tahnoun bin Zayed Al Nahyan, Group 42 partnered with the BGI Group to supply the testing kits to Nevada. However, the US Department of Homeland Security and the State Department raised a warning for Nevada hospitals to not use the Chinese-made testing kits, as there were concerns around the involvement of the Chinese government, test accuracy and privacy of the patients.[251]
Delayed testing
A shortage of trained medical laboratory scientists, assay reagents, analyzers, transport medium, and PPE coupled with high demand had limited initially limited the availability of testing and led to significantly increased turnaround times.[citation needed]
Testing statistics by country
Testing strategies vary by country and over time,[252] with some countries testing very widely,[8] while others have at times focused narrowly on only testing the seriously ill.[6] The country that tests only people showing symptoms will have a higher figure for «Confirmed»/»tested» than the country that also tests others.[253] If two countries are alike in every respect, including which people they test, the one that tests more people will have a higher «Confirmed / population». Studies have also found that countries that test more, relative to the number of deaths, have lower estimated case fatality rates[9] and younger age distributions of cases.[11]
| Country or region | Date[a] | Tested | Units[b] | Confirmed (cases) |
Confirmed / tested, % |
Tested / population, % |
Confirmed / population, % |
Ref. |
|---|---|---|---|---|---|---|---|---|
| 17 Dec 2020 | 154,767 | samples | 49,621 | 32.1 | 0.40 | 0.13 | [254] | |
| 18 Feb 2021 | 428,654 | samples | 96,838 | 22.6 | 15.0 | 3.4 | [255] | |
| 2 Nov 2020 | 230,553 | samples | 58,574 | 25.4 | 0.53 | 0.13 | [256][257] | |
| 23 Feb 2022 | 300,307 | samples | 37,958 | 12.6 | 387 | 49.0 | [258] | |
| 2 Feb 2021 | 399,228 | samples | 20,981 | 5.3 | 1.3 | 0.067 | [259] | |
| 6 Mar 2021 | 15,268 | samples | 832 | 5.4 | 15.9 | 0.86 | [260] | |
| 16 Apr 2022 | 35,716,069 | samples | 9,060,495 | 25.4 | 78.3 | 20.0 | [261] | |
| 29 May 2022 | 3,099,602 | samples | 422,963 | 13.6 | 105 | 14.3 | [262] | |
| 9 Sep 2022 | 78,548,492 | samples | 10,112,229 | 12.9 | 313 | 40.3 | [263] | |
| 4 Jan 2023 | 204,725,396 | samples | 5,719,585 | 2.8 | 2,300 | 64.2 | [264] | |
| 11 May 2022 | 6,838,458 | samples | 792,638 | 11.6 | 69.1 | 8.0 | [265] | |
| 28 Nov 2022 | 259,366 | samples | 37,483 | 14.5 | 67.3 | 9.7 | [266] | |
| 3 Dec 2022 | 10,578,766 | samples | 696,614 | 6.6 | 674 | 44.4 | [267] | |
| 24 Jul 2021 | 7,417,714 | samples | 1,151,644 | 15.5 | 4.5 | 0.70 | [268] | |
| 14 Oct 2022 | 770,100 | samples | 103,014 | 13.4 | 268 | 35.9 | [269] | |
| 9 May 2022 | 13,217,569 | samples | 982,809 | 7.4 | 139 | 10.4 | [270] | |
| 21 Dec 2022 | 36,317,596 | samples | 4,668,248 | 12.9 | 315 | 40.5 | [271] | |
| 8 Jun 2022 | 572,900 | samples | 60,694 | 10.6 | 140 | 14.9 | [272][273] | |
| 4 May 2021 | 595,112 | samples | 7,884 | 1.3 | 5.1 | 0.067 | [274] | |
| 28 Feb 2022 | 1,736,168 | samples | 12,702 | 0.73 | 234 | 1.71 | [275] | |
| 5 Jun 2022 | 4,358,669 | cases | 910,228 | 20.9 | 38.1 | 8.0 | [276] | |
| 27 Sep 2022 | 1,872,934 | samples | 399,887 | 21.4 | 54.7 | 11.7 | [277] | |
| 11 Jan 2022 | 2,026,898 | 232,432 | 11.5 | 89.9 | 10.3 | [278][279] | ||
| 19 Feb 2021 | 23,561,497 | samples | 10,081,676 | 42.8 | 11.2 | 4.8 | [280][281] | |
| 2 Aug 2021 | 153,804 | samples | 338 | 0.22 | 33.5 | 0.074 | [282] | |
| 25 Dec 2022 | 10,888,076 | samples | 1,291,288 | 11.9 | 157 | 18.6 | [283] | |
| 4 Mar 2021 | 158,777 | samples | 12,123 | 7.6 | 0.76 | 0.058 | [256][284] | |
| 5 Jan 2021 | 90,019 | 884 | 0.98 | 0.76 | 0.0074 | [285] | ||
| 1 Aug 2021 | 1,812,706 | 77,914 | 4.3 | 11.2 | 0.48 | [286] | ||
| 18 Feb 2021 | 942,685 | samples | 32,681 | 3.5 | 3.6 | 0.12 | [256] | |
| 26 Nov 2022 | 66,343,123 | samples | 4,423,053 | 6.7 | 175 | 11.7 | [287] | |
| 2 Mar 2021 | 99,027 | samples | 4,020 | 4.1 | 0.72 | 0.029 | [256][288] | |
| 23 Dec 2022 | 47,538,151 | samples | 5,001,737 | 10.5 | 249 | 26.2 | [289] | |
| 31 Jul 2020 | 160,000,000 | cases | 87,655 | 0.055 | 11.1 | 0.0061 | [290][291] | |
| 24 Nov 2022 | 36,875,818 | samples | 6,314,769 | 17.1 | 76.4 | 13.1 | [292][293] | |
| 2 Nov 2021 | 2,575,363 | samples | 561,054 | 21.8 | 51.5 | 11.2 | [294] | |
| 25 Dec 2022 | 5,415,197 | cases | 1,261,997 | 23.3 | 133 | 31.0 | [295] | |
| 25 Dec 2022 | 14,268,594 | samples | 1,111,887 | 7.8 | 126 | 9.8 | [296][297] | |
| 25 Dec 2022 | 27,494,341 | samples | 631,111 | 2.3 | 3,185 | 73.1 | [298] | |
| 23 Dec 2022 | 22,502,031 | samples | 4,577,186 | 20.3 | 210 | 42.8 | [299] | |
| 22 Dec 2022 | 67,509,781 | samples | 3,381,011 | 5.0 | 1,159 | 58.0 | [300][301] | |
| 28 Apr 2022 | 305,941 | 15,631 | 5.1 | 33.2 | 1.7 | [302] | ||
| 20 Jun 2022 | 209,803 | cases | 14,821 | 7.1 | 293 | 20.7 | [303] | |
| 22 Jul 2022 | 3,574,665 | samples | 626,030 | 17.5 | 32.9 | 5.8 | [304] | |
| 28 Feb 2021 | 124,838 | 25,961 | 20.8 | 0.14 | 0.029 | [256][305] | ||
| 23 Jul 2021 | 1,627,189 | samples | 480,720 | 29.5 | 9.5 | 2.8 | [306] | |
| 23 Jul 2021 | 3,137,519 | samples | 283,947 | 9.1 | 3.1 | 0.28 | [256][307] | |
| 18 Mar 2022 | 1,847,861 | samples | 161,052 | 8.7 | 28.5 | 2.5 | [308] | |
| 12 Dec 2022 | 397,874 | 17,089 | 4.3 | 30.4 | 1.3 | [309] | ||
| 20 Dec 2022 | 3,607,122 | samples | 611,350 | 16.9 | 272 | 46.0 | [310] | |
| 8 Dec 2021 | 415,110 | 49,253 | 11.9 | 36.5 | 4.3 | [311] | ||
| 24 Jun 2021 | 2,981,185 | samples | 278,446 | 9.3 | 2.6 | 0.24 | [312] | |
| 27 Feb 2022 | 774,000 | samples | 34,237 | 4.4 | 1,493 | 65.7 | [313] | |
| 24 Nov 2022 | 665,164 | samples | 68,375 | 10.3 | 74.2 | 7.6 | [314] | |
| 14 Jan 2022 | 9,042,453 | samples | 371,135 | 4.1 | 163 | 6.7 | [315] | |
| 15 May 2022 | 272,417,258 | samples | 29,183,646 | 10.7 | 417 | 44.7 | [316] | |
| 23 Jul 2021 | 958,807 | samples | 25,325 | 2.6 | 3.1 | 0.082 | [317] | |
| 15 Feb 2021 | 43,217 | samples | 4,469 | 10.3 | 2.0 | 0.21 | [318] | |
| 3 Nov 2021 | 4,888,787 | samples | 732,965 | 15.0 | 132 | 19.7 | [319] | |
| 7 Jul 2021 | 65,247,345 | samples | 3,733,519 | 5.7 | 77.8 | 4.5 | [320][321] | |
| 3 Jul 2021 | 1,305,749 | samples | 96,708 | 7.4 | 4.2 | 0.31 | [322] | |
| 18 Dec 2022 | 101,576,831 | samples | 5,548,487 | 5.5 | 943 | 51.5 | [323] | |
| 30 Jan 2022 | 164,573 | samples | 10,662 | 6.5 | 293 | 19.0 | [324] | |
| 11 May 2021 | 28,684 | 161 | 0.56 | 25.7 | 0.14 | [325] | ||
| 17 Dec 2022 | 6,463,092 | samples | 1,184,754 | 18.3 | 37.4 | 6.9 | [326] | |
| 21 Jul 2021 | 494,898 | samples | 24,878 | 5.0 | 3.8 | 0.19 | [256][327] | |
| 7 Jul 2022 | 145,231 | 8,400 | 5.8 | 7.7 | 0.45 | [328] | ||
| 15 Jun 2022 | 648,569 | cases | 66,129 | 10.2 | 82.5 | 8.4 | [329] | |
| 26 Nov 2022 | 223,475 | cases | 33,874 | 15.2 | 2.0 | 0.30 | [330] | |
| 26 Nov 2021 | 1,133,782 | samples | 377,859 | 33.3 | 11.8 | 3.9 | [331] | |
| 10 May 2022 | 11,394,556 | samples | 1,909,948 | 16.8 | 118 | 19.8 | [332] | |
| 9 Aug 2022 | 1,988,652 | samples | 203,162 | 10.2 | 546 | 55.8 | [333] | |
| 8 Jul 2022 | 866,177,937 | samples | 43,585,554 | 5.0 | 63 | 31.7 | [334][335] | |
| 9 Jan 2023 | 73,691,812 | cases | 6,723,812 | 9.1 | 27.3 | 2.5 | [336][336] | |
| 31 May 2022 | 52,269,202 | samples | 7,232,268 | 13.8 | 62.8 | 8.7 | [337] | |
| 3 Aug 2022 | 19,090,652 | samples | 2,448,484 | 12.8 | 47.5 | 6.1 | [338] | |
| 13 Dec 2022 | 12,881,518 | samples | 1,684,717 | 13.1 | 262 | 34.2 | [339] | |
| 17 Jan 2022 | 41,373,364 | samples | 1,792,137 | 4.3 | 451 | 19.5 | [340] | |
| 29 Dec 2022 | 262,558,741 | samples | 25,143,705 | 9.6 | 435 | 41.7 | [341] | |
| 3 Mar 2021 | 429,177 | samples | 33,285 | 7.8 | 1.6 | 0.13 | [342] | |
| 30 Sep 2022 | 1,184,973 | samples | 151,931 | 12.8 | 43.5 | 5.6 | [343] | |
| 1 Mar 2021 | 8,487,288 | 432,773 | 5.1 | 6.7 | 0.34 | [344] | ||
| 6 Jun 2021 | 7,407,053 | samples | 739,847 | 10.0 | 69.5 | 6.9 | [345] | |
| 28 May 2021 | 11,575,012 | samples | 385,144 | 3.3 | 62.1 | 2.1 | [346] | |
| 5 Mar 2021 | 1,322,806 | samples | 107,729 | 8.1 | 2.8 | 0.23 | [347] | |
| 31 May 2021 | 611,357 | cases | 107,410 | 17.6 | 33.8 | 5.9 | [348] | |
| 9 Mar 2022 | 7,754,247 | samples | 624,573 | 8.1 | 181 | 14.6 | [349] | |
| 10 Feb 2021 | 695,415 | samples | 85,253 | 12.3 | 10.7 | 1.3 | [350] | |
| 1 Mar 2021 | 114,030 | cases | 45 | 0.039 | 1.6 | 0.00063 | [351] | |
| 5 Sep 2021 | 3,630,095 | samples | 144,518 | 4.0 | 189 | 7.5 | [352] | |
| 14 Jun 2021 | 4,599,186 | samples | 542,649 | 11.8 | 67.4 | 8.0 | [353] | |
| 30 Mar 2022 | 431,221 | 32,910 | 7.6 | 21.5 | 1.6 | [354] | ||
| 17 Jul 2021 | 128,246 | 5,396 | 4.2 | 2.5 | 0.11 | [355] | ||
| 14 Apr 2022 | 2,578,215 | samples | 501,862 | 19.5 | 37.6 | 7.3 | [256][356] | |
| 20 Dec 2022 | 8,992,468 | samples | 1,160,878 | 12.9 | 322 | 41.5 | [357][358] | |
| 12 May 2022 | 4,248,188 | samples | 244,182 | 5.7 | 679 | 39.0 | [359] | |
| 19 Feb 2021 | 119,608 | cases | 19,831 | 16.6 | 0.46 | 0.076 | [360] | |
| 29 Nov 2022 | 624,784 | samples | 88,086 | 14.1 | 3.3 | 0.46 | [361] | |
| 7 Sep 2021 | 23,705,425 | cases | 1,880,734 | 7.9 | 72.3 | 5.7 | [362] | |
| 13 Mar 2022 | 2,216,560 | samples | 174,658 | 7.9 | 398 | 31.3 | [363][364] | |
| 7 Jul 2021 | 322,504 | samples | 14,449 | 4.5 | 1.6 | 0.071 | [256][365] | |
| 8 Sep 2021 | 1,211,456 | samples | 36,606 | 3.0 | 245 | 7.4 | [366] | |
| 16 Apr 2021 | 268,093 | 18,103 | 6.8 | 6.1 | 0.41 | [367] | ||
| 22 Nov 2020 | 289,552 | samples | 494 | 0.17 | 22.9 | 0.039 | [368] | |
| 15 Oct 2021 | 10,503,678 | cases | 3,749,860 | 35.7 | 8.2 | 2.9 | [369] | |
| 20 Apr 2022 | 3,213,594 | samples | 516,864 | 16.1 | 122 | 19.6 | [370] | |
| 10 Jul 2021 | 3,354,200 | cases | 136,053 | 4.1 | 100 | 4.1 | [371] | |
| 10 May 2021 | 394,388 | samples | 98,449 | 25.0 | 62.5 | 15.6 | [372][373] | |
| 17 Dec 2022 | 14,082,633 | cases | 1,270,820 | 9.0 | 38.2 | 3.4 | [374] | |
| 22 Jul 2021 | 688,570 | samples | 105,866 | 15.4 | 2.2 | 0.34 | [375] | |
| 16 Sep 2021 | 4,047,680 | samples | 440,741 | 10.9 | 7.4 | 0.81 | [376] | |
| 4 Jul 2022 | 1,062,663 | samples | 166,229 | 15.6 | 38.7 | 6.1 | [377] | |
| 26 Jul 2022 | 5,804,358 | samples | 984,475 | 17.0 | 20.7 | 3.5 | [378] | |
| 6 Jul 2021 | 14,526,293 | cases | 1,692,834 | 11.7 | 83.4 | 9.7 | [379] | |
| 3 Sep 2021 | 41,962 | samples | 136 | 0.32 | 15.7 | 0.050 | [380] | |
| 18 Dec 2022 | 7,690,738 | samples | 2,027,981 | 26.4 | 154 | 40.7 | [381][382] | |
| 22 Feb 2021 | 79,321 | cases | 4,740 | 6.0 | 0.35 | 0.021 | [383] | |
| 28 Feb 2021 | 1,544,008 | samples | 155,657 | 10.1 | 0.75 | 0.076 | [384] | |
| 25 Nov 2020 | 16,914 | cases | 0 | 0 | 0.066 | 0 | [385] | |
| 1 Jul 2021 | 881,870 | samples | 155,689 | 17.7 | 42.5 | 7.5 | [386][387] | |
| 12 Jul 2022 | 7,096,998 | samples | 103,034 | 1.5 | 2,177 | 31.6 | [388] | |
| 20 Jan 2022 | 9,811,888 | samples | 554,778 | 5.7 | 183 | 10.3 | [389] | |
| 28 Oct 2020 | 509,959 | samples | 114,434 | 22.4 | 11.0 | 2.5 | [390] | |
| 5 Mar 2021 | 9,173,593 | samples | 588,728 | 6.4 | 4.2 | 0.27 | [391] | |
| 5 Feb 2022 | 3,078,533 | samples | 574,105 | 18.6 | 60.9 | 11.4 | [392] | |
| 17 Dec 2022 | 7,361,620 | samples | 1,020,961 | 13.9 | 176 | 24.4 | [393] | |
| 17 Feb 2021 | 47,490 | cases | 961 | 2.0 | 0.53 | 0.011 | [394] | |
| 27 Mar 2022 | 2,609,819 | samples | 647,950 | 24.8 | 36.6 | 9.1 | [395] | |
| 17 Nov 2022 | 36,073,768 | samples | 4,177,786 | 11.6 | 109.9 | 12.7 | [396] | |
| 19 Dec 2022 | 33,903,886 | samples | 4,057,129 | 12.0 | 33.6 | 4.0 | [397][398] | |
| 27 Apr 2022 | 36,064,311 | samples | 5,993,861 | 16.6 | 94.0 | 15.6 | [399] | |
| 5 Jan 2022 | 27,515,490 | samples | 1,499,976 | 5.5 | 268 | 14.6 | [400] | |
| 11 Nov 2022 | 4,061,988 | cases | 473,440 | 11.7 | 141 | 16.4 | [401] | |
| 29 Jan 2021 | 5,405,393 | samples | 724,250 | 13.4 | 27.9 | 3.7 | [402] | |
| 6 Jun 2022 | 295,542,733 | samples | 18,358,459 | 6.2 | 201 | 12.5 | [403][404] | |
| 6 Oct 2021 | 2,885,812 | samples | 98,209 | 3.4 | 22.3 | 0.76 | [405] | |
| 26 Aug 2021 | 30,231 | cases | 995 | 3.3 | 57.6 | 1.9 | [406] | |
| 7 Oct 2022 | 212,132 | samples | 29,550 | 13.9 | 116.6 | 16.2 | [407] | |
| 10 Dec 2022 | 112,841 | cases | 9,500 | 8.4 | 102.4 | 8.6 | [408] | |
| 1 Jan 2023 | 189,365 | samples | 23,176 | 12.2 | 553 | 67.7 | [409] | |
| 26 Apr 2022 | 41,849,069 | samples | 753,632 | 1.8 | 120 | 2.2 | [410] | |
| 12 Jul 2021 | 624,502 | samples | 46,509 | 7.4 | 3.9 | 0.29 | [411] | |
| 24 Dec 2022 | 11,817,342 | cases | 2,440,248 | 20.6 | 170 | 35.0 | [412] | |
| 3 Aug 2021 | 16,206,203 | samples | 65,315 | 0.40 | 284 | 1.1 | [413][414] | |
| 26 Dec 2022 | 7,371,522 | samples | 1,858,747 | 25.2 | 135 | 34.1 | [415] | |
| 26 Dec 2022 | 2,803,682 | samples | 1,301,004 | 46.4 | 134 | 62.1 | [416] | |
| 24 May 2021 | 11,378,282 | cases | 1,637,848 | 14.4 | 19.2 | 2.8 | [417][418] | |
| 1 Mar 2021 | 6,592,010 | samples | 90,029 | 1.4 | 12.7 | 0.17 | [419] | |
| 26 May 2021 | 164,472 | 10,688 | 6.5 | 1.3 | 0.084 | [420] | ||
| 1 Jul 2021 | 54,128,524 | samples | 3,821,305 | 7.1 | 116 | 8.2 | [421][422] | |
| 30 Mar 2021 | 2,384,745 | samples | 93,128 | 3.9 | 10.9 | 0.43 | [423][424] | |
| 7 Jan 2021 | 158,804 | samples | 23,316 | 14.7 | 0.36 | 0.053 | [256] | |
| 24 May 2021 | 9,996,795 | samples | 1,074,751 | 10.8 | 96.8 | 10.4 | [425][426] | |
| 7 Nov 2022 | 23,283,909 | samples | 4,276,836 | 18.4 | 270 | 49.7 | [427] | |
| 18 Dec 2022 | 28,928,047 | samples | 8,588,414 | 29.69 | 122.6 | 36.385 | [428] | |
| 18 Nov 2020 | 3,880 | 509 | 13.1 | 0.0065 | 0.00085 | [256] | ||
| 4 Mar 2021 | 1,579,597 | cases | 26,162 | 1.7 | 2.3 | 0.038 | [429] | |
| 10 Dec 2022 | 802,777 | 39,337 | 4.9 | 9.3 | 0.46 | [430] | ||
| 3 Jan 2022 | 512,730 | cases | 92,997 | 18.1 | 37.6 | 6.8 | [431] | |
| 23 Aug 2021 | 2,893,625 | samples | 703,732 | 24.3 | 24.5 | 6.0 | [432] | |
| 2 Jul 2021 | 61,236,294 | samples | 5,435,831 | 8.9 | 73.6 | 6.5 | [433] | |
| 11 Feb 2021 | 852,444 | samples | 39,979 | 4.7 | 1.9 | 0.087 | [434] | |
| 24 Nov 2021 | 15,648,456 | samples | 3,367,461 | 21.5 | 37.2 | 8.0 | [435] | |
| 4 Jan 2023 | 198,046,693 | samples | 1,047,290 | 0.53 | 2,063 | 10.9 | [436] | |
| 19 May 2022 | 522,526,476 | samples | 22,232,377 | 4.3 | 774 | 32.9 | [437] | |
| 29 Jul 2022 | 929,349,291 | samples | 90,749,469 | 9.8 | 281 | 27.4 | [438][439] | |
| 16 Apr 2022 | 6,089,116 | samples | 895,592 | 14.7 | 175 | 25.8 | [440] | |
| 7 Sep 2020 | 2,630,000 | samples | 43,975 | 1.7 | 7.7 | 0.13 | [441] | |
| 30 Mar 2021 | 3,179,074 | samples | 159,149 | 5.0 | 11.0 | 0.55 | [442] | |
| 28 Aug 2022 | 45,772,571 | samples | 11,403,302 | 24.9 | 46.4 | 11.6 | [443] | |
| 10 Mar 2022 | 3,301,860 | samples | 314,850 | 9.5 | 19.0 | 1.8 | [444] | |
| 15 Oct 2022 | 2,529,087 | samples | 257,893 | 10.2 | 17.0 | 1.7 | [256][445] | |
|
See also
- 2002–2004 SARS outbreak
- Coronavirus breathalyzer
- Coronavirus disease 2019
- COVID-19 misinformation § PCR testing
- COVID-19 pandemic
- Philippine government response to the COVID-19 pandemic § COVID-19 testing controversy
References
This article incorporates public domain material from Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19. Centers for Disease Control and Prevention. Retrieved 5 May 2020.
- ^ «Coronavirus Disease 2019 (COVID-19)». U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Archived from the original on 14 March 2020. Retrieved 9 June 2020.
- ^ Kobokovich A, West R, Gronvall G. «Global Progress on COVID-19 Serology-Based Testing». Johns Hopkins Center for Health Security. Archived from the original on 9 June 2020. Retrieved 9 June 2020.
- ^ a b c Kubina R, Dziedzic A (June 2020). «Molecular and Serological Tests for COVID-19 a Comparative Review of SARS-CoV-2 Coronavirus Laboratory and Point-of-Care Diagnostics». Diagnostics. 10 (6): 434. doi:10.3390/diagnostics10060434. PMC 7345211. PMID 32604919.
- ^ «Test for Past Infection». U.S. Centers for Disease Control and Prevention (CDC). 2020. Archived from the original on 16 May 2020. Retrieved 19 May 2020.
Antibody blood tests, also called antibody tests, check your blood by looking for antibodies, which show if you had a previous infection with the virus. Depending on when someone was infected and the timing of the test, the test may not find antibodies in someone with a current COVID-19 infection.
- ^ a b c Abbasi J (May 2020). «The Promise and Peril of Antibody Testing for COVID-19». JAMA. 323 (19): 1881–1883. doi:10.1001/jama.2020.6170. PMID 32301958. Archived from the original on 20 April 2020. Retrieved 20 April 2020.
- ^ a b Brotschi M (7 March 2020). «Bund sucht nicht mehr alle Corona-Infizierten» [The federal government is no longer looking for all those infected with corona]. Der Bund (in German). ISSN 0774-6156. Archived from the original on 29 March 2020. Retrieved 9 June 2020.
- ^ Van Beusekom M (24 March 2020). «Italian doctors note high COVID-19 death rate, urge action». CIDRAP News. Archived from the original on 9 June 2020. Retrieved 9 June 2020.
- ^ a b Otmani M (22 March 2020). «COVID-19: First results of the voluntary screening in Iceland». Nordic Life Science. Archived from the original on 29 March 2020. Retrieved 9 June 2020.
- ^ a b Ward D (April 2020). «Sampling bias: explaining wide variations in COVID-19 case fatality rates». Preprint. Bern, Switzerland: WardEnvironment. doi:10.13140/RG.2.2.24953.62564/1.
- ^ Henriques M (2 April 2020). «Coronavirus: Why death and mortality rates differ». BBC News. Archived from the original on 2 April 2020. Retrieved 9 June 2020.
- ^ a b Ward D (May 2020). Sampling Bias: Explaining Variations in Age Distributions of COVID-19 Cases. Technical Report (Report). WardEnvironment. doi:10.13140/RG.2.2.27321.19047/2.
- ^ «Why More Younger People Are Testing Positive for COVID-19». Time. Archived from the original on 26 February 2021. Retrieved 18 August 2020.
- ^ Mina MJ, Parker R, Larremore DB (November 2020). «Rethinking Covid-19 Test Sensitivity — A Strategy for Containment». The New England Journal of Medicine. 383 (22): e120. doi:10.1056/NEJMp2025631. PMID 32997903. S2CID 222158786.
- ^ a b «Antigen-detection in the diagnosis of SARS-CoV-2 infection». www.who.int. Retrieved 12 July 2022.
- ^ a b c CDC (11 February 2020). «Guidance for Antigen Testing for SARS-CoV-2 for Healthcare Providers Testing Individuals in the Community». Centers for Disease Control and Prevention. Retrieved 12 July 2022.
- ^ «Siouxsie Wiles & Toby Morris: What we don’t know about Covid-19». The Spinoff. 6 May 2020. Archived from the original on 22 August 2020. Retrieved 6 May 2020.
- ^ «Testing for COVID-19». U.S. Centers for Disease Control and Prevention (CDC). 20 May 2020. Archived from the original on 19 May 2020. Retrieved 20 May 2020.
Two kinds of tests are available for COVID-19: viral tests and antibody tests.
- ^ Tanner T (23 September 2020). «Finland deploys coronavirus-sniffing dogs at main airport». Associated Press. Helsinki. Archived from the original on 27 October 2020. Retrieved 28 October 2020.
- ^ Jones RT, Guest C, Lindsay SW, Kleinschmidt I, Bradley J, Dewhirst S, et al. (December 2020). «Could bio-detection dogs be used to limit the spread of COVID-19 by travellers?». Journal of Travel Medicine. 27 (8). doi:10.1093/jtm/taaa131. PMC 7454791. PMID 32789466.
- ^ Jendrny P, Schulz C, Twele F, Meller S, von Köckritz-Blickwede M, Osterhaus AD, et al. (July 2020). «Scent dog identification of samples from COVID-19 patients — a pilot study». BMC Infectious Diseases. 20 (1): 536. doi:10.1186/s12879-020-05281-3. PMC 7376324. PMID 32703188.
- ^ a b Habibzadeh P, Mofatteh M, Silawi M, Ghavami S, Faghihi MA (September 2021). «Molecular diagnostic assays for COVID-19: an overview». Critical Reviews in Clinical Laboratory Sciences. 58 (6): 385–398. doi:10.1080/10408363.2021.1884640. PMC 7898297. PMID 33595397.
- ^ «RNA Extraction». AssayGenie. Archived from the original on 6 May 2020. Retrieved 7 May 2020.
- ^ a b «How is the COVID-19 Virus Detected using Real Time RT-PCR?». IAEA. 27 March 2020. Archived from the original on 1 May 2020. Retrieved 5 May 2020.
- ^ «Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe». GlobeNewswire News Room. 30 January 2020. Archived from the original on 31 January 2020. Retrieved 1 February 2020.
- ^ a b Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (April 2009). «The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments». Clinical Chemistry. 55 (4): 611–622. doi:10.1373/clinchem.2008.112797. PMID 19246619.
- ^ «Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis» (PDF). Clinical Science. 23 September 2005. Archived (PDF) from the original on 24 November 2020. Retrieved 5 May 2020.
- ^ «The Basics: RT-PCR». ThermoFisher Scientific. Archived from the original on 14 April 2020. Retrieved 5 May 2020.
- ^ Kang XP, Jiang T, Li YQ, Lin F, Liu H, Chang GH, et al. (June 2010). «A duplex real-time RT-PCR assay for detecting H5N1 avian influenza virus and pandemic H1N1 influenza virus». Virology Journal. 7: 113. doi:10.1186/1743-422X-7-113. PMC 2892456. PMID 20515509.
- ^ Joyce C (2002). Quantitative RT-PCR. A review of current methodologies. Methods Mol. Biol. Vol. 193. pp. 83–92. doi:10.1385/1-59259-283-X:083. ISBN 978-1-59259-283-8. PMID 12325527.
- ^ Varkonyi-Gasic E, Hellens RP (2010). «qRT-PCR of Small RNAs». Plant Epigenetics. Methods in Molecular Biology. Vol. 631. pp. 109–22. doi:10.1007/978-1-60761-646-7_10. ISBN 978-1-60761-645-0. PMID 20204872.
- ^ «Accelerated Emergency Use Authorization (Eua) Summary Covid-19 Rt-Pcr Test (Laboratory Corporation of America)». FDA. Archived from the original on 16 January 2021. Retrieved 3 April 2020.
- ^ Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M (April 2010). «A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines». Methods. 50 (4): S1–S5. doi:10.1016/j.ymeth.2010.01.005. PMID 20215014.
- ^ Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. (March 2021). «Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection». The Cochrane Database of Systematic Reviews. 3 (4): CD013705. doi:10.1002/14651858.CD013705.pub2. PMC 8078597. PMID 33760236.
- ^ Dinnes J, Sharma P, Berhane S, van Wyk SS, Nyaaba N, Domen J, et al. (July 2022). «Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection». The Cochrane Database of Systematic Reviews. 2022 (7): CD013705. doi:10.1002/14651858.CD013705.pub3. PMC 9305720. PMID 35866452.
- ^ «Real-Time RT-PCR Panel for Detection 2019-nCoV». U.S. Centers for Disease Control and Prevention (CDC). 29 January 2020. Archived from the original on 30 January 2020. Retrieved 1 February 2020.
- ^ a b c Drosten C (26 March 2020). «Coronavirus-Update Folge 22» [Coronavirus update episode 22] (PDF). NDR. Archived (PDF) from the original on 31 March 2020. Retrieved 2 April 2020.
- ^ a b «Here’s where things stand on COVID-19 tests in the U.S.» Science News. ScienceNews. 17 April 2020. Archived from the original on 28 April 2020. Retrieved 6 May 2020.
- ^ a b c Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q (April 2020). «Saliva: potential diagnostic value and transmission of 2019-nCoV». International Journal of Oral Science. 12 (1): 11. doi:10.1038/s41368-020-0080-z. PMC 7162686. PMID 32300101.
- ^ Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. (May 2003). «Identification of a novel coronavirus in patients with severe acute respiratory syndrome». The New England Journal of Medicine. 348 (20): 1967–1976. doi:10.1056/NEJMoa030747. PMID 12690091.
- ^ Ghoshal U, Vasanth S, Tejan N (June 2020). «A guide to laboratory diagnosis of Corona Virus Disease-19 for the gastroenterologists». Indian Journal of Gastroenterology. 39 (3): 236–242. doi:10.1007/s12664-020-01082-3. PMC 7462729. PMID 32875524.
- ^ «COVID-19 saliva tests: What is the benefit?». Mayo Clinic. 16 April 2020. Archived from the original on 1 May 2020. Retrieved 6 May 2020.
- ^ a b «New Rutgers Saliva Test for Coronavirus Gets FDA Approval». Rutgers.edu. 13 April 2020. Archived from the original on 30 April 2020. Retrieved 1 May 2020.
- ^ «FDA authorizes Covid-19 saliva test for emergency use». CNN. 14 April 2020. Archived from the original on 27 April 2020. Retrieved 1 May 2020.
- ^ Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, et al. (September 2020). «Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2». The New England Journal of Medicine. 383 (13): 1283–1286. doi:10.1056/NEJMc2016359. PMC 7484747. PMID 32857487. S2CID 221358482.
- ^ Service RF (August 2020). «Spit shines for easier coronavirus testing». Science. 369 (6507): 1041–1042. Bibcode:2020Sci…369.1041S. doi:10.1126/science.369.6507.1041. PMID 32855317. S2CID 221358939.
- ^ «Yale University School of Public Health finds saliva samples promising alternative to nasopharyngeal swab». Merck Manual. 29 April 2020. Archived from the original on 28 May 2020. Retrieved 6 April 2020.
- ^ «FDA gives emergency approval to ‘game changer’ COVID-19 saliva test». The Washington Times. Archived from the original on 16 August 2020. Retrieved 15 August 2020.
- ^ «Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization to Yale School of Public Health for SalivaDirect, Which Uses a New Method of Saliva Sample Processing». U.S. Food and Drug Administration (FDA) (Press release). 15 August 2020. Archived from the original on 16 August 2020. Retrieved 6 November 2020.
- ^ a b
One or more of the preceding sentences incorporates text from this source, which is in the public domain: «Risk of False Results with the Curative SARS-Cov-2 Test for COVID-19». U.S. Food and Drug Administration (FDA). 4 January 2021. Archived from the original on 4 January 2021. Retrieved 4 January 2021.
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- COVID-19 Investigation Team (June 2020). «Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States». Nature Medicine. 26 (6): 861–868. doi:10.1038/s41591-020-0877-5. PMID 32327757.
- Young BE, Ong SW, Kalimuddin S, Low JG, Tan SY, Loh J, et al. (April 2020). «Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore». JAMA. 323 (15): 1488–1494. doi:10.1001/jama.2020.3204. PMC 7054855. PMID 32125362.
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. (March 2020). «SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients». The New England Journal of Medicine. 382 (12): 1177–1179. doi:10.1056/NEJMc2001737. PMC 7121626. PMID 32074444.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. (May 2020). «Virological assessment of hospitalized patients with COVID-2019». Nature. 581 (7809): 465–469. Bibcode:2020Natur.581..465W. doi:10.1038/s41586-020-2196-x. PMID 32235945.
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Young et al. (2020)
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- COVID-19 Investigation Team (June 2020). «Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States». Nature Medicine. 26 (6): 861–868. doi:10.1038/s41591-020-0877-5. PMID 32327757.
- ^ Zimmer C (5 May 2020). «With Crispr, a Possible Quick Test for the Coronavirus». The New York Times. ISSN 0362-4331. Archived from the original on 14 May 2020. Retrieved 14 May 2020.
- ^ «STOPCovid». stopcovid.science. Archived from the original on 10 June 2020. Retrieved 14 June 2020.
- ^ Joung J, Ladha A, Saito M, Segel M, Bruneau R, Huang MW, et al. (May 2020). «Point-of-care testing for COVID-19 using SHERLOCK diagnostics». medRxiv: 2020.05.04.20091231. doi:10.1101/2020.05.04.20091231. PMC 7273289. PMID 32511521. Archived from the original on 16 May 2021. Retrieved 2 July 2021.
- ^ a b c d e f «Developing Antibodies and Antigens for COVID-19 Diagnostics». Technology Networks. 6 April 2020. Archived from the original on 30 April 2020. Retrieved 30 April 2020.
- ^ Guglielmi G (September 2020). «Fast coronavirus tests: what they can and can’t do». Nature. 585 (7826): 496–498. Bibcode:2020Natur.585..496G. doi:10.1038/d41586-020-02661-2. PMID 32939084. S2CID 221768935.
- ^ CDC (11 February 2020). «COVID-19 and Your Health». Centers for Disease Control and Prevention. Retrieved 12 July 2022.
- ^ «Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing». whitehouse.gov. 17 April 2020. Archived from the original on 20 January 2021. Retrieved 30 April 2020 – via National Archives.
- ^ Müllender F (11 March 2021). «Grundschulen – Corona-Pool-Tests gelten als kindgerecht, unkompliziert und sicher» (in German). Deutschlandfunk. Archived from the original on 24 July 2021. Retrieved 5 June 2021.
- ^ «NIH launches competition to speed COVID-19 diagnostics». AAAS. 29 April 2020. Archived from the original on 1 May 2020. Retrieved 1 May 2020.
- ^ a b «What to know about the three main types of coronavirus tests». CNN. 29 April 2020. Archived from the original on 10 May 2020. Retrieved 30 April 2020.
- ^ a b Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. (Cochrane COVID-19 Diagnostic Test Accuracy Group) (March 2021). «Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection». The Cochrane Database of Systematic Reviews. 3 (3): CD013705. doi:10.1002/14651858.CD013705.pub2. PMC 8078597. PMID 33760236.
- ^ «Rapid Tests». Rapid Tests. Archived from the original on 31 May 2021. Retrieved 2 July 2021.
- ^ Shaw J (3 August 2020). «Failing the Coronavirus-Testing Test». Harvard Magazine. Archived from the original on 30 June 2021. Retrieved 2 July 2021.
- ^ a b Office of the Commissioner (9 May 2020). «Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients». FDA. Archived from the original on 29 May 2021. Retrieved 2 July 2021.
- ^ a b c d Klasse PJ (9 September 2014). «Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives». Advances in Biology. Hindawi Limited. 2014: 1–24. doi:10.1155/2014/157895. PMC 4835181. PMID 27099867.
- ^ «The next frontier in coronavirus testing: Identifying the full scope of the pandemic, not just individual infections». STAT. 27 March 2020. Archived from the original on 29 June 2020. Retrieved 30 April 2020.
- ^ a b Tang EW, Bobenchik AM, Lu S (September 2020). «Testing for SARS-CoV-2 (COVID-19): A General Review». Rhode Island Medical Journal. 103 (8): 20–23. PMID 32900007.
- ^ «What Immunity to COVID-19 Really Means». Scientific American. 10 April 2020. Archived from the original on 28 April 2020.
- ^ a b Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al. (June 2020). «Antibody tests for identification of current and past infection with SARS-CoV-2». The Cochrane Database of Systematic Reviews. 2020 (6): CD013652. doi:10.1002/14651858.CD013652. PMC 7387103. PMID 32584464. S2CID 220061130.
- ^ «Cellex Emergency Use Authorization». FDA. 1 April 2020. Archived from the original on 9 April 2020. Retrieved 10 April 2020.
- ^ «Will an Antibody Test Allow Us to Go Back to School or Work?». The New York Times. 10 April 2020. Archived from the original on 15 April 2020. Retrieved 15 April 2020.
- ^ «Mount Sinai Emergency Use Authorization». FDA. 15 April 2020. Retrieved 18 April 2020.
- ^ Bauer G (January 2021). «The variability of the serological response to SARS-corona virus-2: Potential resolution of ambiguity through determination of avidity (functional affinity)». Journal of Medical Virology. 93 (1): 311–322. doi:10.1002/jmv.26262. PMC 7361859. PMID 32633840.
- ^ Ravi N, Cortade DL, Ng E, Wang SX (October 2020). «Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape». Biosensors & Bioelectronics. 165: 112454. doi:10.1016/j.bios.2020.112454. PMC 7368663. PMID 32729549.
- ^ Goudouris ES (2020). «Laboratory diagnosis of COVID-19». Jornal de Pediatria. 97 (1): 7–12. doi:10.1016/j.jped.2020.08.001. PMC 7456621. PMID 32882235.
- ^ a b c d «Global Progress on COVID-19 Serology-Based Testing». Johns Hopkins Center for Health Security. Archived from the original on 14 June 2020. Retrieved 14 June 2020.
- ^ a b Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, et al. (September 2020). «A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction». Nature Biotechnology. 38 (9): 1073–1078. doi:10.1038/s41587-020-0631-z. PMID 32704169. S2CID 220720953.
- ^ a b c Mallapaty S (April 2020). «Will antibody tests for the coronavirus really change everything?». Nature. 580 (7805): 571–572. Bibcode:2020Natur.580..571M. doi:10.1038/d41586-020-01115-z. PMID 32313159. S2CID 216048544. Archived from the original on 24 June 2020. Retrieved 20 April 2020.
- ^ a b c «Q&A on COVID-19 Antibody Tests». factcheck.org. 27 April 2020. Archived from the original on 27 April 2020. Retrieved 28 April 2020.
- ^ «Neutralising antibody». Biology-Online. 2008. Archived from the original on 8 July 2018. Retrieved 4 July 2009.
- ^ Schmaljohn AL (July 2013). «Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts». Current HIV Research. 11 (5): 345–353. doi:10.2174/1570162×113116660057. PMID 24191933.
- ^ Rhorer J, Ambrose CS, Dickinson S, Hamilton H, Oleka NA, Malinoski FJ, Wittes J (February 2009). «Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials». Vaccine. Virology Blog. 27 (7): 1101–1110. doi:10.1016/j.vaccine.2008.11.093. PMID 19095024. Archived from the original on 23 April 2020. Retrieved 29 April 2020.
- ^ «expert reaction to announcement by Roche of its new serology test for COVID-19 antibodies». Science Media Centre. 17 April 2020. Archived from the original on 30 April 2020. Retrieved 28 April 2020.
- ^ Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH (September 2007). «Disappearance of antibodies to SARS-associated coronavirus after recovery». The New England Journal of Medicine. NEJM. 357 (11): 1162–1163. doi:10.1056/NEJMc070348. PMID 17855683.
- ^ a b «Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study» (PDF). Journal of Immunology. 19 April 2011. Archived (PDF) from the original on 1 May 2020. Retrieved 1 May 2020.
- ^ Leslie M (May 2020). «T cells found in coronavirus patients ‘bode well’ for long-term immunity». Science. 368 (6493): 809–810. Bibcode:2020Sci…368..809L. doi:10.1126/science.368.6493.809. PMID 32439770. S2CID 218834495.
- ^ Calvo-Henriquez C, Maldonado-Alvarado B, Chiesa-Estomba C, Rivero-Fernández I, Sanz-Rodriguez M, Villarreal IM, et al. (October 2020). «Ethyl alcohol threshold test: a fast, reliable and affordable olfactory Assessment tool for COVID-19 patients». European Archives of Oto-Rhino-Laryngology. 277 (10): 2783–2792. doi:10.1007/s00405-020-06131-3. PMC 7312102. PMID 32583183.
- ^ Hayes J, Exten C, State P (24 December 2020). «At-home DIY smell tests could catch Covid-19 cases». CNN Health. The Conversation. Retrieved 7 September 2021.
- ^ Menni C, Sudre CH, Steves CJ, Ourselin S, Spector TD (November 2020). «Widespread smell testing for COVID-19 has limited application — Authors’ reply». Lancet. 396 (10263): 1630–1631. doi:10.1016/S0140-6736(20)32316-3. PMC 7832202. PMID 33157000.
- ^ a b c Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (July 2020). «Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients». AJR. American Journal of Roentgenology. 215 (1): 87–93. doi:10.2214/AJR.20.23034. PMID 32174129.
Known features of COVID-19 on initial CT include bilateral multilobar ground-glass opacification (GGO) with a peripheral or posterior distribution, mainly in the lower lobes and less frequently within the right middle lobe.
- ^ Manigandan S, Wu MT, Ponnusamy VK, Raghavendra VB, Pugazhendhi A, Brindhadevi K (November 2020). «A systematic review on recent trends in transmission, diagnosis, prevention and imaging features of COVID-19». Process Biochemistry. 98: 233–240. doi:10.1016/j.procbio.2020.08.016. PMC 7439988. PMID 32843849.
- ^ Lee EY, Ng MY, Khong PL (April 2020). «COVID-19 pneumonia: what has CT taught us?». The Lancet. Infectious Diseases. 20 (4): 384–385. doi:10.1016/S1473-3099(20)30134-1. PMC 7128449. PMID 32105641.
- ^ «ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection». American College of Radiology. 22 March 2020. Archived from the original on 13 May 2020. Retrieved 20 May 2020.
- ^ a b c Tabik S, Gomez-Rios A, Martin-Rodriguez JL, Sevillano-Garcia I, Rey-Area M, Charte D, et al. (December 2020). «COVIDGR Dataset and COVID-SDNet Methodology for Predicting COVID-19 Based on Chest X-Ray Images». IEEE Journal of Biomedical and Health Informatics. 24 (12): 3595–3605. doi:10.1109/JBHI.2020.3037127. PMID 33170789. S2CID 219179286.
- ^ Tay YX, Kothan S, Kada S, Cai S, Lai CW (May 2021). «Challenges and optimization strategies in medical imaging service delivery during COVID-19». World Journal of Radiology. 13 (5): 102–121. doi:10.4329/wjr.v13.i5.102. PMC 8188837. PMID 34141091.
- ^ a b Alsharif W, Qurashi A (May 2021). «Effectiveness of COVID-19 diagnosis and management tools: A review». Radiography. 27 (2): 682–687. doi:10.1016/j.radi.2020.09.010. PMC 7505601. PMID 33008761.
- ^ Inui S, Gonoi W, Kurokawa R, Nakai Y, Watanabe Y, Sakurai K, et al. (November 2021). «The role of chest imaging in the diagnosis, management, and monitoring of coronavirus disease 2019 (COVID-19)». Insights into Imaging. 12 (1): 155. doi:10.1186/s13244-021-01096-1. PMC 8561360. PMID 34727257.
- ^ Panwar H, Gupta PK, Siddiqui MK, Morales-Menendez R, Singh V (September 2020). «Application of deep learning for fast detection of COVID-19 in X-Rays using nCOVnet». Chaos, Solitons, and Fractals. 138: 109944. Bibcode:2020CSF…13809944P. doi:10.1016/j.chaos.2020.109944. PMC 7254021. PMID 32536759.
- ^ Inui S, Gonoi W, Kurokawa R, Nakai Y, Watanabe Y, Sakurai K, et al. (November 2021). «The role of chest imaging in the diagnosis, management, and monitoring of coronavirus disease 2019 (COVID-19)». Insights into Imaging. 12 (1): 155. doi:10.1186/s13244-021-01096-1. PMC 8561360. PMID 34727257.
- ^ «Dutch corona blood test from Eindhoven goes international». 19 April 2021. Archived from the original on 27 April 2021. Retrieved 2 July 2021.
- ^ Biesemans B. «Bees in the Netherlands trained to detect COVID-19 infections». Reuters. Archived from the original on 30 June 2021. Retrieved 2 July 2021.
- ^ Henley J (20 May 2021). «Dogs can better detect Covid in humans than lateral flow tests, finds study». The Guardian. Archived from the original on 29 June 2021.
- ^ Grandjean D, Elie C, Gallet C, Julien C, Roger V, Desquilbet L, et al. (8 March 2022). «Diagnostic accuracy of non-invasive detection of SARS-CoV-2 infection by canine olfaction». PLOS ONE. Cold Spring Harbor Laboratory. 17 (6): e0268382. doi:10.1101/2022.03.07.22271219. PMC 9159600. PMID 35648737. S2CID 247291441.
- ^ «Dogs Sniff Out Coronavirus With High Accuracy». Medscape. Reuters. 10 March 2022.
- ^ «Todos Medical Announces Positive Data in Hospitalized and Outpatient Setting for TolloTest, a Novel SARS-CoV-2 3CL Protease Biomarker Assay». Yahoo.
- ^ Roser M, Ritchie H, Ortiz-Ospina E, Hasell J (4 March 2020). «Coronavirus Disease (COVID-19) – Statistics and Research». Our World in Data. Archived from the original on 19 March 2020. Retrieved 2 July 2021 – via ourworldindata.org.
- ^ Schnirring L (11 January 2020). «China releases genetic data on new coronavirus, now deadly». CIDRAP. Archived from the original on 11 January 2020. Retrieved 12 January 2020.
- ^ Shu Y, McCauley J (March 2017). «GISAID: Global initiative on sharing all influenza data — from vision to reality». Euro Surveillance. 22 (13). doi:10.2807/1560-7917.ES.2017.22.13.30494. PMC 5388101. PMID 28382917.
- ^ Ioannidis JP (17 March 2020). «A fiasco in the making? As the coronavirus pandemic takes hold, we are making decisions without reliable data». STAT. Archived from the original on 5 April 2020. Retrieved 22 March 2020.
- ^ «‘Test, Test, Test’: WHO Chief’s Coronavirus Message to World». The New York Times. Reuters. 16 March 2020. Archived from the original on 20 March 2020. Retrieved 16 March 2020.
- ^ Farge E, Revill J (17 March 2020). «‘Test, test, test’: WHO chief’s coronavirus message to world». Reuters. Archived from the original on 3 November 2020. Retrieved 6 November 2020.
- ^ «Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK» (PDF). European Centre for Disease Prevention and Control. 25 March 2020. pp. 15–16. Archived (PDF) from the original on 26 March 2020. Retrieved 29 March 2020.
the current shortages of laboratory consumables and reagents affect diagnostic capacity and hamper the epidemic response at the national and local levels. The laboratories have experienced delayed or missing deliveries of swabbing material, plastic consumables, RNA extraction and RT-PCR reagents, and PPE. This is affecting laboratories in all EU/EEA countries.
- ^ Baird RP (24 March 2020). «Why Widespread Coronavirus Testing Isn’t Coming Anytime Soon». The New Yorker. Archived from the original on 28 March 2020. Retrieved 29 March 2020.
South Dakota, said that her state’s public-health laboratory—the only lab doing COVID-19 testing in the state—had so much trouble securing reagents that it was forced to temporarily stop testing altogether. also noted critical shortages of extraction kits, reagents, and test kits
- ^ Ossola A (25 March 2020). «Here are the coronavirus testing materials that are in short supply in the US». Quartz. Archived from the original on 26 March 2020. Retrieved 29 March 2020.
extract the virus’s genetic material—in this case, RNA—using a set of chemicals that usually come in pre-assembled kits. ‘The big shortage is extraction kits’ There are no easy replacements here: ‘These reagents that are used in extraction are fairly complex chemicals. They have to be very pure, and they have to be in pure solution’
- ^ Temple-Raston D (6 November 2020). «CDC Report: Officials Knew Coronavirus Test Was Flawed But Released It Anyway». NPR. Archived from the original on 11 June 2021. Retrieved 20 March 2021.
- ^ Armario C (7 October 2020). «Peru bet heavily on cheap COVID tests; it didn’t go well». Associated Press. Archived from the original on 14 January 2021. Retrieved 20 March 2021.
- ^ Kiger J (12 March 2020). «Mayo Clinic starts drive-thru testing for COVID-19». PostBulletin.com. Archived from the original on 12 March 2020. Retrieved 13 March 2020.
- ^ Hawkins AJ (11 March 2020). «Some states are offering drive-thru coronavirus testing». The Verge. Archived from the original on 11 March 2020. Retrieved 13 March 2020.
- ^ «South Korea’s Drive-Through Testing For Coronavirus Is Fast – And Free». npr. 11 March 2020. Archived from the original on 20 March 2020. Retrieved 16 March 2020.
- ^ Beaubien J (23 February 2020). «In Age of COVID-19, Hong Kong Innovates To Test And Quarantine Thousands». NPR. Archived from the original on 24 February 2020. Retrieved 26 February 2020.
- ^ «Pooling method allows dozens of COVID-19 tests to run simultaneously». medicalxpress.com. Archived from the original on 22 March 2020. Retrieved 24 March 2020.
- ^ «Israeli team has coronavirus test kit to test dozens of people at once». The Jerusalem Post | JPost.com. Archived from the original on 23 March 2020. Retrieved 24 March 2020.
- ^ Israel21c Staff (19 March 2020). «Israelis introduce method for accelerated COVID-19 testing». Israel21c. Archived from the original on 22 March 2020. Retrieved 24 March 2020.
- ^ «We ‘pool’ coronavirus samples to test 1,000s at a go; we’ve done 30,000 since Sunday – Noguchi». GhanaWeb. 22 April 2020. Archived from the original on 15 May 2020. Retrieved 22 April 2020.
- ^ «Pooling samples boosts Ghana’s COVID-19 testing». WHO Africa. 31 July 2020. Archived from the original on 5 August 2020. Retrieved 31 July 2020.
- ^ «Pooling samples boosts Ghana’s COVID-19 testing». World Health Organization. 30 July 2020. Archived from the original on 21 August 2020. Retrieved 30 July 2020.
- ^ «[Coronavirus] Verified ‘sample pooling’ introduced to prevent herd infection in S. Korea». ajudaily.com. 9 April 2020. Archived from the original on 10 April 2020. Retrieved 19 April 2020.
- ^ «Gov. Ricketts provides update on coronavirus testing». KMTV. 24 March 2020. Archived from the original on 20 April 2020. Retrieved 19 April 2020.
- ^ Lanese N (28 May 2020). «Wuhan tested millions of people for COVID-19 in just days. Could US cities do the same?». livescience.com. Archived from the original on 28 June 2020. Retrieved 28 June 2020.
- ^ «Latest coronavirus update: UP to begin ‘pool testing’ of Covid suspects». Free Press Journal. Archived from the original on 17 April 2020. Retrieved 19 April 2020.
- ^ Yengkhom S. «West Bengal to start pool testing of samples in low-risk zones». The Times of India. Archived from the original on 20 April 2020. Retrieved 19 April 2020.
- ^ «Punjab launches pool testing». Archived from the original on 4 May 2020. Retrieved 19 April 2020.
- ^ «‘Chhattisgarh to adopt pool sample testing’: Health minister TS Singh Deo on Covid-19″. Hindustan Times. 15 April 2020. Archived from the original on 19 April 2020. Retrieved 19 April 2020.
- ^ «Maharashtra to go for pool testing to defeat coronavirus». Deccan Herald. 12 April 2020. Archived from the original on 15 April 2020. Retrieved 19 April 2020.
- ^ «Origami Assays». Origami Assays. 2 April 2020. Archived from the original on 5 April 2020. Retrieved 7 April 2020.
- ^ Pulia MS, O’Brien TP, Hou PC, Schuman A, Sambursky R (August 2020). «Multi-tiered screening and diagnosis strategy for COVID-19: a model for sustainable testing capacity in response to pandemic». Annals of Medicine. 52 (5): 207–214. doi:10.1080/07853890.2020.1763449. PMC 7877955. PMID 32370561. S2CID 218519851.
- ^ «Which States Are Doing Enough Testing? This Benchmark Helps Settle The Debate». NPR.org. 22 April 2020. Archived from the original on 11 May 2020. Retrieved 11 May 2020.
- ^ Lee TB (2 April 2020). «America’s COVID-19 testing has stalled, and that’s a big problem». Ars Technica. Archived from the original on 14 June 2020. Retrieved 5 April 2020.
- ^ a b c d Romer P. «Roadmap to responsibly reopen America» (PDF). Archived (PDF) from the original on 11 May 2020. Retrieved 11 May 2020.
- ^ «ROADMAP TO PANDEMIC RESILIENCE» (PDF). Edmond J. Safra Center for Ethics. 20 April 2020. Archived (PDF) from the original on 20 May 2020. Retrieved 19 May 2020.
- ^ «Certified Service Providers». Pacific Biosciences. Archived from the original on 10 June 2020. Retrieved 18 May 2020.
- ^ «Service Provider Program – US». www.thermofisher.com. ThermoFisher Scientific. Archived from the original on 10 June 2020. Retrieved 18 May 2020.
- ^ «Paul Romer». paulromer.net. Simulating Covid-19: Part 2. Archived from the original on 18 May 2020. Retrieved 19 May 2020.
- ^ Lewis T. «Slovakia Offers a Lesson in How Rapid Testing Can Fight COVID». Scientific American. Archived from the original on 19 April 2021. Retrieved 19 April 2021.
- ^ Pavelka M, Van-Zandvoort K, Abbott S, Sherratt K, Majdan M, Jarčuška P, et al. (May 2021). «The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia». Science. 372 (6542): 635–641. Bibcode:2021Sci…372..635P. doi:10.1126/science.abf9648. PMC 8139426. PMID 33758017.
- ^ «Slovakia’s mass Covid testing cut infection rate by 60%, researchers say». The Guardian. 7 December 2020. Archived from the original on 5 May 2021. Retrieved 30 April 2021.
- ^ Sharif S, Ikram A, et al. (24 June 2020). «Detection of SARs-CoV-2 in wastewater, using the existing environmental surveillance network: An epidemiological gateway to an early warning for COVID-19 in communities». medRxiv. doi:10.1101/2020.06.03.20121426. S2CID 219322544.
- ^ «Coronavirus traces found in March 2019 sewage sample, Spanish study shows». Reuters. 26 June 2020. Retrieved 28 July 2021.
- ^ Kreier F (May 2021). «The myriad ways sewage surveillance is helping fight COVID around the world». Nature. doi:10.1038/d41586-021-01234-1. PMID 33972790. S2CID 234360319.
- ^ Agrawal S, Orschler L, Lackner S (March 2021). «Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany». Scientific Reports. 11 (1): 5372. Bibcode:2021NatSR..11.5372A. doi:10.1038/s41598-021-84914-2. PMC 7940401. PMID 33686189.
- ^ Rooney CM, Moura IB, Wilcox MH (January 2021). «Tracking COVID-19 via sewage». Current Opinion in Gastroenterology. 37 (1): 4–8. doi:10.1097/MOG.0000000000000692. PMID 33074996. S2CID 224811450.
- ^ Larsen DA, Wigginton KR (October 2020). «Tracking COVID-19 with wastewater». Nature Biotechnology. 38 (10): 1151–1153. doi:10.1038/s41587-020-0690-1. PMC 7505213. PMID 32958959.
- ^ Michael-Kordatou I, Karaolia P, Fatta-Kassinos D (October 2020). «Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification». Journal of Environmental Chemical Engineering. 8 (5): 104306. doi:10.1016/j.jece.2020.104306. PMC 7384408. PMID 32834990.
- ^ Seeger C. «Abwasserbasierte EpidemiologieAbwassermonitoring als Frühwarnsystem für Pandemien» (PDF). Retrieved 28 July 2021.
- ^ «[New Product] COVID-19 Kit». kogene.co.kr. 27 February 2020. Archived from the original on 23 April 2020.
- ^ «Letter from FDA». FDA. 27 March 2020. Archived from the original on 28 March 2020. Retrieved 2 April 2020.
- ^ a b ID NOW COVID-19 Archived 16 January 2021 at the Wayback Machine, Instruction for Use, FDA
- ^ «The scramble for the rapid coronavirus tests everybody wants». The Washington Post. 1 April 2020. Archived from the original on 10 February 2021. Retrieved 2 July 2021.
- ^ a b «FDA issues emergency approval of new antigen test that is cheaper, faster and simpler». The Washington Post. 9 May 2020. Archived from the original on 26 January 2021. Retrieved 2 July 2021.
- ^ a b c Sofia 2 SARS Antigen FIA Archived 2 April 2021 at the Wayback Machine Instructions for Use, FDA.gov
- ^ a b c Peplow M (14 June 2021). «COVID-19 test used in UK mass screening program receives stinging rebuke from FDA». Archived from the original on 15 June 2021. Retrieved 2 July 2021.
- ^ FDA Division of Industry and Consumer Education (10 June 2021). «Stop Using Innova Medical Group SARS-CoV-2 Antigen Rapid Qualitative Test: FDA Safety Communication». FDA. Archived from the original on 2 July 2021. Retrieved 2 July 2021.
- ^ Mina MJ, Peto TE, García-Fiñana M, Semple MG, Buchan IE (April 2021). «Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19». Lancet. 397 (10283): 1425–1427. doi:10.1016/S0140-6736(21)00425-6. PMC 8049601. PMID 33609444.
- ^ «NIH Begins Study to Quantify Undetected Cases of Coronavirus Infection | NIH: National Institute of Allergy and Infectious Diseases». niaid.nih.gov. Archived from the original on 10 April 2020. Retrieved 11 April 2020.
- ^ Mandavilli A, Thomas K (10 April 2020). «Will an Antibody Test Allow Us to Go Back to School or Work?». The New York Times. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
- ^ «Quest Diagnostics Launches Consumer-Initiated COVID-19 Antibody Test Through QuestDirect™». Quest Diagnosics. 28 April 2020. Archived from the original on 17 May 2021. Retrieved 2 July 2021.
- ^ Fellmann F. (March 2020). (in German) «Jetzt beginnt die Suche nach den Genesenen» Archived 28 March 2020 at the Wayback Machine. Tages Anzeiger. Retrieved 28 March 2020.
- ^ Herrera T (27 October 2020). «What You Need to Know About the Covid-19 Antibody Test». The New York Times. Retrieved 18 July 2021.
- ^ «EUA Authorized Serology Test Performance». U.S. Food and Drug Administration (FDA). 7 May 2020. Archived from the original on 8 May 2020. Retrieved 8 May 2020.
- ^ Lisboa Bastos M, Tavaziva G, Abidi SK, Campbell JR, Haraoui LP, Johnston JC, et al. (July 2020). «Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis». BMJ. 370: m2516. doi:10.1136/bmj.m2516. PMC 7327913. PMID 32611558.
- ^ Spencer E, Henighan C (1 September 2020). «Overview of BMJ: Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis». CEBM. Archived from the original on 3 October 2020. Retrieved 24 September 2020.
- ^ a b Spencer E, Jefferson T, Brassey J, Heneghan C (11 September 2020). «When is Covid, Covid?». CEBM. Archived from the original on 19 September 2020. Retrieved 19 September 2020.
- ^ Jefferson T, Spencer E, Brassey J, Heneghan C (3 September 2020). «Viral cultures for COVID-19 infectivity assessment. Systematic review». medRxiv. doi:10.1101/2020.08.04.20167932. S2CID 220962177.
{{cite journal}}: CS1 maint: url-status (link) - ^ Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (May 2020). «Detection of SARS-CoV-2 in Different Types of Clinical Specimens». JAMA. 323 (18): 1843–1844. doi:10.1001/jama.2020.3786. PMC 7066521. PMID 32159775.
- ^ a b Ferran M (7 May 2020). «COVID-19 tests are far from perfect, but accuracy isn’t the biggest problem». Popular Science. Archived from the original on 11 May 2020. Retrieved 10 May 2020.
- ^ «Serological testing for SARS-CoV-2 antibodies». American Medical Association. 14 May 2020. Archived from the original on 28 May 2020. Retrieved 29 May 2020.
- ^ «Interim Guidelines for COVID-19 Antibody Testing». U.S. Centers for Disease Control and Prevention (CDC). 23 May 2020. Archived from the original on 29 May 2020. Retrieved 29 May 2020.
- ^ Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J (August 2020). «Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure». Annals of Internal Medicine. 173 (4): 262–267. doi:10.7326/M20-1495. PMC 7240870. PMID 32422057.
- ^ «RT-PCR Testing». www.idsociety.org. Archived from the original on 24 June 2021. Retrieved 16 February 2021.
- ^ Böger B, Fachi MM, Vilhena RO, Cobre AF, Tonin FS, Pontarolo R (January 2021). «Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19». American Journal of Infection Control. 49 (1): 21–29. doi:10.1016/j.ajic.2020.07.011. PMC 7350782. PMID 32659413.
- ^ «Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19». U.S. Centers for Disease Control and Prevention (CDC). 30 April 2020. Archived from the original on 6 June 2021. Retrieved 28 August 2021.
- ^ Xiao AT, Tong YX, Zhang S (November 2020). «Profile of RT-PCR for SARS-CoV-2: A Preliminary Study From 56 COVID-19 Patients». Clinical Infectious Diseases. 71 (16): 2249–2251. doi:10.1093/cid/ciaa460. PMC 7188124. PMID 32306036.
- ^ a b c Engelmann I, Alidjinou EK, Ogiez J, Pagneux Q, Miloudi S, Benhalima I, et al. (March 2021). «Preanalytical Issues and Cycle Threshold Values in SARS-CoV-2 Real-Time RT-PCR Testing: Should Test Results Include These?». ACS Omega. 6 (10): 6528–6536. doi:10.1021/acsomega.1c00166. PMC 7970463. PMID 33748564.
- ^ Fauci A (16 July 2020). «This Week in Virology». YouTube. 4:20.
- ^ Mandavilli A (29 August 2020). «Your Coronavirus Test Is Positive. Maybe It Shouldn’t Be». The New York Times. ISSN 0362-4331. Retrieved 30 August 2021.
- ^ US CDC (20 July 2021). «Real-Time RT-PCR Diagnostic Panel: Instructions for Use». Food and Drug Administration. p. 35. Retrieved 30 August 2021.
- ^ a b van Kasteren PB, van der Veer B, van den Brink S, Wijsman L, de Jonge J, van den Brandt A, et al. (July 2020). «Comparison of seven commercial RT-PCR diagnostic kits for COVID-19». Journal of Clinical Virology. 128: 104412. doi:10.1016/j.jcv.2020.104412. PMC 7206434. PMID 32416600.
- ^ «Chinese Covid-19 test kit outstrips alternatives in Dutch study». South China Morning Post. 20 May 2020. Archived from the original on 23 May 2020. Retrieved 23 May 2020.
- ^ Heneghan C, Jefferson T (1 September 2020). «Virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR». CEBM. Archived from the original on 18 June 2021. Retrieved 19 September 2020.
- ^ Lu J, Peng J, Xiong Q, Liu Z, Lin H, Tan X, et al. (September 2020). «Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR». EBioMedicine. 59: 102960. doi:10.1016/j.ebiom.2020.102960. PMC 7444471. PMID 32853988.
- ^ «SARS-CoV-2 RNA testing: assurance of positive results during periods of low prevalence». GOV.UK. Archived from the original on 6 May 2021. Retrieved 19 September 2020.
- ^ «Study Raises Questions About False Negatives From Quick COVID-19 Test». NPR. 21 April 2020. Archived from the original on 1 May 2020. Retrieved 1 May 2020.
- ^ Thomas K (13 May 2020). «Coronavirus Testing Used by the White House Could Miss Infections». The New York Times. ISSN 0362-4331. Archived from the original on 13 May 2020. Retrieved 14 May 2020.
- ^ «National laboratories». who.int. Archived from the original on 31 January 2020. Retrieved 2 March 2020.
- ^ «PHE novel coronavirus diagnostic test rolled out across UK». GOV.UK. Archived from the original on 7 February 2020. Retrieved 12 April 2020.
In addition to processing samples from suspected cases in this country, PHE is now working as a reference laboratory for WHO, testing samples from countries that do not have assured testing capabilities.
- ^ «Specimen referral for COVID-19 – operational details of WHO reference laboratories providing confirmatory testing for COVID-19» (PDF). World Health Organization. Archived (PDF) from the original on 5 March 2020. Retrieved 29 March 2020.
- ^ «COVID-19: First results of the voluntary screening in Iceland». Nordic Life Science. 27 March 2020. Archived from the original on 29 March 2020. Retrieved 5 April 2020.
- ^ «How an experiment helped one Italian town find ‘submerged infections,’ cut new COVID-19 cases to zero». Nationalpost. 19 March 2020. Retrieved 29 March 2020.
- ^ a b c «PCR拡充が必要 専門家会議が会見 (全文1)» [PCR expansion required Expert meeting (Full text 1)]. THE PAGE (in Japanese). Yahoo!ニュース. 5 May 2020. p. 5. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ a b c d e «「新型コロナウイルス感染拡大阻止 最前線からの報告» [Report from the front line to prevent the spread of new coronavirus infection]. NHK (in Japanese). 15 April 2020. Archived from the original on 19 April 2020. Retrieved 27 May 2020.
- ^ a b c «Did Japan Just Beat the Virus Without Lockdowns or Mass Testing?». Bloomberg.com. 23 May 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ «PCR拡充が必要 専門家会議が会見 (全文1)» [PCR expansion required Expert meeting (Full text 1)]. THE PAGE (in Japanese). Yahoo!ニュース. 5 May 2020. p. 3. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ a b «新型コロナウイルス 感染爆発をどう防ぐか» [How to prevent the outbreak of new coronavirus infection]. NHK (in Japanese). 8 April 2020. Archived from the original on 8 April 2020. Retrieved 27 May 2020.
- ^ «第1波は終息するも欧米からの帰国者経由の第2波が拡大» [The first wave is over, but the second wave is expanding via returnees from Europe and the United States]. 日経メディカル (Nikkei Medical) (in Japanese). 12 May 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ a b «専門家に聞く»新型コロナウイルス»との闘い方と対策» [Ask experts how to fight the «new coronavirus» and countermeasures]. NHK (in Japanese). 27 March 2020. Archived from the original on 8 April 2020. Retrieved 27 May 2020.
- ^ «新型コロナ抗原検査キット、13日から実用化 加藤厚労相が発表 PCRとの併用を想定» [New corona antigen test kit put into practical use from 13th. Minister of Health, Labor and Welfare Kato announced that it will be used in combination with PCR]. 毎日新聞 (Mainichi newspaper ) (in Japanese). 12 May 2020. Archived from the original on 27 May 2020. Retrieved 27 May 2020.
- ^ «コロナ抗原検査が使用可能に、陽性のみ確定診断» [Corona antigen test available, positive only definitive diagnosis]. 日経メディカル (Nikkei Medical) (in Japanese). 12 May 2020. Archived from the original on 21 May 2020. Retrieved 15 May 2020.
- ^ a b «PCR拡充が必要 専門家会議が会見 (全文1)» [PCR expansion required Expert meeting (Full text 1)]. THE PAGE (in Japanese). Yahoo!ニュース. 5 May 2020. p. 4. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ a b «クルーズ船112人治療で「院内感染」ゼロ!「自衛隊中央病院」はなぜ奇跡を起こせたのか» [No «nosocomial infection» with treatment of 112 cruise ships! Why did «Self-Defense Forces Central Hospital» cause a miracle?]. 週刊新潮 (Shukan Shincho) (in Japanese). 30 April 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ «「PCR検査数少ないが、死亡者数・率低い」専門家会議» [«The number of PCR tests is small, but the number of deaths and rate is low» Expert meeting]. m3.com (in Japanese). 5 May 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ^ «調査報告クルーズ船 ウイルス対策のカギは?» [Survey Report What is the key to anti-virus measures for cruise ships?]. NHK (in Japanese). 7 May 2020. Archived from the original on 12 May 2020. Retrieved 24 May 2020.
- ^ «新型コロナウイルス感染症の現在の状況と厚生労働省の対応について(令和2年7月20日版)» [Current status of new coronavirus infection and response by the Ministry of Health, Labor and Welfare (Reiwa 20 July, 2nd edition)] (in Japanese). 厚生労働省. 20 July 2000. Archived from the original on 4 August 2020. Retrieved 1 August 2020.
- ^ «PCR検査能力、4月の3倍 それでも受けにくいわけは» [PCR test capacity, 3 times that of April]. Asahi Shimbun (in Japanese). 28 July 2020. Archived from the original on 31 July 2020. Retrieved 1 August 2020.
- ^ «日本のコロナ検査能力、米英の1割どまり» [Japan’s corona inspection ability, only 10% of the US and UK] (in Japanese). The Nikkei. 21 July 2020. Archived from the original on 31 July 2020. Retrieved 1 August 2020.
- ^ «新型コロナが弱毒化しているという根拠はない» [There is no evidence that the new corona is attenuated] (in Japanese). Yahoo!ニュース. 26 July 2020. Archived from the original on 27 July 2020. Retrieved 1 August 2020.
- ^ «軽症者施設、23都府県で不足 コロナ第2波推計» [Facility for mildly ill people, Insufficient in 23 prefectures Corona second wave estimation] (in Japanese). The Nikkei. 21 July 2020. Archived from the original on 31 July 2020. Retrieved 1 August 2020.
- ^ «患者急増、埋まりつつあるベッド 増床要請に頭抱える病院…スタッフは?一般患者は?経営は?» [The number of patients is increasing rapidly, and the beds are being filled up. Hospitals are having a request to increase the floor space … Staff? General patients? Management?]. Mainichi Shimbun (in Japanese). 22 July 2020. Archived from the original on 29 July 2020. Retrieved 1 August 2020.
- ^ «軽症患者ICUを圧迫 クラスターはほぼ終息 新型コロナで兵庫県対策協» [Squeezing ICU for mildly ill patients The cluster is almost over With the new corona] (in Japanese). 神戸新聞. 25 March 2020. Archived from the original on 22 October 2020. Retrieved 1 August 2020.
- ^ «Over 3 mln COVID-19 tests conducted in Russia». TASS. 27 April 2020. Archived from the original on 11 May 2020. Retrieved 29 April 2020.
- ^ «Popova said explosive growth in incidence was not allowed due to measures taken». TASS. 28 April 2020. Archived from the original on 29 August 2020. Retrieved 29 April 2020.
- ^ «COVID-19 outbreak: Petition to close schools in Singapore garners 7,700 signatures to date». msn.com. Archived from the original on 29 March 2020. Retrieved 29 March 2020.
- ^ «More than 3.6 million people tested during the weekend». The Slovak Spectator. 1 November 2020. Archived from the original on 2 January 2020. Retrieved 2 July 2021.
- ^ Kuhn A (12 March 2020). «Experts Credit South Korea’s Extensive Testing For Curbing Coronavirus Spread». NPR.org. Archived from the original on 16 March 2020. Retrieved 28 June 2020.
- ^ a b «日本が韓国の新型コロナウイルス対策から学べること──(1)検査体制» [What Japan can learn from Korea’s measures against the new coronavirus ── (1) Inspection system]. Newsweek Japan (in Japanese). 2 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ a b «日本が韓国の新型コロナウイルス対策から学べること──(3)情報公開» [What Japan can learn from Korea’s measures against the new coronavirus ── (3) Information disclosure]. Newsweek Japan (in Japanese). 21 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «日本が韓国の新型コロナウイルス対策から学べること──(4)軽症者の隔離・管理対策:「生活治療センター」» [What Japan can learn from Korea’s measures against the new coronavirus ── (4) Isolation and management measures for mildly ill people: «Life Treatment Center»]. Newsweek Japan (in Japanese). 11 May 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ a b «韓国のコロナ対策を称える日本に欠ける視点» [Japan’s lack of perspective to praise South Korea’s measures against corona]. Newsweek Japan (in Japanese). 2 May 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ a b c «韓国式大量検査は徴兵制の賜物…新型コロナが揺さぶる「自由」の価値» [Korean-style mass inspection is a gift of conscription … The value of «freedom» that the new corona shakes] (in Japanese). FNNプライム. 14 April 2020. Archived from the original on 27 April 2020. Retrieved 5 June 2020.
- ^ a b «韓国における新型コロナウィルス防疫事情(韓国)» [New Coronavirus Epidemic Prevention Circumstances in South Korea (Korea)] (in Japanese). 日本商工会議所. 10 May 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «韓国製PCR検査キットが新型コロナから世界を救う日» [The day when the Korean PCR test kit saves the world from the new corona]. Newsweek Japan (in Japanese). 14 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ a b c «新型ウイルス»パンデミック» 医療崩壊を防ぐには» [New virus «pandemic» How to prevent medical collapse]. NHK (in Japanese). 9 April 2020. Archived from the original on 19 April 2020. Retrieved 2 June 2020.
- ^ a b «IT活用でコロナ追跡 韓国、感染者の経路公開» [Corona tracking by utilizing IT South Korea, route disclosure of infected people]. Mainichi Shimbun (in Japanese). 16 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «コロナ対策で浮かび上がる「監視社会」韓国 個人情報をここまでさらしてよいのか» [«Surveillance society» that emerges from corona measures Can South Korea expose personal information to this extent?]. Tokyo Shimbun (in Japanese). 1 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «新型コロナ:「感染追跡」デジタル監視とプライバシーの新しい日常» [New Corona: «Infection Tracking» New Everyday Life in Digital Surveillance and Privacy] (in Japanese). Yahoo!ニュース. 26 March 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «韓国、コロナ隔離者に監視腕輪 「人権侵害」の声» [South Korea, Corona quarantine voice of surveillance bracelet «human rights violations»] (in Japanese). The Nikkei. 17 April 2020. Archived from the original on 29 May 2020. Retrieved 29 May 2020.
- ^ «South Korea is watching quarantined citizens with a smartphone app». MIT Technology Review. 6 March 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ^ «Coronavirus privacy: Are South Korea’s alerts too revealing?». BBC. 5 March 2020. Archived from the original on 6 June 2020. Retrieved 5 June 2020.
- ^ «台湾がコロナ「優等生」になった理由。閣僚に医師出身、デジタル化の一方で強まる監視» [The reason why Taiwan became a corona «honor student». A doctor from a minister, increasing surveillance while digitizing]. Business Insider (in Japanese). 1 May 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ^ «台湾の新型コロナ対策が「善戦」しているワケ» [The reason why Taiwan’s new corona measures are «good fight»]. Wedge Infinity (in Japanese). 28 February 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ^ «台湾が新型コロナの感染拡大を抑制できている理由» [Why Taiwan is able to curb the spread of the new corona]. Wedge Infinity (in Japanese). 28 February 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ^ «新型コロナ対応の「優等生」は「台湾・韓国・ドイツ」» [Why Taiwan is able to curb the spread of the new corona …] (in Japanese). 日経ビジネス (Nikkei Business). 21 April 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ^ «Covid-19: Denmark suspends flights from the Emirates». Le Figaro. Archived from the original on 2 January 2020. Retrieved 22 January 2021.
- ^ «COVID-19 Public Policies #2 ニューヨークはいかにして検査数を増やしたのか» [COVID-19 Public Policies #2 How New York increased the number of inspections]. Office of the City of Yokohama Representative to the Americas (in Japanese). 14 May 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ^ «Coronavirus New York: health officials provide limits on testing patients for COVID-19». Eyewitness News. 21 March 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ^ «マスクも防護服も足りない! ニューヨークの病院で看護師が新型コロナウイルスに感染、死亡» [Not enough masks and protective clothing! A nurse is infected with a new coronavirus and dies at a hospital in New York]. Business Insider Japan (in Japanese). 27 March 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ^ «NY州感染者数、全米2位に 感染爆発で2週間封じ込め作戦へ» [The number of infected people in New York ranks second in the United States.]. Yahoo!ニュース (in Japanese). 11 March 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ^ «Coronavirus clue? Most cases aboard U.S. aircraft carrier are symptom-free». Reuters. 16 April 2020. Archived from the original on 11 December 2020. Retrieved 2 July 2021.
- ^ «Sailors on sidelined USS Theodore Roosevelt get virus for second time». NBC News. Archived from the original on 21 May 2020. Retrieved 21 May 2020.
- ^ «US warned Nevada not to use Chinese COVID tests from UAE». The Associated Press. Retrieved 15 October 2020.
- ^ «Special Report: Italy and South Korea virus outbreaks reveal disparity in deaths and tactics». Reuters. 13 March 2020. Archived from the original on 22 April 2020. Retrieved 22 June 2020.
- ^ «Want to know how many people have the coronavirus? Test randomly». The Conversation. 13 April 2020. Archived from the original on 9 May 2020. Retrieved 7 May 2020.
- ^ «M&E – Health Information System General Directorate – National Diseases Surveillance and Response». MoPH Data Warehouse – Dashboard. 17 December 2020.
- ^ «COVID19/ Ministria e Shëndetësisë: 736 të vaksinuar, 3935 testime, 991 të shëruar, 1112 raste të reja dhe 17 humbje jete në 24 orët e fundit». Ministria e Shëndetësisë dhe Mbrojtjes Sociale [Ministry of Health and Social Protection] (in Albanian). 18 February 2021.
- ^ a b c d e f g h i j k l «Coronavirus Disease 2019 (COVID-19)». Africa CDC.
- ^ «Documentation: Rapport de Situation Sur L’Epidemie de Coronavirus COVID-19». Ministère de la Santé de la Population et de la Réforme Hospitalière [Ministry of Health, Population and Hospital Reform] (in French). 2 November 2020.
- ^ «COVID-19 Dashboard». Government of Andorra. 1 March 2022.
- ^ «COVID-19: Angola Com 58 Novas Infecções e 44 Recuperados». Agência Angola Press (in Portuguese). 4 March 2021.
- ^ «COVID-19 Antigua & Barbuda Dashboard». Official Facebook page of the Ministry of Health & The Environment, Antigua and Barbuda. 6 March 2021.
- ^ «Sala de Situaciόn Coronavirus online» (PDF). Argentina.gob.ar (in Spanish). 16 April 2022.
- ^ Կորոնավիրուսային հիվանդություն (COVID-19). Հիվանդությունների վերահսկման և կանխարգելման ազգային կենտրոն [National Center for Disease Control and Prevention] (in Armenian). 30 May 2022.
- ^ «Coronavirus (COVID-19) current situation and case numbers». Department of Health. 10 September 2022.
- ^ «Coronavirus». AGES Dashboard COVID19 (in German). 5 January 2023.
- ^ «Azərbaycanda Carı Vəzıyyət». Azərbaycan Respublikasının Nazirlər Kabineti [Cabinet of Ministers of the Republic of Azerbaijan] (in Azerbaijani). 11 May 2022.
- ^ «News and Press Releases: COVID-19 Report Update». Government of the Bahamas. 29 November 2022.
- ^ الموقع الرسمي للمستجدات الصحية، مملكة الب9رين. وزارة الصحة [Ministry of Health] (in Arabic). 3 December 2022.
- ^ «Bangladesh Covid-19 Update». Institute of Epidemiology, Disease Control and Research. 24 July 2021.
- ^ «COVID-19 Update». Barbados Government Information Service. 15 October 2022.
- ^ Официальный Минздрав. Официальный канал Министерства здравоохранения Республики Беларусь [Telegram channel of the Ministry of Health of the Republic of Belarus] (in Russian). 9 May 2022.
- ^ «Epistat COVID19 Belgian Dashboard». Sciensano. 22 December 2022.
- ^ «COVID-19 Update». Facebook account of the Ministry of Health and Wellness Belize. 1 November 2021.
- ^ «Coronavirus (COVID-19) By the Numbers». Statistical Institute of Belize. 9 June 2022.
- ^ «Informations coronavirus (covid-19)». Gouvernement de la République du Bénin [Government of the Republic of Benin] (in French). 5 May 2021.
- ^ «National Situational Update on COVID-19». Ministry of Health. 28 February 2022.
- ^ «Reporte COVID-19 en Bolivia». Ministerio de Salud [Ministry of Health] (in Spanish). 5 June 2022.
- ^ «Službene informacije o koronavirusu u BiH». Ministarstvo civilnih poslova Bosne i Hercegovine [Ministry of Civil Affairs of Bosnia and Herzegovina] (in Bosnian). 28 September 2022.
- ^ «COVID-19 Botswana Dashboard». Government of Botswana. 11 January 2022.
- ^ «BW government on Facebook». Government of Botswana. 3 December 2020.
- ^ «COVID-19 Testes». Ministério da Saúde [Ministry of Health] (in Portuguese). 19 February 2021.
- ^ «Coronavírus Brasil». Ministério da Saúde [Ministry of Health] (in Portuguese). 19 February 2021.
- ^ «Press Release on the Current Situation of the COVID-19 Infection in Brunei Darussalam». Ministry of Health Brunei Darussalam. 2 August 2021.
- ^ COVID-19: Единен информационен портал. COVID-19: Единен информационен портал [COVID-19: United information portal] (in Bulgarian). 26 December 2022.
- ^ «Communiqué Coronavirus (COVID-19) au Burkina Faso». Facebook account of the Service d’Information du Gouvernement (SIG) [Government Information Service] (in French). 5 March 2021.
- ^ «Update on COVID-19». Facebook account of the Ministère de la Santé Publique Burundi [Ministry of Public Health Burundi] (in French). 5 January 2021.
- ^ បច្ចុប្បន្នភាពនៃជំងឺកូរ៉ូណាថ្មី COVID-19 នៅប្រទេសកម្ពុជា. Communicable Disease Control Department, Ministry of Health (Cambodia) (in Khmer). 1 August 2021.
- ^ «Coronavirus disease (COVID-19): Outbreak update». Government of Canada. Retrieved 5 December 2022.
- ^ «Communiqué N*320 de la Coordination Nationale de Riposte Sanitaire». Official Facebook account of the Ministère de la Santé Publique du Tchad [Ministry of Public Health of Chad] (in French). 2 March 2021.
- ^ «Cifras Oficiales: COVID-19». Gobierno de Chile [Government of Chile] (in Spanish). 23 December 2022.
- ^ 我国核酸日检测能力达484万份. 中华人民共和国中央人民政府 [The Central People’s Government of the People’s Republic of China] (in Chinese). 6 August 2020.
- ^ «Aug 1: Daily briefing on novel coronavirus cases in China». National Health Commission of the People’s Republic of China. 1 August 2020.
- ^ «#COVID19 en Colombia 28-01-2021». Instituto Nacional de Salud de Colombia [Colombia’s National Institute of Health] (in Spanish). 17 January 2021.
- ^ «#ReporteCOVID19». Cuenta Oficial del Ministerio de Salud y Protección Social de Colombia [Official Account of Health and Social Protection Ministry of Columbia] (in Spanish). 24 November 2022.
- ^ «Situación Nacional COVID-19». Geovisión; Ministerio de Salud, Costa Rica [Ministry of Health, Costa Rica] (in Spanish). 2 November 2021.
- ^ «xxx novih slučajeva u protekla 24 sata». Koronavirus.hr (in Croatian). 26 December 2022.
- ^ «Covid19CubaData». Covid19CubaData (in Spanish). 21 July 2021.
- ^ «Coronavirus en Cuba». Ministerio de Salud Pública [Ministry of Public Health] (in Spanish). 26 December 2022.
- ^ Η εξάπλωση της COVID-19 στην Κύπρο. Πανεπιστήμιο Κύπρου [University of Cyprus] (in Greek). 30 December 2022.
- ^ «Přehled situace v ČR: COVID-19». Ministerstvo zdravotnictví České republiky [The Ministry of Health of the Czech Republic] (in Czech). 24 December 2022.
- ^ «Tal og overvågning over coronavirus/COVID-19 – Sundhedsstyrelsen». Sundhedsstyrelsen [The National Board of Health] (in Danish). 23 December 2022.
- ^ «Statens Serum Institut COVID-19 – Danmark». State20 Serum Institut [The National Board of Health] (in Danish). 15 November 2022.
- ^ «Poit de Presse Sur La Situation COVID19 Par Le Secrétaire De La Santé Dr Meeke Mohamed Moussa». Official Facebook account of the Ministere de la Santé de Djibouti [Djibouti Ministry of Health] (in French). 28 April 2022.
- ^ «Commonwealth of Dominica Coronavirus [COVID-19] Report». Facebook account of the Ministry of Health, Wellness and New Health Investment. 21 June 2022.
- ^ «Boletin Especial 484 COVID 19». Dirección General de Epidemiología [General Directorate of Epidemiology] (in Spanish). 23 July 2022.
- ^ «Situation Épidémiologique en RDC». Stop Coronavirus COVID-19 RDC (in French). 28 February 2021.
- ^ «Situación Nacional Por COVID-19 Infografía N°400» (PDF). Ministerio de Salud Pública [Ministry of Public Health] (in Spanish). 23 July 2021.
- ^ «facebook.com/EgyMohpSpokes». Facebook page for the Egyptian Ministry of Health and Population (MOHP) spokesperson (in Arabic). 23 July 2021.
- ^ «Situación nacional COVID-19». Gobierno de El Salvador [Government of El Salvador] (in Spanish). 19 March 2022.
- ^ «Estadísticas COVID-19» [Ministry of Health and Social Welfare]. Ministerio de Sanidad y Bienestar Social (in Spanish). Equatorial Guinea. 13 December 2022.
- ^ «Koroonakaart». Koroonakaart. 20 December 2022.
- ^ «COVID-19 Eswatini Dashboard». 8 December 2021.
- ^ የኢትዮጵያ የተቀናጀ የኮቪድ-19 መቆጣጠሪያ ስርዓት. covid19.et (in Amharic). 24 July 2021.
- ^ «Corona í Føroyum». Føroya Landsstýri [The Government of the Faroe Islands]. 27 February 2022.
- ^ «COVID-19 Update». Ministry o10 Health & Medical Services. Fiji. 24 November 2022.
- ^ «Confirmed coronavirus cases (COVID-19) in Finland». Terveyden ja hyvinvoinnin laitos (ArcGIS) [National Institute for Health and Welfare (ArcGIS)]. 14 January 2022.
- ^ «info coronavirus covid-19-carte et donnes covid 19 en france». Gouvernement.fr (in French). 15 May 2022.
- ^ «Situation Épidémiologique au Gabon». Info Covid19 Gabon (in French). 23 July 2021.
- ^ «The Gambia COVID-19 Outbreak Situational Report» (PDF). Ministry of Health. 15 February 2021.
- ^ COVID-19 სტატისტიკური მონაცემები. დაავადებათა კონტროლისა და საზოგადოებრივი ჯანმრთელობის ეროვნული ცენტრი [National Center for Disease Control and Public Health] (in Georgian). 3 November 2021.
- ^ «Robert Koch-Institut: COVID-19-Dashboard». Robert Koch-Institut [Robert Koch Institute]. 7 July 2021.
- ^ «Tabellen zu Testzahlen, Testkapazitäten und Probenrückstau pro Woche» (XLSX). Robert Koch-Institut [Robert Koch Institute]. 7 July 2021.
- ^ «Situation Update, COVID-19 Outbreak in Ghana». Ghana Health Service. 3 July 2021.
- ^ Ημερήσια έκθεση επιδημιολογικής επιτήρησης λοίμωξης από το νέο κορωνοϊό (COVID-19). Εθνικός Οργανισμός Δημόσιας Υγείας [National Public Health Organization] (in Greek). 20 December 2022.
- ^ «Coronavirus i Grønland». Naalakkersuisut [Government of Greenland] (in Danish). 30 January 2022.
- ^ «COVID-19 Update | Grenada Dashboard». Ministry of Health Grenada (Facebook). 11 May 2021.
- ^ «Situación de COVID-19 en Guatemala». Ministerio de Salud Pública y Asistencia Social [Ministry of Public Health and Social Assistance] (in Spanish). 18 December 2022.
- ^ «Republique de Guinee COVID-19 Décompte des cas». Official Twitter account of the Agence Nationale de Sécurité Sanitaire [National Agency for Health Security] (in French). 23 July 2021.
- ^ «Situação Epidemiológica Da Covid-19 Na Guiné-Bissau». Official Facebook page of the Alto Comissariado para o Covid-19 [High Commissioner for Covid-19] (in Portuguese). 8 July 2022.
- ^ «Guyana COVID-19 Dashboard». Ministry of Health. 16 June 2022.
- ^ «Surveillance de la COVID-19, Haiti, 2020-2021». Ministère de la Santé Publique et de la Population [Ministry of Public Health and Population] (in French). 7 December 2022.
- ^ «Estadística Nacional de Coronavirus COVID-19». Biblio3eca Virtual en Salud de Honduras [Virtual Health Library of Honduras] (in Spanish). 26 November 2021.
- ^ «Tájékoztató oldal a koronavírusról». Tájékoztató oldal a koronavírusról [Coronavirus Information Page] (in Hungarian). Cabinet Office of the Prime Minister. 11 May 2022.
- ^ «COVID-19 in Iceland – Statistics». Covid.is. 9 August 2022.
- ^ «SARS-CoV-2 (COVID-19) Testing: Status Update». Indian Council of Medical Research. Retrieved 19 September 2021.
- ^ «Ministry of Health and Family Welfare». Ministry of Health and Family Welfare. Retrieved 1 October 2021.
- ^ a b «Peta Sebaran». COVID-19 Handling and National Economic Recovery Committee. Retrieved 9 January 2023.
- ^ «Health Ministry’s Updates on COVID-19». Government of the Islamic Republic of Iran. 1 June 2022.
- ^ «الموقف الوبائي اليومي لجائحة كورونا في العراق ليوم السبت الموافق ٥ كانون الاول ٢٠٢٠». وزارة الصحة العراقية (Facebook) [Iraqi Ministry of Health (Facebook)] (in Arabic). 3 August 2022.
- ^ «Ireland’s COVID-19 Data Hub». gov.ie. 15 December 2022.
- ^ קורונה – לוח בקרה. נגיף הקורונה [Coronavirus] (in Hebrew). Ministry of Health. 17 January 2022.
- ^ «29 Dicembre 2022 – Aggiornamento casi Covid-19» (PDF). Dipartimento della Protezione Civile (GitHub) [Civil Protection Department (GitHub)] (in Italian). 30 December 2022.
- ^ «Point de la situation de la COVID-19 au 3/03/2021». Official Facebook channel of Le Ministère de la Santé et de l’Hygiène Publique [Ministry of Health and Public Hygiene, Ivory Coast] (in French). 3 March 2021.
- ^ «COVID-19 Clinical Management Summary». Ministry of Health & Wellness. 3 October 2022.
- ^ 新型コロナウイルス感染症の現在の状況と厚生労働省の対応について(令和3年3月1日版). 厚生労働省 [The Ministry of Health, Labour and Welfare] (in Japanese). 1 March 2021.
- ^ «corona.moh.gov.jo/en». Jordan Ministry of Health. 6 June 2021.
- ^ Данные по COVID-19 в Казахстане. Национальный центр общественного здравоохранения Министерства здравоохранения Республики Казахстан [National Center of Public Health of the Ministry of Healthcare of the Republic of Kazakhstan] (in Russian). 29 May 2021.
- ^ «twitter.com/MOH_Kenya». Official Twitter Account of the Ministry of Health Kenya. 5 March 2021.
- ^ «facebook.com/IKSHPK». Official Facebook account of the Instituti Kombëtar i Shëndetësisë Publike të Kosovës [National Institute of Public Health of Kosova] (in Albanian). 31 May 2021.
- ^ «twitter.com/KUWAIT_MOH». Kuwait Ministry of Health (Twitter). 9 March 2022.
- ^
За сутки проведено 3436 ПЦР-исследований на коронавирус. Insta official (in Kyrgyz). 10 February 2021. - ^ «ຄະນະສະເພາະກິດ COVID-19». COVID-19 Task Force (in Lao). 1 March 2021.
- ^ «Covid-19 infekcijas izplatība Latvijā». Slimību profilakses un kontroles centrs (ArcGIS) [Center for Disease Prevention and Control (ArcGIS)] (in Latvian). 5 September 2021.
- ^ آخر اﻹحصاءات. فيروس كورونا: COVID-19 [Coronavirus: COVID-19] (in Arabic). Ministry of Information. 14 June 2021.
- ^ «COVID-19 Statistics». Official Twitter account of the National COVID-19 Secretariat (NACOSEC). 31 March 2022.
- ^ «#LiBCOVID19 Case Update». Official Facebook account of the National Public Health Institute of Liberia-NPHIL. 19 July 2021.
- ^ اليومي للوضع الوبائي المحلي لفيروس كورونا المستجد ليوم الأحد 28 فبراير 2021. Official Facebook account of the National Centre for Disease Control (NCDC) — Libya (in Arabic). 16 April 2022.
- ^ «Koronavirusas (COVID-19)». Lietuvos Respublikos sveikatos apsaugos ministerija [Ministry of Health of the Republic of Lithuania] (in Lithuanian). 21 December 2022.
- ^ «Korona Stop». Korona Stop. 16 May 2021.
- ^ «Coronavirus – Rapport Journalier» (PDF). La plate-forme de données luxembourgeoise [The luxembourgish data platform] (in French). Government of Luxembourg. 13 May 2022.
- ^ «COVID-19: Fivoaran’ny antontan’isa teto Madagasikara ny 13 Febroary ka hatramin’ny 19 Febroary 2021». Facebook account of the Ministère de la Santé Publique Madagascar [Ministry of Public Health Madagascar] (in French and Malagasy). 22 February 2021.
- ^ «COVID-19 Daily info update». Facebook page of the Ministry Of Health — Malawi. 29 November 2022.
- ^ «Situasi Terkini». Kementerian Kesihatan Malaysia [Ministry of Health Malaysia] (in Malay). 7 September 2021.
- ^ «COVID-19 Case Updates». Health Protection Agency (Twitter). 13 March 2022.
- ^ «COVID-19 Local Updates». Ministry of Health. 29 January 2021.
- ^ «Communique N°364 du Ministere de la Sante et du Développement Social Sur Le Suivi des Actions de Prevention et de Riposte Face a la Maladie a Coronvirus». Ministère de la Santé et du Développement Social du Mali [Ministry of Health and Social Development of Mali] (in French). 7 July 2021.
- ^ «COVID-19 Malta». Times of Malta (ArcGIS). 8 September 2021.
- ^ «المعطيات العامة للحالة الوبائية». Official Facebook page of the Ministère de la santé /وزارة الصحة [Ministry of Health] (in Arabic). Mauritania. 17 April 2021.
- ^ «Covid-19 : Communiqués». Republic of Mauritius. 23 October 2020.
- ^ «Covid-19 México». Gobierno de México [Government of Mexico] (in Spanish). 15 October 2021.
- ^ «Comunicate». Ministerul Sănătății Muncii și Protecției Sociale [Ministry of Health, Labour and Social Protection] (in Romanian). Moldova. 21 April 2022.
- ^ Нөхцөл байдлын мэдээ COVID-19. Эрүүл Мэндийн Яам [Ministry of Health] (in Mongolian). 10 July 2021.
- ^ «Uživo: COVID-19». Institut za javno zdravlje Crne Gore [Institute of Public Health of Montenegro] (in Montenegrin). 28 July 2020.
- ^ «Novosti». Institut za javno zdravlje Crne Gore [Institute of Public Health of Montenegro] (in Montenegrin). 11 May 2021.
- ^ مرض فيروس كورونا المستجد: الرصد الصحي بالمغرب. البوابة الرسمية لفيروس كورونا بالمغرب [The official portal of coronavirus in Morocco] (in Arabic). 18 December 2022.
- ^ «Boletim diário COVID-19 Nº379». Ministério da Saúde [Ministry of Health] (in Portuguese). 22 July 2021.
- ^ «Coronavirus Disease 2019 (COVID-19) Surveillance Dashboard (Myanmar)». Ministry of Health and Sports (in Burmese). 16 September 2021.
- ^ «COVID-19 update». Official Facebook account of the Ministry of Health and Social Services-Namibia. 5 July 2022.
- ^ «COVID-19 Dashboard». Ministry of Health and Population (Nepal). Retrieved 26 July 2022.
- ^ «Epidemiologische situatie van COVID-19 in Nederland» (PDF). Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment] (in Dutch). 6 July 2021.
- ^ «Info coronavirus Covid-19». Gouvernement de la Nouvelle-Calédonie [Government of New Caledonia] (in French). 4 September 2021.
- ^ «COVID-19: Testing data». Ministry of Health. 19 December 2022.
- ^ «COVID-19: Current cases». Ministry of Health. 19 December 2022.
- ^ «#Covid19Niger Bilan du 22/02/2021». Facebook account of the Ministère de la Santé Publique [Ministry of Public Health] (in French). 22 February 2021.
- ^ «Coronavirus COVID-19 Microsite». Nigeria Centre for Disease Control. 28 February 2021.
- ^ КНДР ввела максимальный уровень карантина. KBS World Radio (in Russian). 2 December 2020.
- ^ Регистрирани 237 Нови Случаи На Ковид 19 – Вкупно Дијагностицирани 84024, Оӡдравени 460 Пациенти – Починати 8 Лица. Министерство за здравство [Ministry of Health] (in Macedonian). 1 July 2021.
- ^ Во последните 24 часа. Министерство за здравство [Ministry of Health] (in Macedonian). 27 June 2021.
- ^ «COVID-19 Genel Durum». Kuzey Kıbrıs Türk Cumhuriyeti Sağlık Bakanlığı [Turkish Republic of Northern Cyprus Ministry of Health] (in Turkish). 13 July 2022.
- ^ «Dags- og ukerapporter om koronavirussykdom (covid-19)». Folkehelseinstituttet [Norwegian Institute of Public Health] (in Norwegian). 20 January 2022.
- ^ «Oman conducts over 500,000 COVID-19 tests since the start of pandemic». The Arabian Stories. 28 October 2020.
- ^ «Pakistan Cases Details». COVID-19 Health Advisory Platform. Ministry of National Health Services Regulations and Coordination. 5 March 2021.
- ^ فايروس كورونا (COVID-19) في فلسطين. فايروس كورونا (COVID-19) في فلسطين [Coronavirus (COVID-19) in Palestine] (in Arabic). 5 February 2022.
- ^ «Compartimos la actualización de datos sobre #COVID19 en nuestro país. Parte 1». Cuenta Oficial de Twitter del Ministerio de Salud de Panama [Official Twitter Account of the Ministry of Health Panama] (in Spanish). 20 December 2022.
- ^ «Official COVID-19 Info Website». Papua New Guinea Joint Agency Task Force, National Control Centre for COVID-19. 20 February 2021.
- ^ «Reportes – COVID19» (in Spanish). Ministe132 280rio de Salud Pública y Bienestar Social (Ministry of Public Health and Social Welfare). 28 March 2022.
- ^ «Sala Situacional». Covid-19 en ″el Perú [Covid-19 in Peru] (in Spanish). 19 November 2022.
- ^ «COVID-19 Tracker». Department of Health (Philippines). 19 December 2022.
- ^ «COVID-19 Tracker». Department of Health (Philippines). 16 April 2021.
- ^ «diagnostyka pod kątem koronawirusa». Official Twitter account of the Ministerstwo Zdrowia [Ministry of Health] (in Polish). 27 April 2022.
- ^ «Ponto de Situação Atual em Portugal». COVID-19 (in Portuguese). Ministry of Health. 5 January 2022.
- ^ «COVID19 Home». Ministry of Public Health. 12 November 2022.
- ^ «Buletin informativ». Ministerul Sănătăţii [Ministry of Health] (in Romanian). 29 January 2021.
- ^ Информационный бюллетень о ситуации и принимаемых мерах по недопущению распространения заболеваний, вызванных новым коронавирусом. Федеральная служба по надзору в сфере защиты прав потребителей и благополучия человека (Роспотребнадзор) [Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)] (in Russian). 7 June 2022.
- ^ стопкоронавирус. Оперативные данные [Stop Coronavirus] (in Russian). 4 June 2022.
- ^ «Amakuru Mashya | Update». Twitter account of the Ministry of Health-Rwanda. 6 October 2021.
- ^ «COVID-19 Updates». Government of St. Kitts and Nevis. 27 August 2021.
- ^ «Saint Lucia’s COVID-19 Dashboard». Ministry of Health and Wellness. 7 October 2022.
- ^ «COVID-19 Report». Ministry of Health, Wellness and the Environment (St. Vincent and the Grenadines). 12 December 2022.
- ^ «Aggiornamento Epidemia COVID-19». Istituto per la Sicurezza Sociale [Institute for Social Security] (in Italian). 2 January 2023.
- ^ «COVID 19 Dashboard: Saudi Arabia». Ministry of Health. 26 April 2022.
- ^ «Riposte à l’épidémie du nouveau coronavirus COVID-19, Sénégal» (PDF). Ministère de la Santé et l’Action sociale [Ministry of Health and Social Action] (in French). 12 July 2021.
- ^ «Coronavirus COVID-19». Ministry of Health of the Republic of Serbia. 25 December 2022.
- ^ «Updates on COVID-19 (Coronavirus Disease 2019) Local Situation». Ministry of Health. 3 August 2021.
- ^ «COVID-19 Situation Report». Ministry of Health. 2 March 2020.
- ^ «Covid-19 in graphs». korona.gov.sk. Office of the Deputy Prime Minister of the Slovak Republic for Investments and Informatization. 27 December 2022.
- ^ «Dnevno spremljanje okužb s SARS-CoV-2 (COVID-19)». Nacionalni inštitut za javno zdravje [National Institute of Public Health] (in Slovenian). 27 December 2022.
- ^ «COVID-19 South African coronavirus news and information». South African Government. 24 May 2021.
- ^ «COVID-19 statistics in South Africa». South Africa Health Twitter Account. 24 May 2021.
- ^ 코로나바이러스감염증-19(COVID-19). 코로나바이러스감염증-19(COVID-19) [Coronavirus infection-19 (COVID-19)] (in Korean). Ministry of Health and Welfare. 1 March 2021.
- ^ «Update on COVID-19 Response». Ministry of Health — South Sudan. 26 May 2021.
- ^ «La pandemia del coronavirus, en datos, mapas y gráficos». RTVE ( Radio y Televisión Española) [RTVE ( Spanish Radio and Television)] (in Spanish). 1 July 2021.
- ^ «Resumen de la situación — Pruebas de laboratorio». Ministerio de Sanidad, Consumo y Bienestar Social [Ministry of Health, Consumption and Social Welfare] (in Spanish). 5 July 2021.
- ^ «COVID-19 Situation Report». Health Promotion Bureau, Sri Lanka. 31 March 2021.
- ^ «COVID-19 : Live Situational Analysis Dashboard of Sri Lanka». Health Promotion Bureau, Sri Lanka. 31 March 2021.
- ^ «Veckorapport om covid-19, vecka 20» (PDF). folkhalsomyndigheten.se (in Swedish). Public Health Agency of Sweden. 28 May 2021. p. 18.
- ^ «Folkhalsomyndigheten Antal fall av Covid-19». folkhalsomyndigheten.se (in Swedish). Public Health Agency of Sweden. 1 February 2021.
- ^ «COVID-19 Switzerland». Federal Office of Public Health FOPH. 8 November 2022.
- ^ «Taiwan Centers for Disease Control». Taiwan Centers for Disease Control. 19 December 2022.
- ^ รายงานสถานการณ์โรคติดเชื้อไวรัสโคโรนา 2019 ฉบับที่ 426 วันที่ 4 มีนาคม 2564 (PDF). Department of Disease Control (in Thai). 4 March 2021.
- ^ «Coronavirus Au Togo». Government of Togo (in French). 11 December 2022.
- ^ «COVID-19 Update Trinidad and Tobago». Ministry of Health. 3 January 2022.
- ^ الأرقام الرئيسيّة المسجّلة بتاريخ 03 فيفري 2021 #كوفيد_19. Official Facebook account of the Ministére de la Santé وزارة الصحة [Ministry of Health, Tunisia] (in Arabic and French). 24 August 2021.
- ^ «Türkıye COVID-19 Hasta Tablosu». Türkiye Cumhuriyeti Sağlık Bakanlığı [Republic of Turkey Ministry of Health] (in Turkish). 2 July 2021.
- ^ «COVID-19 Daily Updates». Facebook page of the Ministry of Health — Uganda. 12 February 2021.
- ^ «COVID-19 pandemic in Ukraine». COVID-19 pandemic in Ukraine. Cabinet of Ministers of Ukraine. 24 November 2021.
- ^
«COVID-19 Updates – Ministry of Health and Prevention – UAE». Ministry of Health & Prevention. 5 January 2023. - ^ «Coronavirus (COVID-19) in the UK». GOV.UK. 19 May 2022.
- ^ «COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University». coronavirus.jhu.edu. 9 August 2021.
- ^ «COVID Data Tracker Weekly Review». Centers for Disease Control and Prevention. 30 July 2022. Retrieved 3 August 2022.
- ^ «Visualizador de casos coronavirus COVID-19 en Uruguay». Sistema Nacional de Emergencias [National Emergency System] (in Spanish). 16 April 2022.
- ^ Дневной прирост случаев COVID-19 продолжает увеличиваться. Gazeta.uz Газета.uz (in Russian). 11 September 2020.
- ^ «Día 353 de la lucha contra la COVID-19». COVID-19 Patria (in Spanish). 30 March 2021.
- ^ «COVID-19 in Viet Nam Situation Report 32». WHO. 30 August 2022. Retrieved 1 September 2022.
- ^ «Daily #COVID19 update». Official Twitter account of the Zambia National Public Health Institute. 10 March 2022.
- ^ «COVID-19 update». Official Twitter account of the Ministry of Health and Child Care (Zimbabwe). 16 October 2022.
Further reading
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. (January 2020). «Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR». Euro Surveillance. 25 (3). doi:10.2807/1560-7917.ES.2020.25.3.2000045. PMC 6988269. PMID 31992387.
- Guglielmi G (July 2020). «The explosion of new coronavirus tests that could help to end the pandemic». Nature. 583 (7817): 506–509. Bibcode:2020Natur.583..506G. doi:10.1038/d41586-020-02140-8. PMID 32681157.
- Kevadiya BD, Machhi J, Herskovitz J, Oleynikov MD, Blomberg WR, Bajwa N, et al. (May 2021). «Diagnostics for SARS-CoV-2 infections». Nature Materials. 20 (5): 593–605. Bibcode:2021NatMa..20..593K. doi:10.1038/s41563-020-00906-z. PMC 8264308. PMID 33589798. S2CID 231930978.
External links
- Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hasell J, MacDonald B, Giattino C, Appel C, Rodés-Guirao L, Roser M (13 July 2020). «Coronavirus (COVID-19) Testing». Our World in Data. – International testing statistics updated twice a week.
- «COVID-19 diagnostic tech tableau». BioCentury. Retrieved 22 June 2020.
- COVID-19 Testing (at least) – now Free for all? (CDC; US Congress; CSPAN video/6:00; 12 March 2020)
- «EUA Authorized Serology Test Performance». U.S. Food and Drug Administration (FDA). 25 May 2021.
- «Global Progress on COVID-19 Serology-Based Testing». Johns Hopkins Center for Health Security.
- «Testing FAQ». Johns Hopkins Coronavirus Resource Center.
- «New Rapid Testing Device for COVID-19 Immunity». Mantracourt Electronics & University of Exeter.
На сегодняшний день не осталось людей, неосведомленных о COVID-19. Весь мир убедился в высокой скорости его распространения и опасности осложнений. Теперь актуальным стал вопрос раннего выявления заболевания для ограничения контактов.
Тест на коронавирус позволяет определить, попал ли возбудитель в кровь пациента. Мы разберем, какими методами он проводится, можно ли пройти его самостоятельно, как интерпретировать результат.
Что такое ПЦР-тест?
ПЦР-тест — это основной метод исследования биологических жидкостей на коронавирус. Он давно используется для выявления всевозможных инфекций в организме человека. ПЦР расшифровывается как полимеразная цепная реакция.
Тест основан на лабораторном изучении фрагмента ДНК во взятом материале. Фрагмент многократно удваивают и наблюдают, повторяется ли в нем искомый участок. Эксперимент показывает, есть ли коронавирус в организме человека. При этом определить его наличие можно даже до появления первых симптомов. Инкубационный период составляет до шести дней. За это время инфекция может поразить большое количество людей. Если вовремя сделать ПЦР-тест, пациент будет изолирован. Это предотвратит распространение вируса.
Кому и как проводят ПЦР-тест на ковид?
Подобные исследования проводят повсеместно с целью сдерживания опасной инфекции. В большинстве случаев это происходит добровольно, но иногда обязанность обусловлена требованиями закона.
Сдать ПЦР-тесты должны все путешественники, прибывающие в Россию. Причем условия одинаковы не для всех. Для иностранцев отрицательный результат теста — фактически пропуск в страну. Соотечественники же могут посетить лабораторию позднее.
Сделать это должен:
- любой человек с признаками ОРВИ;
- член семьи пациента, у которого диагностирована коронавирусная инфекция;
- потенциальный контактный гражданин, который находился в общественных местах без маски или общался с больным;
- медработник, лечивший зараженного.
ПЦР-тест проводится путем забора мазка из носоглотки. Сделать его можно в лаборатории или вызвав врача на дом. Реакцию запускают в лабораторных условиях.
Результат теста: как интерпретировать?
Для полимеразной цепной реакции требуются сутки. Однако с учетом загруженности лабораторий сроки ожидания могут увеличиться до двух-трех дней. Результат трактуется однозначно. Наличие ДНК COVID-19 означает заражение. Отрицательный тест на вирус позволяет допустить его отсутствие в организме.
Почему только допустить? Результат исследования может быть неточным. При получении ложноотрицательного есть вероятность, что:
- забор материала осуществлялся с нарушениями;
- анализ был взят на поздней стадии заболевания;
- вирус не попадал в носоглотку или быстро спустился по дыхательным путям;
- человек продезинфицировал полость рта антисептиком перед забором материала.
Вероятность ложноположительного результата невелика. Такое возможно, если тестирование проводилось в антисанитарных условиях (вирус попал в биоматериал после забора).
Достоверно ли экспресс-тестирование?
Различают такие виды экспресс-теста: исследование методом иммунохроматографии, использование набора реагентов. В первом случае человеку предлагается сдать кровь в медицинском учреждении. У анализа есть преимущества:
- низкая цена по сравнению с ПЦР;
- высокая скорость (до получаса);
- простота реализации.
Однако точность его вызывает сомнения. Экспресс-анализ показывает только присутствие компонентов вируса, а не его самого. Поэтому все положительные пробы важно перепроверять методом ПЦР.
С экспресс-наборами (например, Standard Q Covid-19) человек работает сам. Это не аналог иммунохроматографии: кровь здесь не понадобится, достаточно мазка из носоглотки. Что именно нужно делать — написано в инструкции.
При контакте биоматериала с реагентом происходит реакция с окрашиванием (как с тестом на беременность). Такое исследование на коронавирус достаточно быстрое (до 20 минут), однако не слишком точно.
Тест на антитела
Антитела к коронавирусу, выявленные в организме, показывают его реакцию на возбудитель. Если он вырабатывает специфические иммуноглобулины, это может означать, что:
- человек переболел COVID-19 (в том числе бессимптомно);
- антитела способны бороться с вирусом в случае его повторного проникновения в кровь;
- человек может быть донором и спасать других зараженных, кто переносит заболевание тяжелее.
Если тестирование показало, что антитела к коронавирусу присутствуют, это еще не значит, что их носитель больше никогда не заболеет.
Хотя статистика имеется небольшая, вирусологи пришли к выводу, что положительный тест на антитела может ненадолго приравниваться к вакцинации. Однако это справедливо в течение полугода после выявления инфекции. По истечении этого срока вновь появляются риски, и пациенту рекомендована прививка.
Как расшифровать анализ на антитела?
Обследуемый на антитела может иметь в организме несколько видов белков, вырабатываемых иммунной системой:
- IgM — реакция на любую острую инфекцию. Обычно присутствуют в организме в течение месяца после заражения, однако при COVID-19 этот срок увеличивается до 3 месяцев. Могут сохраняться даже в отсутствие вируса.
- IgA — реакция непосредственно на COVID-19. Их количество должно снижаться по мере выздоровления.
- IgG — запоминающая реакция организма на перенесенную инфекцию. Синтезируются через 6 недель после заражения и формируют иммунитет.
Если в анализе на антитела есть IgA, значит, человек столкнулся с коронавирусом. Если их сопровождают IgG, то больной выздоравливает, если нет — инфекционное заболевание протекает в данный момент. Об устойчивости иммунной системы к вирусу говорит показатель IgG более 1,1.
Возможен ли экспресс-анализ?
Пройти диагностику на антитела можно и в домашних условиях. Например, Leccurate SARS-CoV2 Antibody Test позволяет самостоятельно провести хроматографический иммуноанализ крови. Брать ее можно как из пальца, так и из вены, хотя второй способ без медсестры реализовать сложно. Проходить экспресс-диагностику на антитела лучше натощак. В набор входит одноразовый ланцет для прокола кожи.
Кровь помещается в лунку тестового устройства, где смешивается с буферной жидкостью. Индикаторная реакция происходит в течение 10-20 минут. В зависимости от окрашивания определенных зон, можно обнаружить наличие IgM, IgG или их отсутствие.
Какой метод диагностики лучше?
Формулировать вопрос таким образом не совсем корректно. Речь идет о двух методах с разными задачами. Тест на коронавирус, проводимый через полимеразную цепную реакцию, позволяет определить, болеет обследуемый или нет. Сравнивать его можно только с анализом крови и экспресс-наборами. Картина по итогам полноценного теста более достоверна.
Обследование на антитела параллельно может подсказать, нет ли инфекции в организме на данный момент. Однако в первые дни такой метод неэффективен. Иммунный ответ формируется не сразу, поэтому ошибочный вывод об отсутствии вируса не связан с несовершенством анализа. На это требуется до 14 дней, потом по наличию IgA можно судить о состоянии больного.
Профилактика
Самая главная рекомендация — не стоит намеренно заражаться COVID-19 с целью формирования антител. Коронавирусная инфекция малоизученна — нельзя предсказать, как она будет протекать в конкретном случае. К тому же, антитела формируются не навсегда и вакцинацию не заменяют.
Во избежание случайного заражения важно:
- носить средства индивидуальной защиты в общественных местах;
- пользоваться санитайзером;
- избегать контактов с зараженными;
- укреплять иммунитет;
- обращаться к врачу при первых признаках респираторного заболевания.
Эффективным методом профилактики вирусных заболеваний является вакцинация. Она в России добровольная — обязать делать прививку не может никто. Идти на такой шаг стоит после изучения возможных рисков, определения противопоказаний и сопоставления с ожидаемыми последствиями инфицирования.
Врачи и фармакологи постоянно объясняют, почему нельзя прививаться детям, беременным, аллергикам, людям с тяжелыми реакциями на другие вакцины в анамнезе. При наличии иных ограничений нужна консультация специалиста.
По мере изучения коронавируса появляются все новые методы его диагностики. На первых этапах заболевание выявляли лишь по набору симптомов, который со временем корректировался. Потом добавилась аппаратная диагностика: признаки поражения легких стали выявлять по компьютерной томограмме. Но биохимия и молекулярная биология не бездействовали. Постепенно медицина убедилась, что точный диагноз можно поставить только на основании результатов вирусологического исследования.
Сегодня узнать, заражен ли человек коронавирусной инфекцией, можно несколькими способами. О них и пойдет речь в этом материале. Мы разберемся, как выявить перенесенный ковид в прошлом и оценить устойчивость иммунитета к нему.
Лабораторные тесты на коронавирус (Covid-19)
Сегодня используются разные методы диагностики коронавирусной инфекции. Одни анализы можно сдать только в лаборатории, другие — самостоятельно (даже не придется вызывать врача на дом, достаточно купить специальные наборы в аптеке):
- ПЦР-тест. Лабораторное исследование, показывающее, присутствует ли в биоматериале человека ДНК COVID-19.
- RT-LAMP. Аналог ПЦР-теста, где в центре внимания — не ДНК, а РНК.
- Тест на антиген. Метод экспресс-диагностики, выявляющий присутствие в биоматериале специфических белков вируса.
- Иммунохроматография. Выявление вируса через анализ крови.
- Тест на антитела к коронавирусу. Выясняет, переболел ли человек COVID-19, способна ли его иммунная система сопротивляться инфекции.
Первые четыре метода имеют идентичную задачу. Отличаются они сложностью проведения, затрачиваемым временем, точностью. Пятый их заменить не может.
Диагностика коронавируса (ПЦР)
ПЦР — это полимеразная цепная реакция. Она может делаться только в лабораторных условиях, поскольку требует изучения молекулярной структуры ДНК.
Суть процедуры сводится к следующему:
- Берется мазок из носоглотки человека (для большей точности это должен сделать медицинский работник).
- Выделяется интересующий участок ДНК биоматериала.
- Осуществляется амплификация (многократное удвоение) этого участка до объема, оптимального для визуализации.
- Изучаются визуализированные фрагменты.
Если при копировании участка ДНК в нем продолжает появляться вирус, значит, пациент заражен. Получить результаты ПЦР-теста можно через несколько дней. Во время ожидания (особенно при наличии симптомов) лучше соблюдать самоизоляцию. Сегодня это наиболее достоверный диагностический метод.
Риск получения ошибочного результата
Вероятность получения ложноположительного результата невелика. Вирус может попасть в биоматериал пациента извне при условии нарушения санитарных правил. Поэтому проходить обследование лучше в надежных лабораториях, где подобное исключено.
Ложноотрицательные результаты встречаются чаще. Во избежание их получения перед забором биоматериала нельзя курить и дезинфицировать носоглотку антисептиками.
Исказить клиническую картину также могут:
- ошибки медработника при взятии мазка;
- забор материала на поздней стадии, когда концентрация вируса минимальна;
- отсутствие микроорганизмов в носоглотке.
Последнее возможно, если коронавируса там не было изначально (попал в организм иным путем, например, через кровь). Бывает, что в носоглотке возбудитель не оседает, а сразу продвигается в легкие и выявляется только на компьютерной томографии.
Экспресс-тест на коронавирус
Экспресс-тест можно сделать в домашних условиях. Он не предполагает лабораторных исследований, не требует специального оборудования. Достаточно купить набор в аптеке. Например, к Standard Q Covid-19 прилагается подробная инструкция.
Для проведения экспресс-теста на коронавирус понадобятся:
- стерильный тампон (человек берет у себя мазок сам);
- одноразовая кассета в индивидуальной упаковке;
- пробирка с реактивом;
- насадка с капельницей.
При наличии инфекции в организме контакт биоматериала с реактивом приводит к окрашиванию контрольной полоски. Результат появляется через 20-30 минут. Наибольшую точность тест показывает в первую неделю после заражения. В дальнейшем повышается вероятность получения ложноотрицательного результата. Для уточнения диагноза лучше сделать ПЦР-тест.
Анализ на антитела к коронавирусу
Анализ на антитела к коронавирусу показывает не присутствие инфекции в организме, а ответ иммунной системы на нее. В ответ на заражение она вырабатывает несколько типов иммуноглобулинов:
- IgA являются реакцией непосредственно на коронавирус;
- IgM синтезируются при наличии инфекции в организме (указывают на острое течение заболевания);
- IgG появляются, когда тело «запоминает» вирус и формирует иммунитет к нему.
Недостаточно, чтобы антитела были просто выявлены. Нужно смотреть и на их комбинацию. Подчеркнем, что это исследование не направлено на выявление COVID-19 и не заменяет ПЦР-тест. Сдать такой анализ нужно, чтобы узнать, вырабатывает ли организм антитела к вирусу, а если да, то сколько.
Тесты на антитела
Разные виды тестов на антитела к коронавирусу проводятся дома или в лаборатории. В лаборатории берут кровь из вены и выполняют количественную оценку вырабатываемых иммуноглобулинов.
Домашний тест покажет, есть ли антитела в принципе. Сколько их вырабатывается, с его помощью узнать нельзя: для этого требуются познания в биохимии и специальное оборудование для подсчета. В этом случае достаточно капиллярной крови (из пальца).
В наборах, продающихся в аптеках (например, Leccurate SARS-CoV2 Antibody Test), есть специальный ланцет и пипетка для забора крови. Проколоть себе палец может не каждый, поэтому стоит прибегнуть к помощи близких или вызвать медсестру.
Анализ сдают натощак. Поесть можно максимум за 12 часов до забора крови. Диагностику следует отложить при повышении температуры.
Что показывает тест на антитела?
Наличие иммуноглобулинов означает, что пациент либо болеет сейчас, либо переболел коронавирусом недавно. Если антитела отсутствуют, он либо не заражался никогда, либо микроорганизм попал в тело совсем недавно. Иммунный ответ формируется до двух недель.
Результаты исследования на антитела в количественном выражении интерпретируют так:
- любые иммуноглобулины отсутствуют — заражения не было;
- IgA >1,1 — текущее заболевание в острой форме;
- IgA >1,1 + наличие IgG — начавшееся выздоровление;
- 1,1> IgA >0,8 — неопределенность, требуется повторный забор крови через две недели;
- IgA <0,8 — отсутствие инфекции или совсем недавнее заражение;
- IgG >1,1 — болезнь преодолена, сформирован иммунитет.
Данное исследование не стоит воспринимать как пробу на коронавирус. Если антитела к нему есть, значит, контакт с возбудителем был. Но их отсутствие нельзя однозначно трактовать как избегание вируса.
Наличие антител и прививки
Положительный результат описанного исследования еще не гарантирует невозможность повторного заражения. Иммунитет формируется ориентировочно на полгода. Поэтому в регионах, где установлены ограничения, QR-коды выдаются не только привитым, но и переболевшим в течение последних шести месяцев. Однако присутствие иммуноглобулинов не отменяет необходимость вакцинации. Вопреки распространенному мифу, перед прививкой выяснять их количество не нужно.
Дополнительная диагностика
Перечисленные методы диагностики позволяют установить наличие в организме COVID-19 и иммунный ответ на него. Однако у разных людей заболевание протекает неодинаково: меняется набор симптомов, риски возникновения тех или иных осложнений.
Для подбора схемы лечения врач дополнительно может назначить:
- общий и биохимический анализы крови;
- рентген и/или компьютерную томографию легких;
- кардиограмму;
- почечные пробы;
- печеночные пробы;
- консультации отоларинголога, пульмонолога, кардиолога и других узких специалистов.
Если у выздоровевшего пациента обнаружены антитела в достаточном количестве, это позволяет ему стать донором крови для лечения тяжелых больных. Для этого ему нужно пройти стандартную диагностику во избежание передачи иных вирусов реципиентам.
Коротко обо всех методах диагностики коронавируса
Мы рассмотрели возможные методы обнаружения коронавируса в организме, оценки его устойчивости к данному заболеванию. Подчеркнем, что наличие вируса и выявленные антитела к нему — не одно и то же, поэтому и показания к прохождению обследований отличаются.
При появлении первых признаков ОРВИ, которую легко перепутать с коронавирусом, лучше сразу выбрать полимеразную цепную реакцию. На этом этапе важно выяснить, имеет место банальная простуда или более опасное заболевание. Иммуноглобулинов на первых этапах вообще не бывает, так что искать их нет смысла.
Когда пациент выздоровел, можно оценить, как отреагировал его иммунитет на перенесенное заболевание. Поиск IgG оправдан и в случае, если пациент полагает, что перенес ковидную инфекцию бессимптомно.
Экспресс-тест на коронавирус обладает многими преимуществами. Он помогает оперативно выявить наличие вируса и оградить окружающих, особенно из групп риска, от возможного инфицирования. Чтобы сориентироваться в большом числе различных тестов, необходимо определить, для чего он предназначен и когда необходимо сдать анализ.
Какие существуют экспресс-тесты на COVID-19
Сегодня наличие SARS-CoV-2 — вируса, вызывающего COVID-19, определяют при помощи двух методик:
— ПЦР-тест;
— экспресс-тест на антиген.
ПЦР-тесты
ПЦР или полимеразная цепная реакция — это метод молекулярной диагностики. Он обнаруживают РНК вируса в организме и позволяет узнать, болен ли человек в данный момент или является носителем. Точность этого метода довольно высокая и составляет почти 100%. Но на конечный результат анализа влияет тщательная подготовка материала.
ПЦР-тесты на коронавирус (RT-PCR) нужно обязательно сдавать тем, кто вернулся из-за границы, имел контакты с больными коронавирусом, а также пациентам с диагнозом «внебольничная пневмония», ОРВИ и грипп, сотрудникам медучреждений и людям старше 65 лет — они находятся в группе риска.
Сделать ПЦР-тест можно в государственной поликлинике (при наличии полиса ОМС) и в частных лабораториях. Важное условие — отсутствие явных признаков простуды и контактов с больными в последние несколько дней. Для проведения теста на COVID-19 биологический материал из ротоглотки и полости носа берут амбулаторно либо специалист может выехать на дом. Затем его исследуют в лаборатории с помощью специального оборудования, ждать результатов нужно не менее 24 часов.
Тесты на антиген
Тесты на антигены (RAT) выявляют белки вируса. При помощи небольших одноразовых пластиковых кассет определяют наличие генетического материала вируса аналогично лабораторным методам. Преимущество этого способа в том, что исследование проводится с помощью небольших приборов, которые легко транспортировать и устанавливать вне специализированной лаборатории. В этом случае также исследуют образцы из носа или горла. Анализ основывается на обнаружении антигена вируса и занимает от 5 до 30 минут.
Первые тест-системы давали высокую погрешность, однако современные модели тестов сегодня достаточно точны. При правильном соблюдении порядка сдачи анализов достоверность результатов составляет более 80%.
Сегодня экспресс-тестирование считается удобным и быстрым способом определить наличие вируса в организме. Тест-системы применяются в аэропортах, вокзалах, на крупных предприятиях и в медицинских учреждениях. Анализ требует минимальной подготовки и значительно дешевле ПЦР-тестирования.
Преимущества экспресс-тестов для диагностики COVID-19
Экспресс-тесты в медицинских учреждениях делают с целью подтверждения или исключения инфекции COVID-19 у людей с симптомами или без них. Такие тесты:
- портативные, их можно использовать в любом месте;
- легко выполнимы, с минимальным дополнительным оборудованием или минимумом сложных подготовительных этапов;
- стоят дешевле, чем стандартные лабораторные исследования;
- не требуют специального оборудования и условий проведения;
- быстро предоставляют результаты.
Пациенты с подозрением на COVID-19 должны быстро узнать о возможном наличии инфекции, чтобы получить лечение, информировать находившихся в близком контакте. Чаще всего коронавирусную инфекцию подтверждают тестом ПЦР, но, как говорилось выше, для его проведения нужно специальное оборудование, а для получения результата — не менее 24 часов.
Экспресс-тесты на антиген в медицинских пунктах могут протестировать гораздо большее число людей. Кроме того, анализ можно проводить не только в специализированных медицинских пунктах. При условии, что точность результатов будет высокой, более быстрая диагностика позволит пациентам быстрее принять необходимые меры и снизить распространение COVID-19.
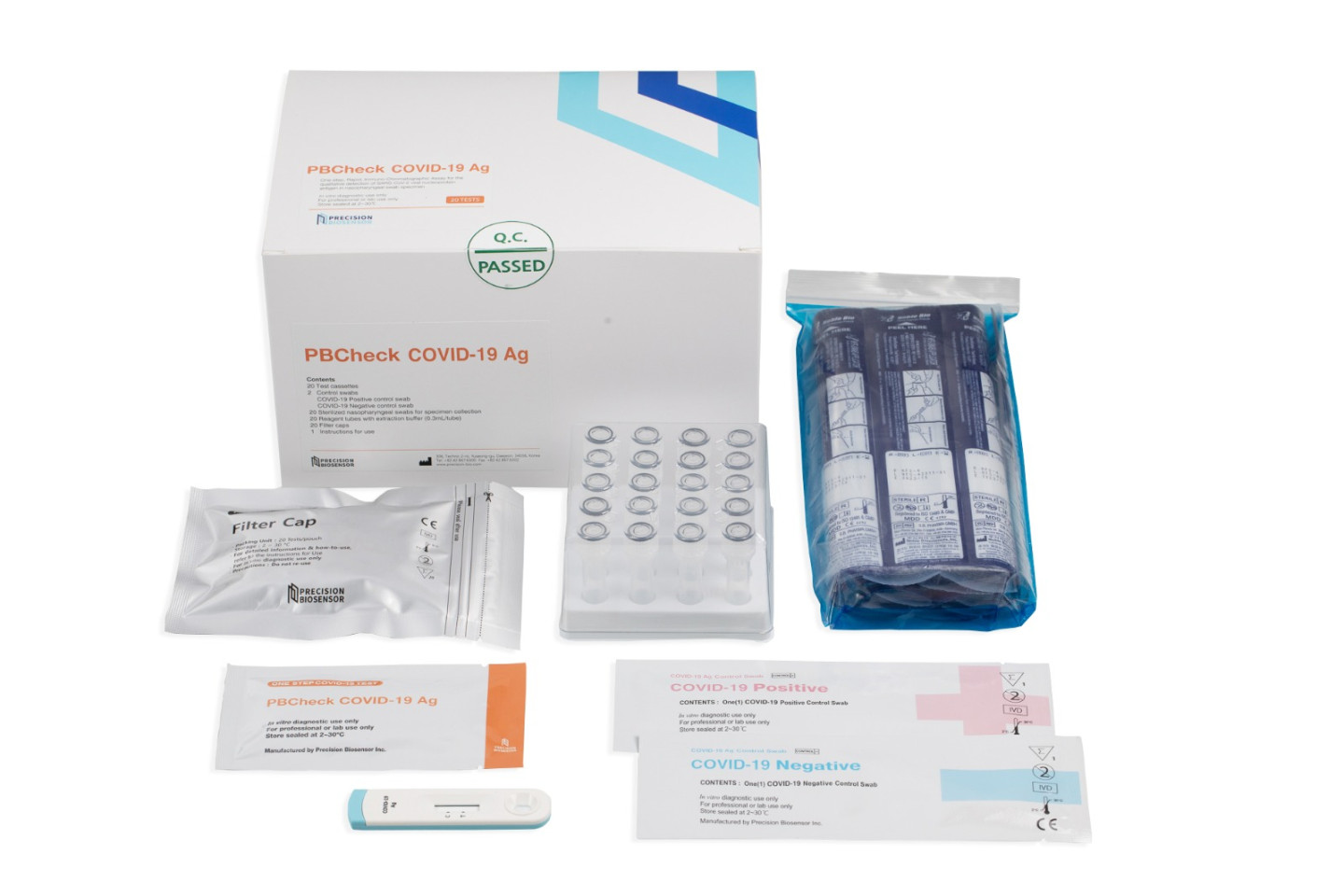
Экспресс-тестирование на антиген как альтернатива ПЦР-тестам
Ученые из Института микробиологии в Лозанне провели исследование: они внедрили тестирование на антиген SARS-CoV-2 в отделении неотложной помощи больницы швейцарского университета [1]. Чувствительность тестов и точность результатов эксперимента проверили на 532 пациентах. Ученые сравнили самые популярные и доступные экспресс-тесты на антиген с ПЦР.
В результате они пришли к выводу, что RAT может представлять собой полезный ресурс в отдельных клинических условиях в качестве дополнительного к ПЦР инструмента. Это поможет при быстрой сортировке больных, особенно во время вспышки заболевания. Но необходимо учитывать более низкую чувствительность теста, особенно у бессимптомных пациентов.
В ходе исследования также выяснили, какие тесты на антигены дают самые точные результаты. Ученые проанализировали самые популярные и доступные к продаже образцы [2]. В ходе эксперимента PBCheck Covid 19 Ag показал лучшие результаты в сравнении с аналогами [3]. У этого теста высокая точность — 88,5%. При этом этот показатель у тестов Panbio Covid-19 Ag Rapid Test составил 87%, а у Standard Q и BD Veritor — 87,2%.
Использование экспресс-тестов на антиген в Швейцарии помогло справиться с очередной волной пандемии. Сортировка пациентов с их помощью позволила швейцарским специалистам быстро изолировать пациентов с положительным результатом анализа на COVID-19 и сэкономить ресурсы. Поэтому многие страны Европы сегодня выбирают экспресс-тесты на антиген как залог эпидемиологической безопасности.
В России также были проведены испытания теста PBCheck Covid 19 Ag. Для этого использовали наборы реагентов с использованием 35 положительных и 35 отрицательных клинических образцов биологического материала (мазков из носа и ротовой полости). В ходе эксперимента установлены следующие диагностические характеристики: диагностическая специфичность 100%, диагностическая чувствительность 92,11% [4].
Как сделать тест на антиген
Экспресс-тестирование на антиген — довольно удобная методика диагностики SARS-CoV-2, так как позволяет проверить наличие инфекции у большого количества людей в максимально короткие сроки.
Антигены коронавируса можно обнаружить в содержимом носоглотки путем иммунохроматографического анализа. Для этого необходимо взять мазок. Вот инструкция, как это правильно сделать:
- введите стерильный тампон в полость носа и осторожно протолкните его до носоглотки;
- сделайте несколько вращательных движений тампоном и извлеките его. При этом старайтесь не касаться дна носовой перегородки;
- опустите тампон с носоглоточным секретом в пробирку с экстракционным буфером;
- прижимая тампон к внутренним стенкам пробирки, осуществите им несколько вращений. Далее подвигайте тампоном вверх-вниз;
- извлеките тампон, оказывая давление на боковые стенки пробирки, чтобы в ней осталось как можно больше исследуемого материала;
- наденьте на пробирку колпачок с капельницей и выдавите две капли на тестовую кассету;
- ожидайте 10 минут и приступайте к определению результатов. Важно помнить, что по прошествии более 15 минут результат может исказиться из-за контакта реагентов с воздухом. В таком случае он будет недействителен;
- удостоверьтесь в наличии цветной полоски на контрольной линии С. При ее отсутствии результат теста некорректен;
- обратите внимание на линию Т: наличие розовой или красной полоски свидетельствует о положительном результате теста. В этом случае вам необходимо обратиться к врачу. Отсутствие полоски на уровне Т означает отрицательный результат теста. В вашем мазке не выявлен антиген COVID-19.
Комментарий эксперта

Сапожкова Жанна Юрьевна, заведующая лаборатории Гемоскрин, кандидат медицинских наук, руководитель Международной Школы Цитологии (МШЦ, Москва); автор более 40 научных публикаций; главный редактор учебных программ МШЦ для аккредитации в НМО; член-корреспондент Международной Академии Цитологии/MIAC
Каков процент точности у современных экспресс-тестов на ковид? Насколько верны результаты?
В диагностике такой серьезной и социально значимой инфекции, как COVID-19, важны точность и высокая специфичность. То есть тесты должны выявлять даже незначительные количества вируса и не должны диагностировать другие инфекции. Практически все современные тесты для диагностики коронавирусной инфекции отвечают требованиям высокой чувствительности и специфичности.
Например, тест PBCheck COVID-19 Ag, с которым работает наша лаборатория, имеет специфичность 100%, то есть никаких других инфекций, кроме коронавирусной, он диагностировать не будет, а чувствительность теста составляет 92,11%, то есть из 100 инфицированных вирус будет определен у 92. Остальные 8 — это могут быть, например, низкокопийные образцы, то есть те образцы, где количество вируса незначительно.
Можно ли равноценно использовать тесты на антиген вместо ПЦР-теста?
Скорее, наравне с ПЦР-тестом. Во многих странах Европы так и происходит. В аптеках можно купить тест, провести его самостоятельно, принести результат и получить справку на выезд в другую страну. Европа валидирует экспресс-тесты и маркирует их знаком СЕ (свидетельствует о том, что продукция отвечает стандартам и обеспечивает свободный допуск товаров на рынки всех стран Евросоюза. — «РБК Стиль»). Наши европейские коллеги считают, что экспресс-тестирование станет одним из возможных факторов, которые помогут пережить очередную волну коронавируса.
В каких ситуациях использование теста на антиген будет предпочтительнее ПЦР?
Это ситуации, когда тест нужен быстро, например, тестирования на мероприятиях или если кто-то из родственников пришел в больницу навестить пациента. В этих случаях экспресс-тесты несравнимо лучше ПЦР, так как дают быстрый ответ с таким же качеством исследования. Еще ситуация, когда, например, есть симптомы, а ПЦР-тест — отрицательный. В этом случае использование экспресс-теста поможет выявить инфекцию.
Если тест на антиген проводится намного проще и быстрее, есть ли вероятность, что в будущем он заменит традиционный ПЦР?
Насколько я могу видеть развитие диагностики в последние десять лет, лабораторные тесты становятся все разнообразнее и быстрее. Экспресс-тестирование относится к POC-тестированию (point of care), это позволяет своевременно, быстро и качественно исключить или подтвердить заболевание, а значит, вовремя принять необходимые меры лечения или профилактики. Так что будущее, безусловно, за экспресс-тестами.
Регистрационное удостоверение на экспресс-тест PBCheck COVID-19 Ag № 2021/14179 действительно до 01.01.2022. Товар предназначен для профессионального использования (медицинскими работниками).