This article is about the metallic element. For other uses, see Iron (disambiguation).
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Iron | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allotropes | see Allotropes of iron | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | lustrous metallic with a grayish tinge | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Fe) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Iron in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d6 4s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 14, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1811 K (1538 °C, 2800 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3134 K (2862 °C, 5182 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.874 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 6.98 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.81 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 340 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.10 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −4, −2, −1, 0, +1,[2] +2, +3, +4, +5,[3] +6, +7[4] (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 126 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | Low spin: 132±3 pm High spin: 152±6 pm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 194 [1] pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of iron |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc)
a=286.65 pm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc)
between 1185–1667 K; a=364.680 pm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 5120 m/s (at r.t.) (electrolytic) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 11.8 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 80.4 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 96.1 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Curie point | 1043 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | ferromagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 211 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 82 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 170 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 608 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 200–1180 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7439-89-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | before 5000 BC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | «Fe»: from Latin ferrum | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of iron
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Iron () is a chemical element with symbol Fe (from Latin: ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth’s outer and inner core. It is the fourth most common element in the Earth’s crust.
In its metallic state, iron is rare in the Earth’s crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth’s crust, although extracting usable metal from them requires kilns or furnaces capable of reaching 1,500 °C (2,730 °F) or higher, about 500 °C (932 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, because of their mechanical properties and low cost. The iron and steel industry is thus very important economically, and iron is the cheapest metal, with a price of a few dollars per kilogram or per pound (see Metal#uses).
Pristine and smooth pure iron surfaces are mirror-like silvery-gray. However, iron reacts readily with oxygen and water to give brown to black hydrated iron oxides, commonly known as rust. Unlike the oxides of some other metals that form passivating layers, rust occupies more volume than the metal and thus flakes off, exposing more fresh surfaces for corrosion. Although iron readily reacts, high purity iron, called electrolytic iron, has better corrosion resistance.
The body of an adult human contains about 4 grams (0.005% body weight) of iron, mostly in hemoglobin and myoglobin. These two proteins play essential roles in vertebrate metabolism, respectively oxygen transport by blood and oxygen storage in muscles. To maintain the necessary levels, human iron metabolism requires a minimum of iron in the diet. Iron is also the metal at the active site of many important redox enzymes dealing with cellular respiration and oxidation and reduction in plants and animals.[5]
Chemically, the most common oxidation states of iron are iron(II) and iron(III). Iron shares many properties of other transition metals, including the other group 8 elements, ruthenium and osmium. Iron forms compounds in a wide range of oxidation states, −2 to +7. Iron also forms many coordination compounds; some of them, such as ferrocene, ferrioxalate, and Prussian blue, have substantial industrial, medical, or research applications.
Characteristics
Allotropes
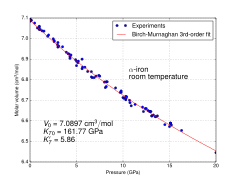
Molar volume vs. pressure for α iron at room temperature
At least four allotropes of iron (differing atom arrangements in the solid) are known, conventionally denoted α, γ, δ, and ε.
The first three forms are observed at ordinary pressures. As molten iron cools past its freezing point of 1538 °C, it crystallizes into its δ allotrope, which has a body-centered cubic (bcc) crystal structure. As it cools further to 1394 °C, it changes to its γ-iron allotrope, a face-centered cubic (fcc) crystal structure, or austenite. At 912 °C and below, the crystal structure again becomes the bcc α-iron allotrope.[6]
The physical properties of iron at very high pressures and temperatures have also been studied extensively,[7][8] because of their relevance to theories about the cores of the Earth and other planets. Above approximately 10 GPa and temperatures of a few hundred kelvin or less, α-iron changes into another hexagonal close-packed (hcp) structure, which is also known as ε-iron. The higher-temperature γ-phase also changes into ε-iron, but does so at higher pressure.
Some controversial experimental evidence exists for a stable β phase at pressures above 50 GPa and temperatures of at least 1500 K. It is supposed to have an orthorhombic or a double hcp structure.[9] (Confusingly, the term «β-iron» is sometimes also used to refer to α-iron above its Curie point, when it changes from being ferromagnetic to paramagnetic, even though its crystal structure has not changed.[6])
The inner core of the Earth is generally presumed to consist of an iron-nickel alloy with ε (or β) structure.[10]
Melting and boiling points
The melting and boiling points of iron, along with its enthalpy of atomization, are lower than those of the earlier 3d elements from scandium to chromium, showing the lessened contribution of the 3d electrons to metallic bonding as they are attracted more and more into the inert core by the nucleus;[11] however, they are higher than the values for the previous element manganese because that element has a half-filled 3d sub-shell and consequently its d-electrons are not easily delocalized. This same trend appears for ruthenium but not osmium.[12]
The melting point of iron is experimentally well defined for pressures less than 50 GPa. For greater pressures, published data (as of 2007) still varies by tens of gigapascals and over a thousand kelvin.[13]
Magnetic properties
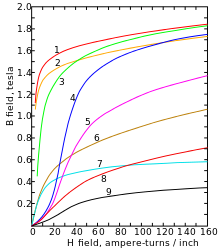
Magnetization curves of 9 ferromagnetic materials, showing saturation. 1. Sheet steel, 2. Silicon steel, 3. Cast steel, 4. Tungsten steel, 5. Magnet steel, 6. Cast iron, 7. Nickel, 8. Cobalt, 9. Magnetite[14]
Below its Curie point of 770 °C (1,420 °F; 1,040 K), α-iron changes from paramagnetic to ferromagnetic: the spins of the two unpaired electrons in each atom generally align with the spins of its neighbors, creating an overall magnetic field.[15] This happens because the orbitals of those two electrons (dz2 and dx2 −. y2) do not point toward neighboring atoms in the lattice, and therefore are not involved in metallic bonding.[6]
In the absence of an external source of magnetic field, the atoms get spontaneously partitioned into magnetic domains, about 10 micrometers across,[16] such that the atoms in each domain have parallel spins, but some domains have other orientations. Thus a macroscopic piece of iron will have a nearly zero overall magnetic field.
Application of an external magnetic field causes the domains that are magnetized in the same general direction to grow at the expense of adjacent ones that point in other directions, reinforcing the external field. This effect is exploited in devices that need to channel magnetic fields to fulfill design function, such as electrical transformers, magnetic recording heads, and electric motors. Impurities, lattice defects, or grain and particle boundaries can «pin» the domains in the new positions, so that the effect persists even after the external field is removed – thus turning the iron object into a (permanent) magnet.[15]
Similar behavior is exhibited by some iron compounds, such as the ferrites including the mineral magnetite, a crystalline form of the mixed iron(II,III) oxide Fe3O4 (although the atomic-scale mechanism, ferrimagnetism, is somewhat different). Pieces of magnetite with natural permanent magnetization (lodestones) provided the earliest compasses for navigation. Particles of magnetite were extensively used in magnetic recording media such as core memories, magnetic tapes, floppies, and disks, until they were replaced by cobalt-based materials.
Isotopes
Iron has four stable isotopes: 54Fe (5.845% of natural iron), 56Fe (91.754%), 57Fe (2.119%) and 58Fe (0.282%). 24 artificial isotopes have also been created. Of these stable isotopes, only 57Fe has a nuclear spin (−1⁄2). The nuclide 54Fe theoretically can undergo double electron capture to 54Cr, but the process has never been observed and only a lower limit on the half-life of 3.1×1022 years has been established.[17]
60Fe is an extinct radionuclide of long half-life (2.6 million years).[18] It is not found on Earth, but its ultimate decay product is its granddaughter, the stable nuclide 60Ni.[17] Much of the past work on isotopic composition of iron has focused on the nucleosynthesis of 60Fe through studies of meteorites and ore formation. In the last decade, advances in mass spectrometry have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotopes of iron. Much of this work is driven by the Earth and planetary science communities, although applications to biological and industrial systems are emerging.[19]
In phases of the meteorites Semarkona and Chervony Kut, a correlation between the concentration of 60Ni, the granddaughter of 60Fe, and the abundance of the stable iron isotopes provided evidence for the existence of 60Fe at the time of formation of the Solar System. Possibly the energy released by the decay of 60Fe, along with that released by 26Al, contributed to the remelting and differentiation of asteroids after their formation 4.6 billion years ago. The abundance of 60Ni present in extraterrestrial material may bring further insight into the origin and early history of the Solar System.[20]
The most abundant iron isotope 56Fe is of particular interest to nuclear scientists because it represents the most common endpoint of nucleosynthesis.[21] Since 56Ni (14 alpha particles) is easily produced from lighter nuclei in the alpha process in nuclear reactions in supernovae (see silicon burning process), it is the endpoint of fusion chains inside extremely massive stars, since addition of another alpha particle, resulting in 60Zn, requires a great deal more energy. This 56Ni, which has a half-life of about 6 days, is created in quantity in these stars, but soon decays by two successive positron emissions within supernova decay products in the supernova remnant gas cloud, first to radioactive 56Co, and then to stable 56Fe. As such, iron is the most abundant element in the core of red giants, and is the most abundant metal in iron meteorites and in the dense metal cores of planets such as Earth.[22] It is also very common in the universe, relative to other stable metals of approximately the same atomic weight.[22][23] Iron is the sixth most abundant element in the universe, and the most common refractory element.[24]
Although a further tiny energy gain could be extracted by synthesizing 62Ni, which has a marginally higher binding energy than 56Fe, conditions in stars are unsuitable for this process. Element production in supernovas greatly favor iron over nickel, and in any case, 56Fe still has a lower mass per nucleon than 62Ni due to its higher fraction of lighter protons.[25] Hence, elements heavier than iron require a supernova for their formation, involving rapid neutron capture by starting 56Fe nuclei.[22]
In the far future of the universe, assuming that proton decay does not occur, cold fusion occurring via quantum tunnelling would cause the light nuclei in ordinary matter to fuse into 56Fe nuclei. Fission and alpha-particle emission would then make heavy nuclei decay into iron, converting all stellar-mass objects to cold spheres of pure iron.[26]
Origin and occurrence in nature
Cosmogenesis
Iron’s abundance in rocky planets like Earth is due to its abundant production during the runaway fusion and explosion of type Ia supernovae, which scatters the iron into space.[27][28]
Metallic iron
A polished and chemically etched piece of an iron meteorite, believed to be similar in composition to the Earth’s metallic core, showing individual crystals of the iron-nickel alloy (Widmanstatten pattern)
Metallic or native iron is rarely found on the surface of the Earth because it tends to oxidize. However, both the Earth’s inner and outer core, that account for 35% of the mass of the whole Earth, are believed to consist largely of an iron alloy, possibly with nickel. Electric currents in the liquid outer core are believed to be the origin of the Earth’s magnetic field. The other terrestrial planets (Mercury, Venus, and Mars) as well as the Moon are believed to have a metallic core consisting mostly of iron. The M-type asteroids are also believed to be partly or mostly made of metallic iron alloy.
The rare iron meteorites are the main form of natural metallic iron on the Earth’s surface. Items made of cold-worked meteoritic iron have been found in various archaeological sites dating from a time when iron smelting had not yet been developed; and the Inuit in Greenland have been reported to use iron from the Cape York meteorite for tools and hunting weapons.[29] About 1 in 20 meteorites consist of the unique iron-nickel minerals taenite (35–80% iron) and kamacite (90–95% iron).[30] Native iron is also rarely found in basalts that have formed from magmas that have come into contact with carbon-rich sedimentary rocks, which have reduced the oxygen fugacity sufficiently for iron to crystallize. This is known as Telluric iron and is described from a few localities, such as Disko Island in West Greenland, Yakutia in Russia and Bühl in Germany.[31]
Mantle minerals
Ferropericlase (Mg,Fe)O, a solid solution of periclase (MgO) and wüstite (FeO), makes up about 20% of the volume of the lower mantle of the Earth, which makes it the second most abundant mineral phase in that region after silicate perovskite (Mg,Fe)SiO3; it also is the major host for iron in the lower mantle.[32] At the bottom of the transition zone of the mantle, the reaction γ-(Mg,Fe)2[SiO4] ↔ (Mg,Fe)[SiO3] + (Mg,Fe)O transforms γ-olivine into a mixture of silicate perovskite and ferropericlase and vice versa. In the literature, this mineral phase of the lower mantle is also often called magnesiowüstite.[33] Silicate perovskite may form up to 93% of the lower mantle,[34] and the magnesium iron form, (Mg,Fe)SiO3, is considered to be the most abundant mineral in the Earth, making up 38% of its volume.[35]
Earth’s crust
While iron is the most abundant element on Earth, most of this iron is concentrated in the inner and outer cores.[36][37] The fraction of iron that is in Earth’s crust only amounts to about 5% of the overall mass of the crust and is thus only the fourth most abundant element in that layer (after oxygen, silicon, and aluminium).[38]
Most of the iron in the crust is combined with various other elements to form many iron minerals. An important class is the iron oxide minerals such as hematite (Fe2O3), magnetite (Fe3O4), and siderite (FeCO3), which are the major ores of iron. Many igneous rocks also contain the sulfide minerals pyrrhotite and pentlandite.[39][40] During weathering, iron tends to leach from sulfide deposits as the sulfate and from silicate deposits as the bicarbonate. Both of these are oxidized in aqueous solution and precipitate in even mildly elevated pH as iron(III) oxide.[41]
Banded iron formation in McKinley Park, Minnesota.
Large deposits of iron are banded iron formations, a type of rock consisting of repeated thin layers of iron oxides alternating with bands of iron-poor shale and chert. The banded iron formations were laid down in the time between 3,700 million years ago and 1,800 million years ago.[42][43]
Materials containing finely ground iron(III) oxides or oxide-hydroxides, such as ochre, have been used as yellow, red, and brown pigments since pre-historical times. They contribute as well to the color of various rocks and clays, including entire geological formations like the Painted Hills in Oregon and the Buntsandstein («colored sandstone», British Bunter).[44] Through Eisensandstein (a jurassic ‘iron sandstone’, e.g. from Donzdorf in Germany)[45] and Bath stone in the UK, iron compounds are responsible for the yellowish color of many historical buildings and sculptures.[46] The proverbial red color of the surface of Mars is derived from an iron oxide-rich regolith.[47]
Significant amounts of iron occur in the iron sulfide mineral pyrite (FeS2), but it is difficult to extract iron from it and it is therefore not exploited.[48] In fact, iron is so common that production generally focuses only on ores with very high quantities of it.[49]
According to the International Resource Panel’s Metal Stocks in Society report, the global stock of iron in use in society is 2,200 kg per capita. More-developed countries differ in this respect from less-developed countries (7,000–14,000 vs 2,000 kg per capita).[50]
Oceans
Ocean science demonstrated the role of the iron in the ancient seas in both marine biota and climate.[51]
Chemistry and compounds
| Oxidation state |
Representative compound |
|---|---|
| −2 (d10) | Disodium tetracarbonylferrate (Collman’s reagent) |
| −1 (d9) | Fe 2(CO)2− 8 |
| 0 (d8) | Iron pentacarbonyl |
| 1 (d7) | Cyclopentadienyliron dicarbonyl dimer («Fp2«) |
| 2 (d6) | Ferrous sulfate, ferrocene |
| 3 (d5) | Ferric chloride, ferrocenium tetrafluoroborate |
| 4 (d4) | Fe(diars) 2Cl2+ 2, Ferryl tetrafluoroborate |
| 5 (d3) | FeO3− 4 |
| 6 (d2) | Potassium ferrate |
| 7 (d1) | [FeO4]– (matrix isolation, 4K) |
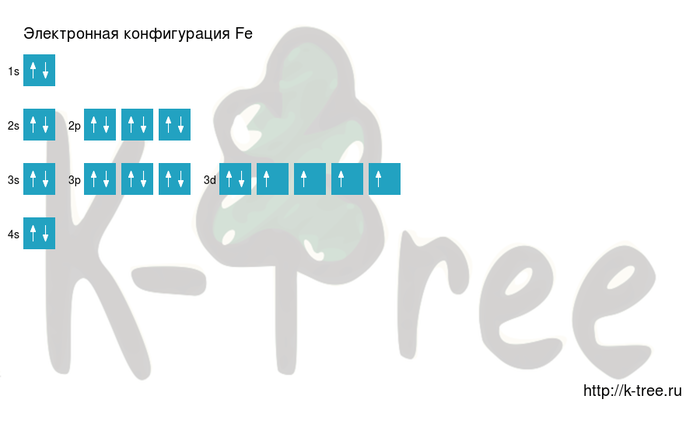
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s.[52] Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity.[53] Its 26 electrons are arranged in the configuration [Ar]3d64s2, of which the 3d and 4s electrons are relatively close in energy, and thus a number of electrons can be ionized.[12]
Iron forms compounds mainly in the oxidation states +2 (iron(II), «ferrous») and +3 (iron(III), «ferric»). Iron also occurs in higher oxidation states, e.g., the purple potassium ferrate (K2FeO4), which contains iron in its +6 oxidation state. The anion [FeO4]– with iron in its +7 oxidation state, along with an iron(V)-peroxo isomer, has been detected by infrared spectroscopy at 4 K after cocondensation of laser-ablated Fe atoms with a mixture of O2/Ar.[54] Iron(IV) is a common intermediate in many biochemical oxidation reactions.[55][56] Numerous organoiron compounds contain formal oxidation states of +1, 0, −1, or even −2. The oxidation states and other bonding properties are often assessed using the technique of Mössbauer spectroscopy.[57] Many mixed valence compounds contain both iron(II) and iron(III) centers, such as magnetite and Prussian blue (Fe4(Fe[CN]6)3).[56] The latter is used as the traditional «blue» in blueprints.[58]
Iron is the first of the transition metals that cannot reach its group oxidation state of +8, although its heavier congeners ruthenium and osmium can, with ruthenium having more difficulty than osmium.[6] Ruthenium exhibits an aqueous cationic chemistry in its low oxidation states similar to that of iron, but osmium does not, favoring high oxidation states in which it forms anionic complexes.[6] In the second half of the 3d transition series, vertical similarities down the groups compete with the horizontal similarities of iron with its neighbors cobalt and nickel in the periodic table, which are also ferromagnetic at room temperature and share similar chemistry. As such, iron, cobalt, and nickel are sometimes grouped together as the iron triad.[53]
Unlike many other metals, iron does not form amalgams with mercury. As a result, mercury is traded in standardized 76 pound flasks (34 kg) made of iron.[59]
Iron is by far the most reactive element in its group; it is pyrophoric when finely divided and dissolves easily in dilute acids, giving Fe2+. However, it does not react with concentrated nitric acid and other oxidizing acids due to the formation of an impervious oxide layer, which can nevertheless react with hydrochloric acid.[6] High purity iron, called electrolytic iron, is considered to be resistant to rust, due to its oxide layer.
Binary compounds
Oxides and sulfides
Ferrous or iron(II) oxide,
FeO
Ferric or iron(III) oxide
Fe2O3
Ferrosoferric or iron(II,III) oxide
Fe3O4
Iron forms various oxide and hydroxide compounds; the most common are iron(II,III) oxide (Fe3O4), and iron(III) oxide (Fe2O3). Iron(II) oxide also exists, though it is unstable at room temperature. Despite their names, they are actually all non-stoichiometric compounds whose compositions may vary.[60] These oxides are the principal ores for the production of iron (see bloomery and blast furnace). They are also used in the production of ferrites, useful magnetic storage media in computers, and pigments. The best known sulfide is iron pyrite (FeS2), also known as fool’s gold owing to its golden luster.[56] It is not an iron(IV) compound, but is actually an iron(II) polysulfide containing Fe2+ and S2−
2 ions in a distorted sodium chloride structure.[60]
Halides
Hydrated iron(III) chloride (ferric chloride)
The binary ferrous and ferric halides are well-known. The ferrous halides typically arise from treating iron metal with the corresponding hydrohalic acid to give the corresponding hydrated salts.[56]
- Fe + 2 HX → FeX2 + H2 (X = F, Cl, Br, I)
Iron reacts with fluorine, chlorine, and bromine to give the corresponding ferric halides, ferric chloride being the most common.[61]
- 2 Fe + 3 X2 → 2 FeX3 (X = F, Cl, Br)
Ferric iodide is an exception, being thermodynamically unstable due to the oxidizing power of Fe3+ and the high reducing power of I−:[61]
- 2 I− + 2 Fe3+ → I2 + 2 Fe2+ (E0 = +0.23 V)
Ferric iodide, a black solid, is not stable in ordinary conditions, but can be prepared through the reaction of iron pentacarbonyl with iodine and carbon monoxide in the presence of hexane and light at the temperature of −20 °C, with oxygen and water excluded.[61]Complexes of ferric iodide with some soft bases are known to be stable compounds.[62][63]
Solution chemistry
Comparison of colors of solutions of ferrate (left) and permanganate (right)
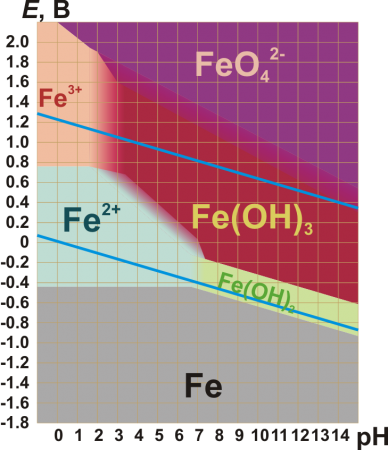
The standard reduction potentials in acidic aqueous solution for some common iron ions are given below:[6]
| [Fe(H2O)6]2+ + 2 e− | ⇌ Fe | E0 = −0.447 V |
| [Fe(H2O)6]3+ + e− | ⇌ [Fe(H2O)6]2+ | E0 = +0.77 V |
| FeO2− 4 + 8 H3O+ + 3 e− |
⇌ [Fe(H2O)6]3+ + 6 H2O | E0 = +2.20 V |
The red-purple tetrahedral ferrate(VI) anion is such a strong oxidizing agent that it oxidizes ammonia to nitrogen (N2) and water to oxygen[61]
- 4 FeO2−
4 + 34 H
2O → 4 [Fe(H2O)6]3+ + 20 OH−
+ 3 O2
The pale-violet hexaquo complex [Fe(H2O)6]3+ is an acid such that above above pH 0 it is fully hydrolyzed:[64]
| [Fe(H2O)6]3+ | ⇌ [Fe(H2O)5(OH)]2+ + H+ | K = 10−3.05 mol dm−3 |
| [Fe(H2O)5(OH)]2+ | ⇌ [Fe(H2O)4(OH)2]+ + H+ | K = 10−3.26 mol dm−3 |
| 2[Fe(H2O)6]3+ | ⇌ [Fe(H2O)4(OH)]4+2 + 2H+ + 2H2O | K = 10−2.91 mol dm−3 |
As pH rises above 0 the above yellow hydrolyzed species form and as it rises above 2–3, reddish-brown hydrous iron(III) oxide precipitates out of solution. Although Fe3+ has a d5 configuration, its absorption spectrum is not like that of Mn2+ with its weak, spin-forbidden d–d bands, because Fe3+ has higher positive charge and is more polarizing, lowering the energy of its ligand-to-metal charge transfer absorptions. Thus, all the above complexes are rather strongly colored, with the single exception of the hexaquo ion – and even that has a spectrum dominated by charge transfer in the near ultraviolet region.[64] On the other hand, the pale green iron(II) hexaquo ion [Fe(H2O)6]2+ does not undergo appreciable hydrolysis. Carbon dioxide is not evolved when carbonate anions are added, which instead results in white iron(II) carbonate being precipitated out. In excess carbon dioxide this forms the slightly soluble bicarbonate, which occurs commonly in groundwater, but it oxidises quickly in air to form iron(III) oxide that accounts for the brown deposits present in a sizeable number of streams.[65]
Coordination compounds
Due to its electronic structure, iron has a very large coordination and organometallic chemistry.
Many coordination compounds of iron are known. A typical six-coordinate anion is hexachloroferrate(III), [FeCl6]3−, found in the mixed salt tetrakis(methylammonium) hexachloroferrate(III) chloride.[66][67] Complexes with multiple bidentate ligands have geometric isomers. For example, the trans-chlorohydridobis(bis-1,2-(diphenylphosphino)ethane)iron(II) complex is used as a starting material for compounds with the Fe(dppe)2 moiety.[68][69] The ferrioxalate ion with three oxalate ligands (shown at right) displays helical chirality with its two non-superposable geometries labelled Λ (lambda) for the left-handed screw axis and Δ (delta) for the right-handed screw axis, in line with IUPAC conventions.[64] Potassium ferrioxalate is used in chemical actinometry and along with its sodium salt undergoes photoreduction applied in old-style photographic processes. The dihydrate of iron(II) oxalate has a polymeric structure with co-planar oxalate ions bridging between iron centres with the water of crystallisation located forming the caps of each octahedron, as illustrated below.[70]
Crystal structure of iron(II) oxalate dihydrate, showing iron (gray), oxygen (red), carbon (black), and hydrogen (white) atoms.
Blood-red positive thiocyanate test for iron(III)
Iron(III) complexes are quite similar to those of chromium(III) with the exception of iron(III)’s preference for O-donor instead of N-donor ligands. The latter tend to be rather more unstable than iron(II) complexes and often dissociate in water. Many Fe–O complexes show intense colors and are used as tests for phenols or enols. For example, in the ferric chloride test, used to determine the presence of phenols, iron(III) chloride reacts with a phenol to form a deep violet complex:[64]
- 3 ArOH + FeCl3 → Fe(OAr)3 + 3 HCl (Ar = aryl)
Among the halide and pseudohalide complexes, fluoro complexes of iron(III) are the most stable, with the colorless [FeF5(H2O)]2− being the most stable in aqueous solution. Chloro complexes are less stable and favor tetrahedral coordination as in [FeCl4]−; [FeBr4]− and [FeI4]− are reduced easily to iron(II). Thiocyanate is a common test for the presence of iron(III) as it forms the blood-red [Fe(SCN)(H2O)5]2+. Like manganese(II), most iron(III) complexes are high-spin, the exceptions being those with ligands that are high in the spectrochemical series such as cyanide. An example of a low-spin iron(III) complex is [Fe(CN)6]3−. Iron shows a great variety of electronic spin states, including every possible spin quantum number value for a d-block element from 0 (diamagnetic) to 5⁄2 (5 unpaired electrons). This value is always half the number of unpaired electrons. Complexes with zero to two unpaired electrons are considered low-spin and those with four or five are considered high-spin.[60]
Iron(II) complexes are less stable than iron(III) complexes but the preference for O-donor ligands is less marked, so that for example [Fe(NH3)6]2+ is known while [Fe(NH3)6]3+ is not. They have a tendency to be oxidized to iron(III) but this can be moderated by low pH and the specific ligands used.[65]
Organometallic compounds
Organoiron chemistry is the study of organometallic compounds of iron, where carbon atoms are covalently bound to the metal atom. They are many and varied, including cyanide complexes, carbonyl complexes, sandwich and half-sandwich compounds.
Prussian blue or «ferric ferrocyanide», Fe4[Fe(CN)6]3, is an old and well-known iron-cyanide complex, extensively used as pigment and in several other applications. Its formation can be used as a simple wet chemistry test to distinguish between aqueous solutions of Fe2+ and Fe3+ as they react (respectively) with potassium ferricyanide and potassium ferrocyanide to form Prussian blue.[56]
Another old example of an organoiron compound is iron pentacarbonyl, Fe(CO)5, in which a neutral iron atom is bound to the carbon atoms of five carbon monoxide molecules. The compound can be used to make carbonyl iron powder, a highly reactive form of metallic iron. Thermolysis of iron pentacarbonyl gives triiron dodecacarbonyl, Fe3(CO)12, a complex with a cluster of three iron atoms at its core. Collman’s reagent, disodium tetracarbonylferrate, is a useful reagent for organic chemistry; it contains iron in the −2 oxidation state. Cyclopentadienyliron dicarbonyl dimer contains iron in the rare +1 oxidation state.[71]
Structural formula of ferrocene and a powdered sample
A landmark in this field was the discovery in 1951 of the remarkably stable sandwich compound ferrocene Fe(C5H5)2, by Pauson and Kealy[72] and independently by Miller and colleagues,[73] whose surprising molecular structure was determined only a year later by Woodward and Wilkinson[74] and Fischer.[75]
Ferrocene is still one of the most important tools and models in this class.[76]
Iron-centered organometallic species are used as catalysts. The Knölker complex, for example, is a transfer hydrogenation catalyst for ketones.[77]
Industrial uses
The iron compounds produced on the largest scale in industry are iron(II) sulfate (FeSO4·7H2O) and iron(III) chloride (FeCl3). The former is one of the most readily available sources of iron(II), but is less stable to aerial oxidation than Mohr’s salt ((NH4)2Fe(SO4)2·6H2O). Iron(II) compounds tend to be oxidized to iron(III) compounds in the air.[56]
History
Development of iron metallurgy
Iron is one of the elements undoubtedly known to the ancient world.[78] It has been worked, or wrought, for millennia. However, iron artefacts of great age are much rarer than objects made of gold or silver due to the ease with which iron corrodes.[79] The technology developed slowly, and even after the discovery of smelting it took many centuries for iron to replace bronze as the metal of choice for tools and weapons.
Meteoritic iron
Beads made from meteoric iron in 3500 BC or earlier were found in Gerzeh, Egypt by G.A. Wainwright.[80] The beads contain 7.5% nickel, which is a signature of meteoric origin since iron found in the Earth’s crust generally has only minuscule nickel impurities.
Meteoric iron was highly regarded due to its origin in the heavens and was often used to forge weapons and tools.[80] For example, a dagger made of meteoric iron was found in the tomb of Tutankhamun, containing similar proportions of iron, cobalt, and nickel to a meteorite discovered in the area, deposited by an ancient meteor shower.[81][82][83] Items that were likely made of iron by Egyptians date from 3000 to 2500 BC.[79]
Meteoritic iron is comparably soft and ductile and easily cold forged but may get brittle when heated because of the nickel content.[84]
Wrought iron
The symbol for Mars has been used since antiquity to represent iron.
The iron pillar of Delhi is an example of the iron extraction and processing methodologies of early India.
The first iron production started in the Middle Bronze Age, but it took several centuries before iron displaced bronze. Samples of smelted iron from Asmar, Mesopotamia and Tall Chagar Bazaar in northern Syria were made sometime between 3000 and 2700 BC.[85] The Hittites established an empire in north-central Anatolia around 1600 BC. They appear to be the first to understand the production of iron from its ores and regard it highly in their society.[86] The Hittites began to smelt iron between 1500 and 1200 BC and the practice spread to the rest of the Near East after their empire fell in 1180 BC.[85] The subsequent period is called the Iron Age.
Artifacts of smelted iron are found in India dating from 1800 to 1200 BC,[87] and in the Levant from about 1500 BC (suggesting smelting in Anatolia or the Caucasus).[88][89] Alleged references (compare history of metallurgy in South Asia) to iron in the Indian Vedas have been used for claims of a very early usage of iron in India respectively to date the texts as such. The rigveda term ayas (metal) refers to copper, while iron which is called as śyāma ayas, literally «black copper», first is mentioned in the post-rigvedic Atharvaveda.[90]
Some archaeological evidence suggests iron was smelted in Zimbabwe and southeast Africa as early as the eighth century BC.[91] Iron working was introduced to Greece in the late 11th century BC, from which it spread quickly throughout Europe.[92]
Iron sickle from Ancient Greece.
The spread of ironworking in Central and Western Europe is associated with Celtic expansion. According to Pliny the Elder, iron use was common in the Roman era.[80] In the lands of what is now considered China, iron appears approximately 700–500 BC.[93] Iron smelting may have been introduced into China through Central Asia.[94] The earliest evidence of the use of a blast furnace in China dates to the 1st century AD,[95] and cupola furnaces were used as early as the Warring States period (403–221 BC).[96] Usage of the blast and cupola furnace remained widespread during the Tang and Song dynasties.[97]
During the Industrial Revolution in Britain, Henry Cort began refining iron from pig iron to wrought iron (or bar iron) using innovative production systems. In 1783 he patented the puddling process for refining iron ore. It was later improved by others, including Joseph Hall.[98]
Cast iron
Cast iron was first produced in China during 5th century BC,[99] but was hardly in Europe until the medieval period.[100][101] The earliest cast iron artifacts were discovered by archaeologists in what is now modern Luhe County, Jiangsu in China. Cast iron was used in ancient China for warfare, agriculture, and architecture.[102] During the medieval period, means were found in Europe of producing wrought iron from cast iron (in this context known as pig iron) using finery forges. For all these processes, charcoal was required as fuel.[103]
Medieval blast furnaces were about 10 feet (3.0 m) tall and made of fireproof brick; forced air was usually provided by hand-operated bellows.[101] Modern blast furnaces have grown much bigger, with hearths fourteen meters in diameter that allow them to produce thousands of tons of iron each day, but essentially operate in much the same way as they did during medieval times.[103]
In 1709, Abraham Darby I established a coke-fired blast furnace to produce cast iron, replacing charcoal, although continuing to use blast furnaces. The ensuing availability of inexpensive iron was one of the factors leading to the Industrial Revolution. Toward the end of the 18th century, cast iron began to replace wrought iron for certain purposes, because it was cheaper. Carbon content in iron was not implicated as the reason for the differences in properties of wrought iron, cast iron, and steel until the 18th century.[85]
Since iron was becoming cheaper and more plentiful, it also became a major structural material following the building of the innovative first iron bridge in 1778. This bridge still stands today as a monument to the role iron played in the Industrial Revolution. Following this, iron was used in rails, boats, ships, aqueducts, and buildings, as well as in iron cylinders in steam engines.[103] Railways have been central to the formation of modernity and ideas of progress[104] and various languages refer to railways as iron road (e.g. French chemin de fer, German Eisenbahn, Turkish demiryolu, Russian железная дорога, Chinese, Japanese, and Korean 鐵道, Vietnamese đường sắt).
Steel
Steel (with smaller carbon content than pig iron but more than wrought iron) was first produced in antiquity by using a bloomery. Blacksmiths in Luristan in western Persia were making good steel by 1000 BC.[85] Then improved versions, Wootz steel by India and Damascus steel were developed around 300 BC and AD 500 respectively. These methods were specialized, and so steel did not become a major commodity until the 1850s.[105]
New methods of producing it by carburizing bars of iron in the cementation process were devised in the 17th century. In the Industrial Revolution, new methods of producing bar iron without charcoal were devised and these were later applied to produce steel. In the late 1850s, Henry Bessemer invented a new steelmaking process, involving blowing air through molten pig iron, to produce mild steel. This made steel much more economical, thereby leading to wrought iron no longer being produced in large quantities.[106]
Foundations of modern chemistry
In 1774, Antoine Lavoisier used the reaction of water steam with metallic iron inside an incandescent iron tube to produce hydrogen in his experiments leading to the demonstration of the conservation of mass, which was instrumental in changing chemistry from a qualitative science to a quantitative one.[107]
Symbolic role
«Gold gab ich für Eisen» – «I gave gold for iron». German-American brooch from WWI.
Iron plays a certain role in mythology and has found various usage as a metaphor and in folklore. The Greek poet Hesiod’s Works and Days (lines 109–201) lists different ages of man named after metals like gold, silver, bronze and iron to account for successive ages of humanity.[108] The Iron Age was closely related with Rome, and in Ovid’s Metamorphoses
The Virtues, in despair, quit the earth; and the depravity of man becomes universal and complete. Hard steel succeeded then.
An example of the importance of iron’s symbolic role may be found in the German Campaign of 1813. Frederick William III commissioned then the first Iron Cross as military decoration. Berlin iron jewellery reached its peak production between 1813 and 1815, when the Prussian royal family urged citizens to donate gold and silver jewellery for military funding. The inscription Gold gab ich für Eisen (I gave gold for iron) was used as well in later war efforts.[109]
Production of metallic iron
Iron furnace in Columbus, Ohio, 1922
Laboratory routes
For a few limited purposes when it is needed, pure iron is produced in the laboratory in small quantities by reducing the pure oxide or hydroxide with hydrogen, or forming iron pentacarbonyl and heating it to 250 °C so that it decomposes to form pure iron powder.[41] Another method is electrolysis of ferrous chloride onto an iron cathode.[110]
Main industrial route
| Country | Iron ore | Pig iron | Direct iron | Steel |
|---|---|---|---|---|
| 1,114.9 | 549.4 | 573.6 | ||
| 393.9 | 4.4 | 5.2 | ||
| 305.0 | 25.1 | 0.011 | 26.5 | |
| 66.9 | 87.5 | |||
| 257.4 | 38.2 | 23.4 | 63.5 | |
| 92.1 | 43.9 | 4.7 | 60.0 | |
| 65.8 | 25.7 | 29.9 | ||
| 0.1 | 27.3 | 48.6 | ||
| 0.4 | 20.1 | 0.38 | 32.7 | |
| World | 1,594.9 | 914.0 | 64.5 | 1,232.4 |
Nowadays, the industrial production of iron or steel consists of two main stages. In the first stage, iron ore is reduced with coke in a blast furnace, and the molten metal is separated from gross impurities such as silicate minerals. This stage yields an alloy—pig iron—that contains relatively large amounts of carbon. In the second stage, the amount of carbon in the pig iron is lowered by oxidation to yield wrought iron, steel, or cast iron.[112] Other metals can be added at this stage to form alloy steels.
17th century Chinese illustration of workers at a blast furnace, making wrought iron from pig iron[113]
How iron was extracted in the 19th century
Blast furnace processing
The blast furnace is loaded with iron ores, usually hematite Fe2O3 or magnetite Fe3O4, along with coke (coal that has been separately baked to remove volatile components) and flux (limestone or dolomite). «Blasts» of air pre-heated to 900 °C (sometimes with oxygen enrichment) is blown through the mixture, in sufficient amount to turn the carbon into carbon monoxide:[112]
This reaction raises the temperature to about 2000 °C. The carbon monoxide reduces the iron ore to metallic iron[112]
Some iron in the high-temperature lower region of the furnace reacts directly with the coke:[112]
The flux removes silicaceous minerals in the ore, which would otherwise clog the furnace: The heat of the furnace decomposes the carbonates to calcium oxide, which reacts with any excess silica to form a slag composed of calcium silicate CaSiO3 or other products. At the furnace’s temperature, the metal and the slag are both molten. They collect at the bottom as two immiscible liquid layers (with the slag on top), that are then easily separated.[112] The slag can be used as a material in road construction or to improve mineral-poor soils for agriculture.[101]
Steelmaking thus remains one of the largest industrial contributors of CO2 emissions in the world.[114]
This heap of iron ore pellets will be used in steel production.
Steelmaking
A pot of molten iron being used to make steel
The pig iron produced by the blast furnace process contains up to 4–5% carbon (by mass), with small amounts of other impurities like sulfur, magnesium, phosphorus, and manganese. This high level of carbon makes it relatively weak and brittle. Reducing the amount of carbon to 0.002–2.1% produces steel, which may be up to 1000 times harder than pure iron. A great variety of steel articles can then be made by cold working, hot rolling, forging, machining, etc. Removing the impurities from pig iron, but leaving 2–4% carbon, results in cast iron, which is cast by foundries into articles such as stoves, pipes, radiators, lamp-posts, and rails.[112]
Steel products often undergo various heat treatments after they are forged to shape. Annealing consists of heating them to 700–800 °C for several hours and then gradual cooling. It makes the steel softer and more workable.[115]
Direct iron reduction
Owing to environmental concerns, alternative methods of processing iron have been developed. «Direct iron reduction» reduces iron ore to a ferrous lump called «sponge» iron or «direct» iron that is suitable for steelmaking.[101] Two main reactions comprise the direct reduction process:
Natural gas is partially oxidized (with heat and a catalyst):[101]
Iron ore is then treated with these gases in a furnace, producing solid sponge iron:[101]
Silica is removed by adding a limestone flux as described above.[101]
Thermite process
Ignition of a mixture of aluminium powder and iron oxide yields metallic iron via the thermite reaction:
Alternatively pig iron may be made into steel (with up to about 2% carbon) or wrought iron (commercially pure iron). Various processes have been used for this, including finery forges, puddling furnaces, Bessemer converters, open hearth furnaces, basic oxygen furnaces, and electric arc furnaces. In all cases, the objective is to oxidize some or all of the carbon, together with other impurities. On the other hand, other metals may be added to make alloy steels.[103]
Applications
As structural material
Iron is the most widely used of all the metals, accounting for over 90% of worldwide metal production. Its low cost and high strength often make it the material of choice to withstand stress or transmit forces, such as the construction of machinery and machine tools, rails, automobiles, ship hulls, concrete reinforcing bars, and the load-carrying framework of buildings. Since pure iron is quite soft, it is most commonly combined with alloying elements to make steel.[116]
Mechanical properties
| Material | TS (MPa) |
BH (Brinell) |
|---|---|---|
| Iron whiskers | 11000 | |
| Ausformed (hardened) steel |
2930 | 850–1200 |
| Martensitic steel | 2070 | 600 |
| Bainitic steel | 1380 | 400 |
| Pearlitic steel | 1200 | 350 |
| Cold-worked iron | 690 | 200 |
| Small-grain iron | 340 | 100 |
| Carbon-containing iron | 140 | 40 |
| Pure, single-crystal iron | 10 | 3 |
The mechanical properties of iron and its alloys are extremely relevant to their structural applications. Those properties can be evaluated in various ways, including the Brinell test, the Rockwell test and the Vickers hardness test.
The properties of pure iron are often used to calibrate measurements or to compare tests.[118][119] However, the mechanical properties of iron are significantly affected by the sample’s purity: pure, single crystals of iron are actually softer than aluminium,[117] and the purest industrially produced iron (99.99%) has a hardness of 20–30 Brinell.[120] The pure iron (99.9%~99.999%), especially called electrolytic iron, is industrially produced by electrolytic refining.
An increase in the carbon content will cause a significant increase in the hardness and tensile strength of iron. Maximum hardness of 65 Rc is achieved with a 0.6% carbon content, although the alloy has low tensile strength.[121] Because of the softness of iron, it is much easier to work with than its heavier congeners ruthenium and osmium.[12]
Iron-carbon phase diagram
Types of steels and alloys
α-Iron is a fairly soft metal that can dissolve only a small concentration of carbon (no more than 0.021% by mass at 910 °C).[122] Austenite (γ-iron) is similarly soft and metallic but can dissolve considerably more carbon (as much as 2.04% by mass at 1146 °C). This form of iron is used in the type of stainless steel used for making cutlery, and hospital and food-service equipment.[16]
Commercially available iron is classified based on purity and the abundance of additives. Pig iron has 3.5–4.5% carbon[123] and contains varying amounts of contaminants such as sulfur, silicon and phosphorus. Pig iron is not a saleable product, but rather an intermediate step in the production of cast iron and steel. The reduction of contaminants in pig iron that negatively affect material properties, such as sulfur and phosphorus, yields cast iron containing 2–4% carbon, 1–6% silicon, and small amounts of manganese.[112] Pig iron has a melting point in the range of 1420–1470 K, which is lower than either of its two main components, and makes it the first product to be melted when carbon and iron are heated together.[6] Its mechanical properties vary greatly and depend on the form the carbon takes in the alloy.[12]
«White» cast irons contain their carbon in the form of cementite, or iron carbide (Fe3C).[12] This hard, brittle compound dominates the mechanical properties of white cast irons, rendering them hard, but unresistant to shock. The broken surface of a white cast iron is full of fine facets of the broken iron carbide, a very pale, silvery, shiny material, hence the appellation. Cooling a mixture of iron with 0.8% carbon slowly below 723 °C to room temperature results in separate, alternating layers of cementite and α-iron, which is soft and malleable and is called pearlite for its appearance. Rapid cooling, on the other hand, does not allow time for this separation and creates hard and brittle martensite. The steel can then be tempered by reheating to a temperature in between, changing the proportions of pearlite and martensite. The end product below 0.8% carbon content is a pearlite-αFe mixture, and that above 0.8% carbon content is a pearlite-cementite mixture.[12]
In gray iron the carbon exists as separate, fine flakes of graphite, and also renders the material brittle due to the sharp edged flakes of graphite that produce stress concentration sites within the material.[124] A newer variant of gray iron, referred to as ductile iron, is specially treated with trace amounts of magnesium to alter the shape of graphite to spheroids, or nodules, reducing the stress concentrations and vastly increasing the toughness and strength of the material.[124]
Wrought iron contains less than 0.25% carbon but large amounts of slag that give it a fibrous characteristic.[123] It is a tough, malleable product, but not as fusible as pig iron. If honed to an edge, it loses it quickly. Wrought iron is characterized by the presence of fine fibers of slag entrapped within the metal. Wrought iron is more corrosion resistant than steel. It has been almost completely replaced by mild steel for traditional «wrought iron» products and blacksmithing.
Mild steel corrodes more readily than wrought iron, but is cheaper and more widely available. Carbon steel contains 2.0% carbon or less,[125] with small amounts of manganese, sulfur, phosphorus, and silicon. Alloy steels contain varying amounts of carbon as well as other metals, such as chromium, vanadium, molybdenum, nickel, tungsten, etc. Their alloy content raises their cost, and so they are usually only employed for specialist uses. One common alloy steel, though, is stainless steel. Recent developments in ferrous metallurgy have produced a growing range of microalloyed steels, also termed ‘HSLA’ or high-strength, low alloy steels, containing tiny additions to produce high strengths and often spectacular toughness at minimal cost.[125][126][127]
Alloys with high purity elemental makeups (such as alloys of electrolytic iron) have specifically enhanced properties such as ductility, tensile strength, toughness, fatigue strength, heat resistance, and corrosion resistance.
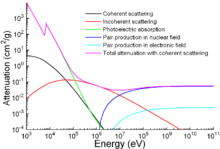
Apart from traditional applications, iron is also used for protection from ionizing radiation. Although it is lighter than another traditional protection material, lead, it is much stronger mechanically. The attenuation of radiation as a function of energy is shown in the graph.[128]
The main disadvantage of iron and steel is that pure iron, and most of its alloys, suffer badly from rust if not protected in some way, a cost amounting to over 1% of the world’s economy.[129] Painting, galvanization, passivation, plastic coating and bluing are all used to protect iron from rust by excluding water and oxygen or by cathodic protection. The mechanism of the rusting of iron is as follows:[129]
- Cathode: 3 O2 + 6 H2O + 12 e− → 12 OH−
- Anode: 4 Fe → 4 Fe2+ + 8 e−; 4 Fe2+ → 4 Fe3+ + 4 e−
- Overall: 4 Fe + 3 O2 + 6 H2O → 4 Fe3+ + 12 OH− → 4 Fe(OH)3 or 4 FeO(OH) + 4 H2O
The electrolyte is usually iron(II) sulfate in urban areas (formed when atmospheric sulfur dioxide attacks iron), and salt particles in the atmosphere in seaside areas.[129]
Catalysts and reagents
Because Fe is inexpensive and nontoxic, much effort has been devoted to the development of Fe-based catalysts and reagents. Iron is however less common as a catalyst in commercial processes than more expensive metals.[130] In biology, Fe-containing enzymes are pervasive.[131]
Iron catalysts are traditionally used in the Haber–Bosch process for the production of ammonia and the Fischer–Tropsch process for conversion of carbon monoxide to hydrocarbons for fuels and lubricants.[132] Powdered iron in an acidic medium is used in the Bechamp reduction, the conversion of nitrobenzene to aniline.[133]
Iron compounds
Iron(III) oxide mixed with aluminium powder can be ignited to create a thermite reaction, used in welding large iron parts (like rails) and purifying ores. Iron(III) oxide and oxyhydroxide are used as reddish and ocher pigments.
Iron(III) chloride finds use in water purification and sewage treatment, in the dyeing of cloth, as a coloring agent in paints, as an additive in animal feed, and as an etchant for copper in the manufacture of printed circuit boards.[134] It can also be dissolved in alcohol to form tincture of iron, which is used as a medicine to stop bleeding in canaries.[135]
Iron(II) sulfate is used as a precursor to other iron compounds. It is also used to reduce chromate in cement. It is used to fortify foods and treat iron deficiency anemia. Iron(III) sulfate is used in settling minute sewage particles in tank water. Iron(II) chloride is used as a reducing flocculating agent, in the formation of iron complexes and magnetic iron oxides, and as a reducing agent in organic synthesis.[134]
Sodium nitroprusside is a drug used as a vasodilator. It is on the World Health Organization’s List of Essential Medicines.[136]
Biological and pathological role
Iron is required for life.[5][137][138] The iron–sulfur clusters are pervasive and include nitrogenase, the enzymes responsible for biological nitrogen fixation. Iron-containing proteins participate in transport, storage and used of oxygen.[5] Iron proteins are involved in electron transfer.[139]
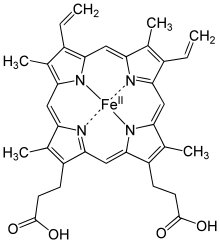
Simplified structure of Heme b; in the protein additional ligand(s) are attached to Fe.
Examples of iron-containing proteins in higher organisms include hemoglobin, cytochrome (see high-valent iron), and catalase.[5][140] The average adult human contains about 0.005% body weight of iron, or about four grams, of which three quarters is in hemoglobin – a level that remains constant despite only about one milligram of iron being absorbed each day,[139] because the human body recycles its hemoglobin for the iron content.[141]
Microbial growth may be assisted by oxidation of iron(II) or by reduction of iron (III).[142]
Biochemistry
Iron acquisition poses a problem for aerobic organisms because ferric iron is poorly soluble near neutral pH. Thus, these organisms have developed means to absorb iron as complexes, sometimes taking up ferrous iron before oxidising it back to ferric iron.[5] In particular, bacteria have evolved very high-affinity sequestering agents called siderophores.[143][144][145]
After uptake in human cells, iron storage is precisely regulated.[5][146] A major component of this regulation is the protein transferrin, which binds iron ions absorbed from the duodenum and carries it in the blood to cells.[5][147] Transferrin contains Fe3+ in the middle of a distorted octahedron, bonded to one nitrogen, three oxygens and a chelating carbonate anion that traps the Fe3+ ion: it has such a high stability constant that it is very effective at taking up Fe3+ ions even from the most stable complexes. At the bone marrow, transferrin is reduced from Fe3+ and Fe2+ and stored as ferritin to be incorporated into hemoglobin.[139]
The most commonly known and studied bioinorganic iron compounds (biological iron molecules) are the heme proteins: examples are hemoglobin, myoglobin, and cytochrome P450.[5] These compounds participate in transporting gases, building enzymes, and transferring electrons.[139] Metalloproteins are a group of proteins with metal ion cofactors. Some examples of iron metalloproteins are ferritin and rubredoxin.[139] Many enzymes vital to life contain iron, such as catalase,[148] lipoxygenases,[149] and IRE-BP.[150]
Hemoglobin is an oxygen carrier that occurs in red blood cells and contributes their color, transporting oxygen in the arteries from the lungs to the muscles where it is transferred to myoglobin, which stores it until it is needed for the metabolic oxidation of glucose, generating energy.[5] Here the hemoglobin binds to carbon dioxide, produced when glucose is oxidized, which is transported through the veins by hemoglobin (predominantly as bicarbonate anions) back to the lungs where it is exhaled.[139] In hemoglobin, the iron is in one of four heme groups and has six possible coordination sites; four are occupied by nitrogen atoms in a porphyrin ring, the fifth by an imidazole nitrogen in a histidine residue of one of the protein chains attached to the heme group, and the sixth is reserved for the oxygen molecule it can reversibly bind to.[139] When hemoglobin is not attached to oxygen (and is then called deoxyhemoglobin), the Fe2+ ion at the center of the heme group (in the hydrophobic protein interior) is in a high-spin configuration. It is thus too large to fit inside the porphyrin ring, which bends instead into a dome with the Fe2+ ion about 55 picometers above it. In this configuration, the sixth coordination site reserved for the oxygen is blocked by another histidine residue.[139]
When deoxyhemoglobin picks up an oxygen molecule, this histidine residue moves away and returns once the oxygen is securely attached to form a hydrogen bond with it. This results in the Fe2+ ion switching to a low-spin configuration, resulting in a 20% decrease in ionic radius so that now it can fit into the porphyrin ring, which becomes planar.[139] (Additionally, this hydrogen bonding results in the tilting of the oxygen molecule, resulting in a Fe–O–O bond angle of around 120° that avoids the formation of Fe–O–Fe or Fe–O2–Fe bridges that would lead to electron transfer, the oxidation of Fe2+ to Fe3+, and the destruction of hemoglobin.) This results in a movement of all the protein chains that leads to the other subunits of hemoglobin changing shape to a form with larger oxygen affinity. Thus, when deoxyhemoglobin takes up oxygen, its affinity for more oxygen increases, and vice versa.[139] Myoglobin, on the other hand, contains only one heme group and hence this cooperative effect cannot occur. Thus, while hemoglobin is almost saturated with oxygen in the high partial pressures of oxygen found in the lungs, its affinity for oxygen is much lower than that of myoglobin, which oxygenates even at low partial pressures of oxygen found in muscle tissue.[139] As described by the Bohr effect (named after Christian Bohr, the father of Niels Bohr), the oxygen affinity of hemoglobin diminishes in the presence of carbon dioxide.[139]
Carbon monoxide and phosphorus trifluoride are poisonous to humans because they bind to hemoglobin similarly to oxygen, but with much more strength, so that oxygen can no longer be transported throughout the body. Hemoglobin bound to carbon monoxide is known as carboxyhemoglobin. This effect also plays a minor role in the toxicity of cyanide, but there the major effect is by far its interference with the proper functioning of the electron transport protein cytochrome a.[139] The cytochrome proteins also involve heme groups and are involved in the metabolic oxidation of glucose by oxygen. The sixth coordination site is then occupied by either another imidazole nitrogen or a methionine sulfur, so that these proteins are largely inert to oxygen – with the exception of cytochrome a, which bonds directly to oxygen and thus is very easily poisoned by cyanide.[139] Here, the electron transfer takes place as the iron remains in low spin but changes between the +2 and +3 oxidation states. Since the reduction potential of each step is slightly greater than the previous one, the energy is released step-by-step and can thus be stored in adenosine triphosphate. Cytochrome a is slightly distinct, as it occurs at the mitochondrial membrane, binds directly to oxygen, and transports protons as well as electrons, as follows:[139]
- 4 Cytc2+ + O2 + 8H+
inside → 4 Cytc3+ + 2 H2O + 4H+
outside
Although the heme proteins are the most important class of iron-containing proteins, the iron–sulfur proteins are also very important, being involved in electron transfer, which is possible since iron can exist stably in either the +2 or +3 oxidation states. These have one, two, four, or eight iron atoms that are each approximately tetrahedrally coordinated to four sulfur atoms; because of this tetrahedral coordination, they always have high-spin iron. The simplest of such compounds is rubredoxin, which has only one iron atom coordinated to four sulfur atoms from cysteine residues in the surrounding peptide chains. Another important class of iron–sulfur proteins is the ferredoxins, which have multiple iron atoms. Transferrin does not belong to either of these classes.[139]
The ability of sea mussels to maintain their grip on rocks in the ocean is facilitated by their use of organometallic iron-based bonds in their protein-rich cuticles. Based on synthetic replicas, the presence of iron in these structures increased elastic modulus 770 times, tensile strength 58 times, and toughness 92 times. The amount of stress required to permanently damage them increased 76 times.[152]
Nutrition
Diet
Iron is pervasive, but particularly rich sources of dietary iron include red meat, oysters, beans, poultry, fish, leaf vegetables, watercress, tofu, and blackstrap molasses.[5] Bread and breakfast cereals are sometimes specifically fortified with iron.[5][153]
Iron provided by dietary supplements is often found as iron(II) fumarate, although iron(II) sulfate is cheaper and is absorbed equally well.[134] Elemental iron, or reduced iron, despite being absorbed at only one-third to two-thirds the efficiency (relative to iron sulfate),[154] is often added to foods such as breakfast cereals or enriched wheat flour. Iron is most available to the body when chelated to amino acids[155] and is also available for use as a common iron supplement. Glycine, the least expensive amino acid, is most often used to produce iron glycinate supplements.[156]
Dietary recommendations
The U.S. Institute of Medicine (IOM) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for iron in 2001.[5] The current EAR for iron for women ages 14–18 is 7.9 mg/day, 8.1 for ages 19–50 and 5.0 thereafter (post menopause). For men the EAR is 6.0 mg/day for ages 19 and up. The RDA is 15.0 mg/day for women ages 15–18, 18.0 for 19–50 and 8.0 thereafter. For men, 8.0 mg/day for ages 19 and up. RDAs are higher than EARs so as to identify amounts that will cover people with higher than average requirements. RDA for pregnancy is 27 mg/day and, for lactation, 9 mg/day.[5] For children ages 1–3 years 7 mg/day, 10 for ages 4–8 and 8 for ages 9–13. As for safety, the IOM also sets Tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. In the case of iron the UL is set at 45 mg/day. Collectively the EARs, RDAs and ULs are referred to as Dietary Reference Intakes.[157]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women the PRI is 13 mg/day ages 15–17 years, 16 mg/day for women ages 18 and up who are premenopausal and 11 mg/day postmenopausal. For pregnancy and lactation, 16 mg/day. For men the PRI is 11 mg/day ages 15 and older. For children ages 1 to 14 the PRI increases from 7 to 11 mg/day. The PRIs are higher than the U.S. RDAs, with the exception of pregnancy.[158] The EFSA reviewed the same safety question did not establish a UL.[159]
Infants may require iron supplements if they are bottle-fed cow’s milk.[160] Frequent blood donors are at risk of low iron levels and are often advised to supplement their iron intake.[161]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For iron labeling purposes 100% of the Daily Value was 18 mg, and as of May 27, 2016 remained unchanged at 18 mg.[162][163] A table of the old and new adult daily values is provided at Reference Daily Intake.
Deficiency
Iron deficiency is the most common nutritional deficiency in the world.[5][164][165][166] When loss of iron is not adequately compensated by adequate dietary iron intake, a state of latent iron deficiency occurs, which over time leads to iron-deficiency anemia if left untreated, which is characterised by an insufficient number of red blood cells and an insufficient amount of hemoglobin.[167] Children, pre-menopausal women (women of child-bearing age), and people with poor diet are most susceptible to the disease. Most cases of iron-deficiency anemia are mild, but if not treated can cause problems like fast or irregular heartbeat, complications during pregnancy, and delayed growth in infants and children.[168]
Excess
Iron uptake is tightly regulated by the human body, which has no regulated physiological means of excreting iron. Only small amounts of iron are lost daily due to mucosal and skin epithelial cell sloughing, so control of iron levels is primarily accomplished by regulating uptake.[169] Regulation of iron uptake is impaired in some people as a result of a genetic defect that maps to the HLA-H gene region on chromosome 6 and leads to abnormally low levels of hepcidin, a key regulator of the entry of iron into the circulatory system in mammals.[170] In these people, excessive iron intake can result in iron overload disorders, known medically as hemochromatosis.[5] Many people have an undiagnosed genetic susceptibility to iron overload, and are not aware of a family history of the problem. For this reason, people should not take iron supplements unless they suffer from iron deficiency and have consulted a doctor. Hemochromatosis is estimated to be the cause of 0.3 to 0.8% of all metabolic diseases of Caucasians.[171]
Overdoses of ingested iron can cause excessive levels of free iron in the blood. High blood levels of free ferrous iron react with peroxides to produce highly reactive free radicals that can damage DNA, proteins, lipids, and other cellular components. Iron toxicity occurs when the cell contains free iron, which generally occurs when iron levels exceed the availability of transferrin to bind the iron. Damage to the cells of the gastrointestinal tract can also prevent them from regulating iron absorption, leading to further increases in blood levels. Iron typically damages cells in the heart, liver and elsewhere, causing adverse effects that include coma, metabolic acidosis, shock, liver failure, coagulopathy, long-term organ damage, and even death.[172] Humans experience iron toxicity when the iron exceeds 20 milligrams for every kilogram of body mass; 60 milligrams per kilogram is considered a lethal dose.[173] Overconsumption of iron, often the result of children eating large quantities of ferrous sulfate tablets intended for adult consumption, is one of the most common toxicological causes of death in children under six.[173] The Dietary Reference Intake (DRI) sets the Tolerable Upper Intake Level (UL) for adults at 45 mg/day. For children under fourteen years old the UL is 40 mg/day.[174]
The medical management of iron toxicity is complicated, and can include use of a specific chelating agent called deferoxamine to bind and expel excess iron from the body.[172][175][176]
ADHD
Some research has suggested that low thalamic iron levels may play a role in the pathophysiology of ADHD.[177] Some researchers have found that iron supplementation can be effective especially in the inattentive subtype of the disorder.[178] One study also showed that iron may be able to decrease the risk of cardiovascular events during treatment with ADHD drugs.[179]
Some researchers in the 2000s suggested a link between low levels of iron in the blood and ADHD. A 2012 study found no such correlation.[180]
Cancer
The role of iron in cancer defense can be described as a «double-edged sword» because of its pervasive presence in non-pathological processes.[181] People having chemotherapy may develop iron deficiency and anemia, for which intravenous iron therapy is used to restore iron levels.[182] Iron overload, which may occur from high consumption of red meat,[5] may initiate tumor growth and increase susceptibility to cancer onset,[182] particularly for colorectal cancer.[5]
Marine systems
Iron plays an essential role in marine systems and can act as a limiting nutrient for planktonic activity.[183] Because of this, too much of a decrease in iron may lead to a decrease in growth rates in phytoplanktonic organisms such as diatoms.[184] Iron can also be oxidized by marine microbes under conditions that are high in iron and low in oxygen.[185]
Iron can enter marine systems through adjoining rivers and directly from the atmosphere. Once iron enters the ocean, it can be distributed throughout the water column through ocean mixing and through recycling on the cellular level.[186] In the arctic, sea ice plays a major role in the store and distribution of iron in the ocean, depleting oceanic iron as it freezes in the winter and releasing it back into the water when thawing occurs in the summer.[187] The iron cycle can fluctuate the forms of iron from aqueous to particle forms altering the availability of iron to primary producers.[188] Increased light and warmth increases the amount of iron that is in forms that are usable by primary producers.[189]
See also
- El Mutún in Bolivia, where 10% of the world’s accessible iron ore is located
- Iron and steel industry
- Iron cycle
- Iron nanoparticle
- Iron–platinum nanoparticle
- Iron fertilization – proposed fertilization of oceans to stimulate phytoplankton growth
- Iron-oxidizing bacteria
- List of countries by iron production
- Pelletising – process of creation of iron ore pellets
- Rustproof iron
- Steel
References
- ^ «Standard Atomic Weights: Iron». CIAAW. 1993.
- ^ Ram, R. S.; Bernath, P. F. (2003). «Fourier transform emission spectroscopy of the g4Δ–a4Δ system of FeCl». Journal of Molecular Spectroscopy. 221 (2): 261. Bibcode:2003JMoSp.221..261R. doi:10.1016/S0022-2852(03)00225-X.
- ^ Demazeau, G.; Buffat, B.; Pouchard, M.; Hagenmuller, P. (1982). «Recent developments in the field of high oxidation states of transition elements in oxides stabilization of six-coordinated Iron(V)». Zeitschrift für anorganische und allgemeine Chemie. 491: 60–66. doi:10.1002/zaac.19824910109.
- ^ Lu, J.; Jian, J.; Huang, W.; Lin, H.; Li, J; Zhou, M. (2016). «Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4−«. Physical Chemistry Chemical Physics. 18 (45): 31125–31131. Bibcode:2016PCCP…1831125L. doi:10.1039/C6CP06753K. PMID 27812577.
- ^ a b c d e f g h i j k l m n o p q «Iron». Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, Oregon. April 2016. Retrieved 6 March 2018.
- ^ a b c d e f g h Greenwood & Earnshaw 1997, pp. 1075–79.
- ^ Hirose, K., Tateno, S. (2010). «The Structure of Iron in Earth’s Inner Core». Science. American Association for the Advancement of Science. 330 (6002): 359–361. Bibcode:2010Sci…330..359T. doi:10.1126/science.1194662. PMID 20947762. S2CID 206528628.
- ^ Chamati, Gaminchev (2014). «Dynamic stability of Fe under high pressure». Journal of Physics. IOP Publishing. 558 (1): 012013. Bibcode:2014JPhCS.558a2013G. doi:10.1088/1742-6596/558/1/012013.
- ^ Boehler, Reinhard (2000). «High-pressure experiments and the phase diagram of lower mantle and core materials». Reviews of Geophysics. American Geophysical Union. 38 (2): 221–45. Bibcode:2000RvGeo..38..221B. doi:10.1029/1998RG000053. S2CID 33458168.
- ^ Stixrude, Lars; Wasserman, Evgeny; Cohen, Ronald E. (10 November 1997). «Composition and temperature of Earth’s inner core». Journal of Geophysical Research: Solid Earth. 102 (B11): 24729–39. Bibcode:1997JGR…10224729S. doi:10.1029/97JB02125.
- ^ Greenwood & Earnshaw 1997, p. 1116.
- ^ a b c d e f Greenwood & Earnshaw 1997, pp. 1074–75.
- ^ Boehler, Reinhard; Ross, M. (2007). «Properties of Rocks and Minerals_High-Pressure Melting». Mineral Physics. Treatise on Geophysics. Vol. 2. Elsevier. pp. 527–41. doi:10.1016/B978-044452748-6.00047-X. ISBN 9780444527486.
- ^ Steinmetz, Charles (1917). «fig. 42». Theory and Calculation of Electric Circuits. McGraw-Hill.
- ^ a b Cullity; C. D. Graham (2008). Introduction to Magnetic Materials, 2nd. New York: Wiley–IEEE. p. 116. ISBN 978-0-471-47741-9.
- ^ a b Bramfitt, B.L.; Benscoter, Arlan O. (2002). «The Iron Carbon Phase Diagram». Metallographer’s guide: practice and procedures for irons and steels. ASM International. pp. 24–28. ISBN 978-0-87170-748-2.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), «The NUBASE evaluation of nuclear and decay properties», Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729….3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ Rugel, G.; Faestermann, T.; Knie, K.; Korschinek, G.; Poutivtsev, M.; Schumann, D.; Kivel, N.; Günther-Leopold, I.; Weinreich, R.; Wohlmuther, M. (2009). «New Measurement of the 60Fe Half-Life». Physical Review Letters. 103 (7): 072502. Bibcode:2009PhRvL.103g2502R. doi:10.1103/PhysRevLett.103.072502. PMID 19792637.
- ^ Dauphas, N.; Rouxel, O. (2006). «Mass spectrometry and natural variations of iron isotopes» (PDF). Mass Spectrometry Reviews. 25 (4): 515–50. Bibcode:2006MSRv…25..515D. doi:10.1002/mas.20078. PMID 16463281. Archived from the original (PDF) on 10 June 2010.
- ^ Mostefaoui, S.; Lugmair, G.W.; Hoppe, P.; El Goresy, A. (2004). «Evidence for live 60Fe in meteorites». New Astronomy Reviews. 48 (1–4): 155–59. Bibcode:2004NewAR..48..155M. doi:10.1016/j.newar.2003.11.022.
- ^ Fewell, M. P. (1995). «The atomic nuclide with the highest mean binding energy». American Journal of Physics. 63 (7): 653. Bibcode:1995AmJPh..63..653F. doi:10.1119/1.17828.
- ^ a b c Greenwood & Earnshaw 1997, p. 12.
- ^ Woosley, S.; Janka, T. (2006). «The physics of core collapse supernovae». Nature Physics. 1 (3): 147–54. arXiv:astro-ph/0601261. Bibcode:2005NatPh…1..147W. doi:10.1038/nphys172. S2CID 118974639.
- ^ McDonald, I.; Sloan, G. C.; Zijlstra, A. A.; Matsunaga, N.; Matsuura, M.; Kraemer, K. E.; Bernard-Salas, J.; Markwick, A. J. (2010). «Rusty Old Stars: A Source of the Missing Interstellar Iron?». The Astrophysical Journal Letters. 717 (2): L92–L97. arXiv:1005.3489. Bibcode:2010ApJ…717L..92M. doi:10.1088/2041-8205/717/2/L92. S2CID 14437704.
- ^ Bautista, Manuel A.; Pradhan, Anil K. (1995). «Iron and Nickel Abundances in H~II Regions and Supernova Remnants». Bulletin of the American Astronomical Society. 27: 865. Bibcode:1995AAS…186.3707B.
- ^ Dyson, Freeman J. (1979). «Time without end: Physics and biology in an open universe». Reviews of Modern Physics. 51 (3): 447–60. Bibcode:1979RvMP…51..447D. doi:10.1103/RevModPhys.51.447.
- ^ Aron, Jacob. «Supernova space bullets could have seeded Earth’s iron core». New Scientist. Retrieved 2 October 2020.
- ^ Croswell, Ken. «Iron in the Fire: The Little-Star Supernovae That Could». Scientific American. Retrieved 3 January 2021.
- ^ Buchwald, V F (1992). «On the Use of Iron by the Eskimos in Greenland». Materials Characterization. 29 (2): 139–176. doi:10.1016/1044-5803(92)90112-U.
- ^ Emiliani, Cesare (1992). Planet earth: cosmology, geology, and the evolution of life and environment. Cambridge University Press. p. 152. Bibcode:1992pecg.book…..E. ISBN 978-0-521-40949-0.
- ^ Pernet-Fisher, J.; Day, J.M.D.; Howarth, G.H.; Ryabov, V.V.; Taylor, L.A. (2017). «Atmospheric outgassing and native-iron formation during carbonaceous sediment–basalt melt interactions». Earth and Planetary Science Letters. 460: 201–212. Bibcode:2017E&PSL.460..201P. doi:10.1016/j.epsl.2016.12.022.
- ^ Stark, Anne M. (20 September 2007) Researchers locate mantle’s spin transition zone, leading to clues about earth’s structure. Lawrence Livermore National Laboratory
- ^ Ferropericlase. Mindat.org
- ^ Murakami, M.; Ohishi Y.; Hirao N.; Hirose K. (2012). «A perovskitic lower mantle inferred from high-pressure, high-temperature sound velocity data». Nature. 485 (7396): 90–94. Bibcode:2012Natur.485…90M. doi:10.1038/nature11004. PMID 22552097. S2CID 4387193.
- ^ Sharp, T. (27 November 2014). «Bridgmanite – named at last». Science. 346 (6213): 1057–58. Bibcode:2014Sci…346.1057S. doi:10.1126/science.1261887. PMID 25430755. S2CID 206563252.
- ^ Kong, L. T.; Li, J. F.; Shi, Q. W.; Huang, H. J.; Zhao, K. (6 March 2012). «Dynamical stability of iron under high-temperature and high-pressure conditions». EPL. 97 (5): 56004p1–56004p5. Bibcode:2012EL…..9756004K. doi:10.1209/0295-5075/97/56004. S2CID 121861429.
- ^ Gaminchev, K. G.; Chamati, H. (3 December 2014). «Dynamic stability of Fe under high pressure». J. Phys. 558 (1): 012013(1–7). Bibcode:2014JPhCS.558a2013G. doi:10.1088/1742-6596/558/1/012013.
- ^ Morgan, John W. & Anders, Edward (1980). «Chemical composition of Earth, Venus, and Mercury». Proc. Natl. Acad. Sci. 77 (12): 6973–77. Bibcode:1980PNAS…77.6973M. doi:10.1073/pnas.77.12.6973. PMC 350422. PMID 16592930.
- ^ «Pyrrhotite». Mindat.org. Retrieved 7 July 2009.
- ^ Klein, Cornelis and Cornelius S. Hurlbut, Jr. (1985) Manual of Mineralogy, Wiley, 20th ed, pp. 278–79 ISBN 0-471-80580-7
- ^ a b Greenwood & Earnshaw 1997, p. 1071.
- ^ Lyons, T. W.; Reinhard, C. T. (2009). «Early Earth: Oxygen for heavy-metal fans». Nature. 461 (7261): 179–181. Bibcode:2009Natur.461..179L. doi:10.1038/461179a. PMID 19741692. S2CID 205049360.
- ^ Cloud, P. (1973). «Paleoecological Significance of the Banded Iron-Formation». Economic Geology. 68 (7): 1135–43. doi:10.2113/gsecongeo.68.7.1135.
- ^ Dickinson, Robert E. (1964). Germany: A regional and economic geography (2nd ed.). London: Methuen.
- ^ Naturwerksteine in Baden-Württemberg. Landesamt für Geologie, Rohstoffe und Bergbau, Baden-Württemberg
- ^ «Tales From The Riverbank». Minerva Stone Conservation. Archived from the original on 28 September 2015. Retrieved 22 September 2015.
- ^ Klingelhöfer, G.; Morris, R. V.; Souza, P. A.; Rodionov, D.; Schröder, C. (2007). «Two earth years of Mössbauer studies of the surface of Mars with MIMOS II». Hyperfine Interactions. 170 (1–3): 169–77. Bibcode:2006HyInt.170..169K. doi:10.1007/s10751-007-9508-5. S2CID 98227499.
- ^ Winderlich, R.; Peter, W. (1954). Lehrbuch der Chemie für Höhere Lehranstalten : Einheitsausgabe für Unter- und Oberstufe (in German). Wiesbaden. p. 75. ISBN 978-3-663-04370-6. OCLC 913701506.
- ^ Bertau, Martin (2013). Industrielle Anorganische Chemie (in German). Weinheim: Wiley-VCH. p. 696. ISBN 978-3-527-64956-3. OCLC 855858511.
- ^ Metal Stocks in Society: Scientific synthesis, 2010, International Resource Panel, UNEP
- ^ Stoll, Heather (17 February 2020). «30 years of the iron hypothesis of ice ages». Nature. Springer Science and Business Media LLC. 578 (7795): 370–371. doi:10.1038/d41586-020-00393-x. ISSN 0028-0836.
- ^ Greenwood & Earnshaw 1997, p. 905.
- ^ a b Greenwood & Earnshaw 1997, p. 1070.
- ^ Lu, Jun-Bo; Jian, Jiwen; Huang, Wei; Lin, Hailu; Li, Jun; Zhou, Mingfei (16 November 2016). «Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4−«. Phys. Chem. Chem. Phys. 18 (45): 31125–31131. Bibcode:2016PCCP…1831125L. doi:10.1039/c6cp06753k. PMID 27812577.
- ^ Nam, Wonwoo (2007). «High-Valent Iron(IV)–Oxo Complexes of Heme and Non-Heme Ligands in Oxygenation Reactions» (PDF). Accounts of Chemical Research. 40 (7): 522–531. doi:10.1021/ar700027f. PMID 17469792. Archived from the original (PDF) on 15 June 2021. Retrieved 22 February 2022.
- ^ a b c d e f Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). «Iron». Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 1125–46. ISBN 3-11-007511-3.
- ^ Reiff, William Michael; Long, Gary J. (1984). «Mössbauer Spectroscopy and the Coordination Chemistry of Iron». Mössbauer spectroscopy applied to inorganic chemistry. Springer. pp. 245–83. ISBN 978-0-306-41647-7.
- ^ Ware, Mike (1999). «An introduction in monochrome». Cyanotype: the history, science and art of photographic printing in Prussian blue. NMSI Trading Ltd. pp. 11–19. ISBN 978-1-900747-07-3.
- ^ Gmelin, Leopold (1852). «Mercury and Iron». Hand-book of chemistry. Vol. 6. Cavendish Society. pp. 128–29.
- ^ a b c Greenwood & Earnshaw 1997, p. 1079.
- ^ a b c d Greenwood & Earnshaw 1997, pp. 1082–84.
- ^ Siegfried Pohl, Ulrich Bierbach, Wolfgang Saak; «FeI3SC(NMe2)2, a Neutral Thiourea Complex of Iron(III) Iodide», Angewandte Chemie International Edition in English (1989) 28 (6), 776-777. https://doi.org/10.1002/anie.198907761
- ^ Nicholas A. Barnes, Stephen M.Godfrey, Nicholas Ho, Charles A.McAuliffe, Robin G.Pritchard; «Facile synthesis of a rare example of an iron(III) iodide complex, [FeI3(AsMe3)2], from the reaction of Me3AsI2 with unactivated iron powder», Polyhedron (2013) 55, 67-72. https://doi.org/10.1016/j.poly.2013.02.066
- ^ a b c d Greenwood & Earnshaw 1997, pp. 1088–91.
- ^ a b Greenwood & Earnshaw 1997, pp. 1091–97.
- ^ Clausen, C.A.; Good, M.L. (1968). «Stabilization of the hexachloroferrate(III) anion by the methylammonium cation». Inorganic Chemistry. 7 (12): 2662–63. doi:10.1021/ic50070a047.
- ^ James, B.D.; Bakalova, M.; Lieseganga, J.; Reiff, W.M.; Hockless, D.C.R.; Skelton, B.W.; White, A.H. (1996). «The hexachloroferrate(III) anion stabilized in hydrogen bonded packing arrangements. A comparison of the X-ray crystal structures and low temperature magnetism of tetrakis(methylammonium) hexachloroferrate(III) chloride (I) and tetrakis(hexamethylenediammonium) hexachloroferrate(III) tetrachloroferrate(III) tetrachloride (II)«. Inorganica Chimica Acta. 247 (2): 169–74. doi:10.1016/0020-1693(95)04955-X.
- ^ Giannoccaro, P.; Sacco, A. (1977). Bis[ethylenebis(diphenylphosphine)]-Hydridoiron Complexes. Inorg. Synth. Inorganic Syntheses. Vol. 17. pp. 69–72. doi:10.1002/9780470132487.ch19. ISBN 978-0-470-13248-7.
- ^ Lee, J.; Jung, G.; Lee, S.W. (1998). «Structure of trans-chlorohydridobis(diphenylphosphinoethane)iron(II)». Bull. Korean Chem. Soc. 19 (2): 267–69. doi:10.1007/BF02698412. S2CID 35665289.
- ^ Echigo, Takuya; Kimata, Mitsuyoshi (2008). «Single-crystal X-ray diffraction and spectroscopic studies on humboldtine and lindbergite: weak Jahn–Teller effect of Fe2+ ion». Phys. Chem. Minerals. 35 (8): 467–75. Bibcode:2008PCM….35..467E. doi:10.1007/s00269-008-0241-7. S2CID 98739882.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 1282–86. ISBN 978-0-08-022057-4..
- ^ Kealy, T.J.; Pauson, P.L. (1951). «A New Type of Organo-Iron Compound». Nature. 168 (4285): 1039–40. Bibcode:1951Natur.168.1039K. doi:10.1038/1681039b0. S2CID 4181383.
- ^ Miller, S. A.; Tebboth, J. A.; Tremaine, J. F. (1952). «114. Dicyclopentadienyliron». J. Chem. Soc.: 632–635. doi:10.1039/JR9520000632.
- ^ Wilkinson, G.; Rosenblum, M.; Whiting, M. C.; Woodward, R. B. (1952). «The Structure of Iron Bis-Cyclopentadienyl». J. Am. Chem. Soc. 74 (8): 2125–2126. doi:10.1021/ja01128a527.
- ^ Okuda, Jun (28 December 2016). «Ferrocene – 65 Years After». European Journal of Inorganic Chemistry. 2017 (2): 217–219. doi:10.1002/ejic.201601323. ISSN 1434-1948.
- ^ Greenwood & Earnshaw 1997, p. 1104.
- ^ Bullock, R.M. (11 September 2007). «An Iron Catalyst for Ketone Hydrogenations under Mild Conditions». Angew. Chem. Int. Ed. 46 (39): 7360–63. doi:10.1002/anie.200703053. PMID 17847139.
- ^ Weeks 1968, p. 4.
- ^ a b Weeks 1968, p. 29.
- ^ a b c Weeks 1968, p. 31.
- ^ Bjorkman, Judith Kingston (1973). «Meteors and Meteorites in the ancient Near East». Meteoritics. 8 (2): 91–132. Bibcode:1973Metic…8…91B. doi:10.1111/j.1945-5100.1973.tb00146.x.
- ^ Comelli, Daniela; d’Orazio, Massimo; Folco, Luigi; El-Halwagy, Mahmud; Frizzi, Tommaso; Alberti, Roberto; Capogrosso, Valentina; Elnaggar, Abdelrazek; Hassan, Hala; Nevin, Austin; Porcelli, Franco; Rashed, Mohamed G; Valentini, Gianluca (2016). «The meteoritic origin of Tutankhamun’s iron dagger blade». Meteoritics & Planetary Science. 51 (7): 1301–09. Bibcode:2016M&PS…51.1301C. doi:10.1111/maps.12664.
- ^ Walsh, Declan (2 June 2016). «King Tut’s Dagger Made of ‘Iron From the Sky,’ Researchers Say». The New York Times. Archived from the original on 3 January 2022. Retrieved 4 June 2016.
the blade’s composition of iron, nickel and cobalt was an approximate match for a meteorite that landed in northern Egypt. The result «strongly suggests an extraterrestrial origin»
- ^ Ure, Andrew (1843). Technisches wörterbuch oder Handbuch der Gewerbskunde … : Bearb. nach Dr. Andrew Ure’s Dictionary of arts, manufactures and mines (in German). G. Haase. p. 492.
- ^ a b c d Weeks 1968, p. 32.
- ^ McNutt, Paula (1990 1). The Forging of Israel: Iron Technology, Symbolism and Tradition in Ancient Society. A&C Black.
- ^ Tewari, Rakesh. «The origins of Iron Working in India: New evidence from the Central Ganga plain and the Eastern Vindhyas» (PDF). State Archaeological Department. Retrieved 23 May 2010.
- ^ Photos, E. (1989). «The Question of Meteoritic versus Smelted Nickel-Rich Iron: Archaeological Evidence and Experimental Results». World Archaeology. Taylor & Francis, Ltd. 20 (3): 403–21. doi:10.1080/00438243.1989.9980081. JSTOR 124562.
- ^ Muhly, James D. (2003). «Metalworking/Mining in the Levant». In Lake, Richard Winona (ed.). Near Eastern Archaeology IN: Eisenbrauns. Vol. 180. pp. 174–83.
- ^ Witzel, Michael (2001), «Autochthonous Aryans? The Evidence from Old Indian and Iranian Texts», in Electronic Journal of Vedic Studies (EJVS) 7-3, pp. 1–93
- ^ Weeks, p. 33, quoting Cline, Walter (1937) «Mining and Metallurgy in Negro Africa,» George Banta Publishing Co., Menasha, Wis., pp. 17–23.
- ^ Riederer, Josef; Wartke, Ralf-B. (2009) «Iron», Cancik, Hubert; Schneider, Helmuth (eds.): Brill’s New Pauly, Brill.
- ^ Sawyer, Ralph D. and Sawyer, Mei-chün (1993). The Seven Military Classics of Ancient China. Boulder: Westview. ISBN 0-465-00304-4. p. 10.
- ^ Pigott, Vincent C. (1999). The Archaeometallurgy of the Asian Old World. Philadelphia: University of Pennsylvania Museum of Archaeology and Anthropology. ISBN 0-924171-34-0, p. 8.
- ^ Golas, Peter J. (1999). Science and Civilisation in China: Volume 5, Chemistry and Chemical Technology, Part 13, Mining. Cambridge University Press. p. 152. ISBN 978-0-521-58000-7.
earliest blast furnace discovered in China from about the first century AD
- ^ Pigott, Vincent C. (1999). The Archaeometallurgy of the Asian Old World. Philadelphia: University of Pennsylvania Museum of Archaeology and Anthropology. ISBN 0-924171-34-0, p. 191.
- ^ The Coming of the Ages of Steel. Brill Archive. 1961. p. 54.
- ^ Mott, R.A (2014). «Dry and Wet Puddling». Transactions of the Newcomen Society. 49: 156–57. doi:10.1179/tns.1977.011.
- ^ Wagner, Donald B. (2003). «Chinese blast furnaces from the 10th to the 14th century» (PDF). Historical Metallurgy. 37 (1): 25–37. Archived from the original (PDF) on 7 January 2018. Retrieved 7 January 2018. originally published in Wagner, Donald B. (2001). «Chinese blast furnaces from the 10th to the 14th century». West Asian Science, Technology, and Medicine. 18: 41–74. doi:10.1163/26669323-01801008.
- ^ Giannichedda, Enrico (2007): «Metal production in Late Antiquity», in Technology in Transition AD 300–650 Lavan, L.; Zanini, E. and Sarantis, A.(eds.), Brill, Leiden; ISBN 90-04-16549-5, p. 200.
- ^ a b c d e f g Biddle, Verne; Parker, Gregory. Chemistry, Precision and Design. A Beka Book, Inc.
- ^ Wagner, Donald B. (1993). Iron and Steel in Ancient China. Brill. pp. 335–340. ISBN 978-90-04-09632-5.
- ^ a b c d Greenwood & Earnshaw 1997, p. 1072.
- ^ Schivelbusch, G. (1986) The Railway Journey: Industrialization and Perception of Time and Space in the 19th Century. Oxford: Berg.
- ^ Spoerl, Joseph S. A Brief History of Iron and Steel Production Archived 2 June 2010 at the Wayback Machine. Saint Anselm College
- ^ Enghag, Per (8 January 2008). Encyclopedia of the Elements: Technical Data – History – Processing – Applications. pp. 190–91. ISBN 978-3-527-61234-5.
- ^ Whitaker, Robert D (1975). «An historical note on the conservation of mass». Journal of Chemical Education. 52 (10): 658. Bibcode:1975JChEd..52..658W. doi:10.1021/ed052p658.
- ^ Fontenrose, Joseph (1974). «Work, Justice, and Hesiod’s Five Ages». Classical Philology. 69 (1): 1–16. doi:10.1086/366027. JSTOR 268960. S2CID 161808359.
- ^ Schmidt, Eva (1981) Der preußische Eisenkunstguss. (Art of Prussian cast iron) Technik, Geschichte, Werke, Künstler. Verlag Mann, Berlin, ISBN 3-7861-1130-8
- ^ Lux, H. (1963) «Metallic Iron» in Handbook of Preparative Inorganic Chemistry, 2nd Ed. G. Brauer (ed.), Academic Press, NY. Vol. 2. pp. 1490–91.
- ^ Steel Statistical Yearbook 2010. World Steel Association
- ^ a b c d e f g Greenwood & Earnshaw 1997, p. 1073.
- ^ Song Yingxing (1637): The Tiangong Kaiwu encyclopedia.
- ^ Wang, Peng; Ryberg, Morten; Yang, Yi; Feng, Kuishuang; Kara, Sami; Hauschild, Michael; Chen, Wei-Qiang (6 April 2021). «Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts». Nature Communications. 12 (1): 2066. Bibcode:2021NatCo..12.2066W. doi:10.1038/s41467-021-22245-6. ISSN 2041-1723. PMC 8024266. PMID 33824307.
- ^ Verhoeven, J.D. (1975) Fundamentals of Physical Metallurgy, Wiley, New York, p. 326
- ^ Greenwood & Earnshaw 1997, pp. 1070–71.
- ^ a b Kohl, Walter H. (1995). Handbook of materials and techniques for vacuum devices. Springer. pp. 164–67. ISBN 1-56396-387-6.
- ^ a b Kuhn, Howard; Medlin, Dana; et al., eds. (2000). ASM Handbook – Mechanical Testing and Evaluation (PDF). Vol. 8. ASM International. p. 275. ISBN 0-87170-389-0. Archived from the original (PDF) on 9 February 2019. Retrieved 22 February 2022.
- ^ «Hardness Conversion Chart». Maryland Metrics. Archived from the original on 18 June 2015. Retrieved 23 May 2010.
- ^ Takaji, Kusakawa; Toshikatsu, Otani (1964). «Properties of Various Pure Irons: Study on pure iron I». Tetsu-to-Hagane. 50 (1): 42–47. doi:10.2355/tetsutohagane1955.50.1_42.
- ^ Raghavan, V. (2004). Materials Science and Engineering. PHI Learning Pvt. Ltd. p. 218. ISBN 81-203-2455-2.
- ^ Martin, John Wilson (2007). Concise encyclopedia of the structure of materials. Elsevier. p. 183. ISBN 978-0-08-045127-5.
- ^ a b Camp, James McIntyre; Francis, Charles Blaine (1920). The Making, Shaping and Treating of Steel. Pittsburgh: Carnegie Steel Company. pp. 173–74. ISBN 1-147-64423-3.
- ^ a b Smith, William F.; Hashemi, Javad (2006), Foundations of Materials Science and Engineering (4th ed.), McGraw-Hill, p. 431, ISBN 0-07-295358-6.
- ^ a b «Classification of Carbon and Low-Alloy Steels». Archived from the original on 2 January 2011. Retrieved 5 January 2008.
- ^ HSLA Steel, 15 November 2002, archived from the original on 30 December 2009, retrieved 11 October 2008.
- ^ Oberg, E.; et al. (1996), «Machinery’s Handbook», New York: Industrial Press (25th ed.), Industrial Press Inc: 440–42, Bibcode:1984msh..book…..R
- ^ Rokni, Sayed H.; Cossairt, J. Donald; Liu, James C. (January 2008). «Radiation Shielding at High-Energy Electron and Proton Accelerators» (PDF). Retrieved 6 August 2016.
- ^ a b c Greenwood & Earnshaw 1997, p. 1076.
- ^ Fürstner, Alois (2016). «Iron Catalysis in Organic Synthesis: A Critical Assessment of What It Takes to Make This Base Metal a Multitasking Champion». ACS Central Science. 2 (11): 778–789. doi:10.1021/acscentsci.6b00272. PMC 5140022. PMID 27981231.
- ^ Bullock, R. Morris; et al. (2020). «Using nature’s blueprint to expand catalysis with Earth-abundant metals». Science. 369 (6505): eabc3183. doi:10.1126/science.abc3183. PMC 7875315. PMID 32792370.
- ^ Kolasinski, Kurt W. (2002). «Where are Heterogenous Reactions Important». Surface science: foundations of catalysis and nanoscience. John Wiley and Sons. pp. 15–16. ISBN 978-0-471-49244-3.
- ^ McKetta, John J. (1989). «Nitrobenzene and Nitrotoluene». Encyclopedia of Chemical Processing and Design: Volume 31 – Natural Gas Liquids and Natural Gasoline to Offshore Process Piping: High Performance Alloys. CRC Press. pp. 166–67. ISBN 978-0-8247-2481-8.
- ^ a b c Wildermuth, Egon; Stark, Hans; Friedrich, Gabriele; Ebenhöch, Franz Ludwig; Kühborth, Brigitte; Silver, Jack; Rituper, Rafael (2000). «Iron Compounds». Ullmann’s Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a14_591. ISBN 3-527-30673-0.
- ^ Stroud, Robert (1933). Diseases of Canaries. Canary Publishers Company. p. 203. ISBN 978-1-4465-4656-7.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Dlouhy, Adrienne C.; Outten, Caryn E. (2013). Banci, Lucia (ed.). Metallomics and the Cell. Metal Ions in Life Sciences. Vol. 12. Springer. pp. 241–78. doi:10.1007/978-94-007-5561-1_8. ISBN 978-94-007-5560-4. PMC 3924584. PMID 23595675. electronic-book ISBN 978-94-007-5561-1
- ^
Yee, Gereon M.; Tolman, William B. (2015). Peter M.H. Kroneck; Martha E. Sosa Torres (eds.). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. Vol. 15. Springer. pp. 131–204. doi:10.1007/978-3-319-12415-5_5. PMID 25707468. - ^ a b c d e f g h i j k l m n o p Greenwood & Earnshaw 1997, pp. 1098–104.
- ^ Lippard, S.J.; Berg, J.M. (1994). Principles of Bioinorganic Chemistry. Mill Valley: University Science Books. ISBN 0-935702-73-3.
- ^ Kikuchi, G.; Yoshida, T.; Noguchi, M. (2005). «Heme oxygenase and heme degradation». Biochemical and Biophysical Research Communications. 338 (1): 558–67. doi:10.1016/j.bbrc.2005.08.020. PMID 16115609.
- ^
Uebe, René; Schüler, Dirk; «The Formation of Iron Biominerals «, pp 159-184 in «Metals, Microbes and Minerals: The Biogeochemical Side of Life» (2021) pp xiv + 341. Walter de Gruyter, Berlin. Editors Kroneck, Peter M.H. and Sosa Torres, Martha. DOI 10.1515/9783110589771-006 - ^ Neilands, J.B. (1995). «Siderophores: structure and function of microbial iron transport compounds». The Journal of Biological Chemistry. 270 (45): 26723–26. doi:10.1074/jbc.270.45.26723. PMID 7592901.
- ^ Neilands, J.B. (1981). «Microbial Iron Compounds». Annual Review of Biochemistry. 50 (1): 715–31. doi:10.1146/annurev.bi.50.070181.003435. PMID 6455965.
- ^ Boukhalfa, Hakim; Crumbliss, Alvin L. (2002). «Chemical aspects of siderophore mediated iron transport». BioMetals. 15 (4): 325–39. doi:10.1023/A:1020218608266. PMID 12405526. S2CID 19697776.
- ^ Nanami, M.; Ookawara, T.; Otaki, Y.; Ito, K.; Moriguchi, R.; Miyagawa, K.; Hasuike, Y.; Izumi, M.; Eguchi, H.; Suzuki, K.; Nakanishi, T. (2005). «Tumor necrosis factor-α-induced iron sequestration and oxidative stress in human endothelial cells». Arteriosclerosis, Thrombosis, and Vascular Biology. 25 (12): 2495–501. doi:10.1161/01.ATV.0000190610.63878.20. PMID 16224057.
- ^ Rouault, Tracey A. (2003). «How Mammals Acquire and Distribute Iron Needed for Oxygen-Based Metabolism». PLOS Biology. 1 (3): e9. doi:10.1371/journal.pbio.0000079. PMC 300689. PMID 14691550.
- ^ Boon EM, Downs A, Marcey D. «Proposed Mechanism of Catalase». Catalase: H2O2: H2O2 Oxidoreductase: Catalase Structural Tutorial. Retrieved 11 February 2007.
- ^ Boyington JC, Gaffney BJ, Amzel LM (1993). «The three-dimensional structure of an arachidonic acid 15-lipoxygenase». Science. 260 (5113): 1482–86. Bibcode:1993Sci…260.1482B. doi:10.1126/science.8502991. PMID 8502991.
- ^ Gray, N.K.; Hentze, M.W. (August 1994). «Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs». EMBO J. 13 (16): 3882–91. doi:10.1002/j.1460-2075.1994.tb06699.x. PMC 395301. PMID 8070415.
- ^ Gregory B. Vásquez; Xinhua Ji; Clara Fronticelli; Gary L. Gilliland (1998). «Human Carboxyhemoglobin at 2.2 Å Resolution: Structure and Solvent Comparisons of R-State, R2-State and T-State Hemoglobins». Acta Crystallogr. D. 54 (3): 355–66. doi:10.1107/S0907444997012250. PMID 9761903.
- ^ Sanderson, K (2017). «Mussels’ iron grip inspires strong and stretchy polymer». Chemical & Engineering News. American Chemical Society. 95 (44): 8. doi:10.1021/cen-09544-notw3. Retrieved 2 November 2017.
- ^ Food Standards Agency – Eat well, be well – Iron deficiency Archived 8 August 2006 at the Wayback Machine. Eatwell.gov.uk (5 March 2012). Retrieved on 27 June 2012.
- ^ Hoppe, M.; Hulthén, L.; Hallberg, L. (2005). «The relative bioavailability in humans of elemental iron powders for use in food fortification». European Journal of Nutrition. 45 (1): 37–44. doi:10.1007/s00394-005-0560-0. PMID 15864409. S2CID 42983904.
- ^ Pineda, O.; Ashmead, H. D. (2001). «Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate». Nutrition. 17 (5): 381–4. doi:10.1016/S0899-9007(01)00519-6. PMID 11377130.
- ^ Ashmead, H. DeWayne (1989). Conversations on Chelation and Mineral Nutrition. Keats Publishing. ISBN 0-87983-501-X.
- ^ Institute of Medicine (US) Panel on Micronutrients (2001). «Iron» (PDF). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Iron. National Academy Press. pp. 290–393. ISBN 0-309-07279-4. PMID 25057538. Archived from the original (PDF) on 9 September 2017. Retrieved 9 March 2017.
- ^ «Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies» (PDF). European Food Safety Authority. 2017.
- ^ «Tolerable Upper Intake Levels For Vitamins And Minerals» (PDF). European Food Safety Authority. 2006.
- ^ «Iron Deficiency Anemia». MediResource. Retrieved 17 December 2008.
- ^ Milman, N. (1996). «Serum ferritin in Danes: studies of iron status from infancy to old age, during blood donation and pregnancy». International Journal of Hematology. 63 (2): 103–35. doi:10.1016/0925-5710(95)00426-2. PMID 8867722.
- ^ «Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982» (PDF).
- ^ «Daily Value Reference of the Dietary Supplement Label Database (DSLD)». Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- ^ Centers for Disease Control and Prevention (2002). «Iron deficiency – United States, 1999–2000». MMWR. 51 (40): 897–99. PMID 12418542.
- ^ Hider, Robert C.; Kong, Xiaole (2013). «Chapter 8. Iron: Effect of Overload and Deficiency». In Astrid Sigel, Helmut Sigel and Roland K.O. Sigel (ed.). Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. Vol. 13. Springer. pp. 229–94. doi:10.1007/978-94-007-7500-8_8. PMID 24470094.
- ^ Dlouhy, Adrienne C.; Outten, Caryn E. (2013). «Chapter 8.4 Iron Uptake, Trafficking and Storage». In Banci, Lucia (ed.). Metallomics and the Cell. Metal Ions in Life Sciences. Vol. 12. Springer. pp. 241–78. doi:10.1007/978-94-007-5561-1_8. ISBN 978-94-007-5560-4. PMC 3924584. PMID 23595675. electronic-book ISBN 978-94-007-5561-1
- ^ CDC Centers for Disease Control and Prevention (3 April 1998). «Recommendations to Prevent and Control Iron Deficiency in the United States». Morbidity and Mortality Weekly Report. 47 (RR3): 1. Retrieved 12 August 2014.
- ^ Centers for Disease Control and Prevention. «Iron and Iron Deficiency». Retrieved 12 August 2014.
- ^ Ramzi S. Cotran; Vinay Kumar; Tucker Collins; Stanley Leonard Robbins (1999). Robbins pathologic basis of disease. Saunders. ISBN 978-0-7216-7335-6. Retrieved 27 June 2012.
- ^ Ganz T (August 2003). «Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation». Blood. 102 (3): 783–8. doi:10.1182/blood-2003-03-0672. PMID 12663437. S2CID 28909635.
- ^ Durupt, S.; Durieu, I.; Nové-Josserand, R.; Bencharif, L.; Rousset, H.; Vital Durand, D. (2000). «Hereditary hemochromatosis». Rev Méd Interne. 21 (11): 961–71. doi:10.1016/S0248-8663(00)00252-6. PMID 11109593.
- ^ a b Cheney, K.; Gumbiner, C.; Benson, B.; Tenenbein, M. (1995). «Survival after a severe iron poisoning treated with intermittent infusions of deferoxamine». J Toxicol Clin Toxicol. 33 (1): 61–66. doi:10.3109/15563659509020217. PMID 7837315.
- ^ a b «Toxicity, Iron». Medscape. Retrieved 23 May 2010.
- ^ Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals (PDF), Food and Nutrition Board, Institute of Medicine, National Academies, 2004, archived from the original (PDF) on 14 March 2013, retrieved 9 June 2009
- ^ Tenenbein, M. (1996). «Benefits of parenteral deferoxamine for acute iron poisoning». J Toxicol Clin Toxicol. 34 (5): 485–89. doi:10.3109/15563659609028005. PMID 8800185.
- ^ Wu H, Wu T, Xu X, Wang J, Wang J (May 2011). «Iron toxicity in mice with collagenase-induced intracerebral hemorrhage». J Cereb Blood Flow Metab. 31 (5): 1243–50. doi:10.1038/jcbfm.2010.209. PMC 3099628. PMID 21102602.
- ^ Robberecht, Harry; et al. (2020). «Magnesium, Iron, Zinc, Copper and Selenium Status in Attention-Deficit/Hyperactivity Disorder (ADHD)». Molecules. 25 (19): 4440. doi:10.3390/molecules25194440. PMC 7583976. PMID 32992575.
- ^ Soto-Insuga, V; et al. (2013). «[Role of iron in the treatment of attention deficit-hyperactivity disorder]». An Pediatr (Barc). 79 (4): 230–235. doi:10.1016/j.anpedi.2013.02.008. PMID 23582950.
- ^ Parisi, Pasquale; Villa, Maria Pia; Donfrancesco, Renato; Miano, Silvia; Paolino, Maria Chiara; Cortese, Samuele (August 2012). «Could treatment of iron deficiency both improve ADHD and reduce cardiovascular risk during treatment with ADHD drugs?». Medical Hypotheses. 79 (2): 246–249. doi:10.1016/j.mehy.2012.04.049. PMID 22632845.
- ^ Donfrancesco, Renato; Parisi, Pasquale; Vanacore, Nicola; Martines, Francesca; Sargentini, Vittorio; Cortese, Samuele (May 2013). «Iron and ADHD: Time to Move Beyond Serum Ferritin Levels». Journal of Attention Disorders. 17 (4): 347–357. doi:10.1177/1087054711430712. ISSN 1087-0547. PMID 22290693. S2CID 22445593.
- ^ Thévenod, Frank (2018). «Chapter 15. Iron and Its Role in Cancer Defense: A Double-Edged Sword». In Sigel, Astrid; Sigel, Helmut; Freisinger, Eva; Sigel, Roland K. O. (eds.). Metallo-Drugs: Development and Action of Anticancer Agents. Metal Ions in Life Sciences. Vol. 18. Berlin: de Gruyter GmbH. pp. 437–67. doi:10.1515/9783110470734-021. PMID 29394034.
- ^ a b Beguin, Y; Aapro, M; Ludwig, H; Mizzen, L; Osterborg, A (2014). «Epidemiological and nonclinical studies investigating effects of iron in carcinogenesis—a critical review». Critical Reviews in Oncology/Hematology. 89 (1): 1–15. doi:10.1016/j.critrevonc.2013.10.008. PMID 24275533.
- ^ Morel, F.M.M., Hudson, R.J.M., & Price, N.M. (1991). Limitation of productivity by trace metals in the sea. Limnology and Oceanography, 36(8), 1742-1755. doi:10.4319/lo.1991.36.8.1742
- ^ Brezezinski, M.A., Baines, S.B., Balch, W.M., Beucher, C.P., Chai, F., Dugdale, R.C., Krause, J.W., Landry, M.R., Marchi, A., Measures, C.I., Nelson, D.M., Parker, A.E., Poulton, A.J., Selph, K.E., Strutton, P.G., Taylor, A.G., & Twining, B.S.(2011). Co-limitation of diatoms by iron and silicic acid in the equatorial Pacific. Deep-Sea Research Part II: Topical Studies in Oceanography, 58(3-4), 493-511. doi:10.1016/j.dsr2.2010.08.005
- ^ Field, E. K., Kato, S., Findlay, A. J., MacDonald, D. J., Chiu, B. K., Luther, G. W., & Chan, C. S. (2016). Planktonic marine iron oxidizers drive iron mineralization under low-oxygen conditions. Geobiology, 14(5), 499-508. doi:10.1111/gbi.12189
- ^ Wells, M.L., Price, N.M., & Bruland, K.W. (1995). Iron chemistry in seawater and its relationship to phytoplankton: a workshop report. Marine Chemistry, 48(2), 157-182. doi:10.1016/0304-4203(94)00055-I
- ^ Lannuzel, D., Vancoppenolle, M., van der Merwe, P., de Jong, J., Meiners, K.M., Grotti, M., Nishioska, J., & Schoemann. (2016). Iron in sea ice: Review and new insights. Elementa: Science of the Anthropocene, 4 000130.
doi:10.12952/journal.elementa.000130 - ^ Raiswell, R. 2011. Iron Transport from the Continents to the Open Ocean: The Aging–Rejuvenation Cycle. Elements, 7(2), 101–106. doi:10.2113/gselements.7.2.101
- ^ Tagliabue, A., Bopp, L., Aumont,O., & Arrigo, K.R. (2009). Influence of light and temperature on the marine iron cycle: From theoretical to global modeling. Global Biogeochemical Cycles, 23.
doi:10.1029/2008GB003214
Bibliography
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Weeks, Mary Elvira; Leichester, Henry M. (1968). «Elements known to the ancients». Discovery of the elements. Easton, PA: Journal of Chemical Education. pp. 29–40. ISBN 0-7661-3872-0. LCCN 68-15217.
Further reading
- H.R. Schubert, History of the British Iron and Steel Industry … to 1775 AD (Routledge, London, 1957)
- R.F. Tylecote, History of Metallurgy (Institute of Materials, London 1992).
- R.F. Tylecote, «Iron in the Industrial Revolution» in J. Day and R.F. Tylecote, The Industrial Revolution in Metals (Institute of Materials 1991), 200–60.
External links
Wikiquote has quotations related to Iron.
Look up iron in Wiktionary, the free dictionary.
Wikimedia Commons has media related to Iron.
- It’s Elemental – Iron
- Iron at The Periodic Table of Videos (University of Nottingham)
- Metallurgy for the non-Metallurgist
- Iron by J.B. Calvert
This article is about the metallic element. For other uses, see Iron (disambiguation).
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Iron | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allotropes | see Allotropes of iron | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | lustrous metallic with a grayish tinge | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Fe) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Iron in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d6 4s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 14, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1811 K (1538 °C, 2800 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3134 K (2862 °C, 5182 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.874 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 6.98 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.81 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 340 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.10 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −4, −2, −1, 0, +1,[2] +2, +3, +4, +5,[3] +6, +7[4] (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 126 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | Low spin: 132±3 pm High spin: 152±6 pm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 194 [1] pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of iron |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc)
a=286.65 pm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc)
between 1185–1667 K; a=364.680 pm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 5120 m/s (at r.t.) (electrolytic) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 11.8 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 80.4 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 96.1 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Curie point | 1043 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | ferromagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 211 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 82 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 170 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 608 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 200–1180 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7439-89-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | before 5000 BC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | «Fe»: from Latin ferrum | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of iron
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Iron () is a chemical element with symbol Fe (from Latin: ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth’s outer and inner core. It is the fourth most common element in the Earth’s crust.
In its metallic state, iron is rare in the Earth’s crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth’s crust, although extracting usable metal from them requires kilns or furnaces capable of reaching 1,500 °C (2,730 °F) or higher, about 500 °C (932 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, because of their mechanical properties and low cost. The iron and steel industry is thus very important economically, and iron is the cheapest metal, with a price of a few dollars per kilogram or per pound (see Metal#uses).
Pristine and smooth pure iron surfaces are mirror-like silvery-gray. However, iron reacts readily with oxygen and water to give brown to black hydrated iron oxides, commonly known as rust. Unlike the oxides of some other metals that form passivating layers, rust occupies more volume than the metal and thus flakes off, exposing more fresh surfaces for corrosion. Although iron readily reacts, high purity iron, called electrolytic iron, has better corrosion resistance.
The body of an adult human contains about 4 grams (0.005% body weight) of iron, mostly in hemoglobin and myoglobin. These two proteins play essential roles in vertebrate metabolism, respectively oxygen transport by blood and oxygen storage in muscles. To maintain the necessary levels, human iron metabolism requires a minimum of iron in the diet. Iron is also the metal at the active site of many important redox enzymes dealing with cellular respiration and oxidation and reduction in plants and animals.[5]
Chemically, the most common oxidation states of iron are iron(II) and iron(III). Iron shares many properties of other transition metals, including the other group 8 elements, ruthenium and osmium. Iron forms compounds in a wide range of oxidation states, −2 to +7. Iron also forms many coordination compounds; some of them, such as ferrocene, ferrioxalate, and Prussian blue, have substantial industrial, medical, or research applications.
Characteristics
Allotropes
Molar volume vs. pressure for α iron at room temperature
At least four allotropes of iron (differing atom arrangements in the solid) are known, conventionally denoted α, γ, δ, and ε.
The first three forms are observed at ordinary pressures. As molten iron cools past its freezing point of 1538 °C, it crystallizes into its δ allotrope, which has a body-centered cubic (bcc) crystal structure. As it cools further to 1394 °C, it changes to its γ-iron allotrope, a face-centered cubic (fcc) crystal structure, or austenite. At 912 °C and below, the crystal structure again becomes the bcc α-iron allotrope.[6]
The physical properties of iron at very high pressures and temperatures have also been studied extensively,[7][8] because of their relevance to theories about the cores of the Earth and other planets. Above approximately 10 GPa and temperatures of a few hundred kelvin or less, α-iron changes into another hexagonal close-packed (hcp) structure, which is also known as ε-iron. The higher-temperature γ-phase also changes into ε-iron, but does so at higher pressure.
Some controversial experimental evidence exists for a stable β phase at pressures above 50 GPa and temperatures of at least 1500 K. It is supposed to have an orthorhombic or a double hcp structure.[9] (Confusingly, the term «β-iron» is sometimes also used to refer to α-iron above its Curie point, when it changes from being ferromagnetic to paramagnetic, even though its crystal structure has not changed.[6])
The inner core of the Earth is generally presumed to consist of an iron-nickel alloy with ε (or β) structure.[10]
Melting and boiling points
The melting and boiling points of iron, along with its enthalpy of atomization, are lower than those of the earlier 3d elements from scandium to chromium, showing the lessened contribution of the 3d electrons to metallic bonding as they are attracted more and more into the inert core by the nucleus;[11] however, they are higher than the values for the previous element manganese because that element has a half-filled 3d sub-shell and consequently its d-electrons are not easily delocalized. This same trend appears for ruthenium but not osmium.[12]
The melting point of iron is experimentally well defined for pressures less than 50 GPa. For greater pressures, published data (as of 2007) still varies by tens of gigapascals and over a thousand kelvin.[13]
Magnetic properties
Magnetization curves of 9 ferromagnetic materials, showing saturation. 1. Sheet steel, 2. Silicon steel, 3. Cast steel, 4. Tungsten steel, 5. Magnet steel, 6. Cast iron, 7. Nickel, 8. Cobalt, 9. Magnetite[14]
Below its Curie point of 770 °C (1,420 °F; 1,040 K), α-iron changes from paramagnetic to ferromagnetic: the spins of the two unpaired electrons in each atom generally align with the spins of its neighbors, creating an overall magnetic field.[15] This happens because the orbitals of those two electrons (dz2 and dx2 −. y2) do not point toward neighboring atoms in the lattice, and therefore are not involved in metallic bonding.[6]
In the absence of an external source of magnetic field, the atoms get spontaneously partitioned into magnetic domains, about 10 micrometers across,[16] such that the atoms in each domain have parallel spins, but some domains have other orientations. Thus a macroscopic piece of iron will have a nearly zero overall magnetic field.
Application of an external magnetic field causes the domains that are magnetized in the same general direction to grow at the expense of adjacent ones that point in other directions, reinforcing the external field. This effect is exploited in devices that need to channel magnetic fields to fulfill design function, such as electrical transformers, magnetic recording heads, and electric motors. Impurities, lattice defects, or grain and particle boundaries can «pin» the domains in the new positions, so that the effect persists even after the external field is removed – thus turning the iron object into a (permanent) magnet.[15]
Similar behavior is exhibited by some iron compounds, such as the ferrites including the mineral magnetite, a crystalline form of the mixed iron(II,III) oxide Fe3O4 (although the atomic-scale mechanism, ferrimagnetism, is somewhat different). Pieces of magnetite with natural permanent magnetization (lodestones) provided the earliest compasses for navigation. Particles of magnetite were extensively used in magnetic recording media such as core memories, magnetic tapes, floppies, and disks, until they were replaced by cobalt-based materials.
Isotopes
Iron has four stable isotopes: 54Fe (5.845% of natural iron), 56Fe (91.754%), 57Fe (2.119%) and 58Fe (0.282%). 24 artificial isotopes have also been created. Of these stable isotopes, only 57Fe has a nuclear spin (−1⁄2). The nuclide 54Fe theoretically can undergo double electron capture to 54Cr, but the process has never been observed and only a lower limit on the half-life of 3.1×1022 years has been established.[17]
60Fe is an extinct radionuclide of long half-life (2.6 million years).[18] It is not found on Earth, but its ultimate decay product is its granddaughter, the stable nuclide 60Ni.[17] Much of the past work on isotopic composition of iron has focused on the nucleosynthesis of 60Fe through studies of meteorites and ore formation. In the last decade, advances in mass spectrometry have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotopes of iron. Much of this work is driven by the Earth and planetary science communities, although applications to biological and industrial systems are emerging.[19]
In phases of the meteorites Semarkona and Chervony Kut, a correlation between the concentration of 60Ni, the granddaughter of 60Fe, and the abundance of the stable iron isotopes provided evidence for the existence of 60Fe at the time of formation of the Solar System. Possibly the energy released by the decay of 60Fe, along with that released by 26Al, contributed to the remelting and differentiation of asteroids after their formation 4.6 billion years ago. The abundance of 60Ni present in extraterrestrial material may bring further insight into the origin and early history of the Solar System.[20]
The most abundant iron isotope 56Fe is of particular interest to nuclear scientists because it represents the most common endpoint of nucleosynthesis.[21] Since 56Ni (14 alpha particles) is easily produced from lighter nuclei in the alpha process in nuclear reactions in supernovae (see silicon burning process), it is the endpoint of fusion chains inside extremely massive stars, since addition of another alpha particle, resulting in 60Zn, requires a great deal more energy. This 56Ni, which has a half-life of about 6 days, is created in quantity in these stars, but soon decays by two successive positron emissions within supernova decay products in the supernova remnant gas cloud, first to radioactive 56Co, and then to stable 56Fe. As such, iron is the most abundant element in the core of red giants, and is the most abundant metal in iron meteorites and in the dense metal cores of planets such as Earth.[22] It is also very common in the universe, relative to other stable metals of approximately the same atomic weight.[22][23] Iron is the sixth most abundant element in the universe, and the most common refractory element.[24]
Although a further tiny energy gain could be extracted by synthesizing 62Ni, which has a marginally higher binding energy than 56Fe, conditions in stars are unsuitable for this process. Element production in supernovas greatly favor iron over nickel, and in any case, 56Fe still has a lower mass per nucleon than 62Ni due to its higher fraction of lighter protons.[25] Hence, elements heavier than iron require a supernova for their formation, involving rapid neutron capture by starting 56Fe nuclei.[22]
In the far future of the universe, assuming that proton decay does not occur, cold fusion occurring via quantum tunnelling would cause the light nuclei in ordinary matter to fuse into 56Fe nuclei. Fission and alpha-particle emission would then make heavy nuclei decay into iron, converting all stellar-mass objects to cold spheres of pure iron.[26]
Origin and occurrence in nature
Cosmogenesis
Iron’s abundance in rocky planets like Earth is due to its abundant production during the runaway fusion and explosion of type Ia supernovae, which scatters the iron into space.[27][28]
Metallic iron
A polished and chemically etched piece of an iron meteorite, believed to be similar in composition to the Earth’s metallic core, showing individual crystals of the iron-nickel alloy (Widmanstatten pattern)
Metallic or native iron is rarely found on the surface of the Earth because it tends to oxidize. However, both the Earth’s inner and outer core, that account for 35% of the mass of the whole Earth, are believed to consist largely of an iron alloy, possibly with nickel. Electric currents in the liquid outer core are believed to be the origin of the Earth’s magnetic field. The other terrestrial planets (Mercury, Venus, and Mars) as well as the Moon are believed to have a metallic core consisting mostly of iron. The M-type asteroids are also believed to be partly or mostly made of metallic iron alloy.
The rare iron meteorites are the main form of natural metallic iron on the Earth’s surface. Items made of cold-worked meteoritic iron have been found in various archaeological sites dating from a time when iron smelting had not yet been developed; and the Inuit in Greenland have been reported to use iron from the Cape York meteorite for tools and hunting weapons.[29] About 1 in 20 meteorites consist of the unique iron-nickel minerals taenite (35–80% iron) and kamacite (90–95% iron).[30] Native iron is also rarely found in basalts that have formed from magmas that have come into contact with carbon-rich sedimentary rocks, which have reduced the oxygen fugacity sufficiently for iron to crystallize. This is known as Telluric iron and is described from a few localities, such as Disko Island in West Greenland, Yakutia in Russia and Bühl in Germany.[31]
Mantle minerals
Ferropericlase (Mg,Fe)O, a solid solution of periclase (MgO) and wüstite (FeO), makes up about 20% of the volume of the lower mantle of the Earth, which makes it the second most abundant mineral phase in that region after silicate perovskite (Mg,Fe)SiO3; it also is the major host for iron in the lower mantle.[32] At the bottom of the transition zone of the mantle, the reaction γ-(Mg,Fe)2[SiO4] ↔ (Mg,Fe)[SiO3] + (Mg,Fe)O transforms γ-olivine into a mixture of silicate perovskite and ferropericlase and vice versa. In the literature, this mineral phase of the lower mantle is also often called magnesiowüstite.[33] Silicate perovskite may form up to 93% of the lower mantle,[34] and the magnesium iron form, (Mg,Fe)SiO3, is considered to be the most abundant mineral in the Earth, making up 38% of its volume.[35]
Earth’s crust
While iron is the most abundant element on Earth, most of this iron is concentrated in the inner and outer cores.[36][37] The fraction of iron that is in Earth’s crust only amounts to about 5% of the overall mass of the crust and is thus only the fourth most abundant element in that layer (after oxygen, silicon, and aluminium).[38]
Most of the iron in the crust is combined with various other elements to form many iron minerals. An important class is the iron oxide minerals such as hematite (Fe2O3), magnetite (Fe3O4), and siderite (FeCO3), which are the major ores of iron. Many igneous rocks also contain the sulfide minerals pyrrhotite and pentlandite.[39][40] During weathering, iron tends to leach from sulfide deposits as the sulfate and from silicate deposits as the bicarbonate. Both of these are oxidized in aqueous solution and precipitate in even mildly elevated pH as iron(III) oxide.[41]
Banded iron formation in McKinley Park, Minnesota.
Large deposits of iron are banded iron formations, a type of rock consisting of repeated thin layers of iron oxides alternating with bands of iron-poor shale and chert. The banded iron formations were laid down in the time between 3,700 million years ago and 1,800 million years ago.[42][43]
Materials containing finely ground iron(III) oxides or oxide-hydroxides, such as ochre, have been used as yellow, red, and brown pigments since pre-historical times. They contribute as well to the color of various rocks and clays, including entire geological formations like the Painted Hills in Oregon and the Buntsandstein («colored sandstone», British Bunter).[44] Through Eisensandstein (a jurassic ‘iron sandstone’, e.g. from Donzdorf in Germany)[45] and Bath stone in the UK, iron compounds are responsible for the yellowish color of many historical buildings and sculptures.[46] The proverbial red color of the surface of Mars is derived from an iron oxide-rich regolith.[47]
Significant amounts of iron occur in the iron sulfide mineral pyrite (FeS2), but it is difficult to extract iron from it and it is therefore not exploited.[48] In fact, iron is so common that production generally focuses only on ores with very high quantities of it.[49]
According to the International Resource Panel’s Metal Stocks in Society report, the global stock of iron in use in society is 2,200 kg per capita. More-developed countries differ in this respect from less-developed countries (7,000–14,000 vs 2,000 kg per capita).[50]
Oceans
Ocean science demonstrated the role of the iron in the ancient seas in both marine biota and climate.[51]
Chemistry and compounds
| Oxidation state |
Representative compound |
|---|---|
| −2 (d10) | Disodium tetracarbonylferrate (Collman’s reagent) |
| −1 (d9) | Fe 2(CO)2− 8 |
| 0 (d8) | Iron pentacarbonyl |
| 1 (d7) | Cyclopentadienyliron dicarbonyl dimer («Fp2«) |
| 2 (d6) | Ferrous sulfate, ferrocene |
| 3 (d5) | Ferric chloride, ferrocenium tetrafluoroborate |
| 4 (d4) | Fe(diars) 2Cl2+ 2, Ferryl tetrafluoroborate |
| 5 (d3) | FeO3− 4 |
| 6 (d2) | Potassium ferrate |
| 7 (d1) | [FeO4]– (matrix isolation, 4K) |
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s.[52] Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity.[53] Its 26 electrons are arranged in the configuration [Ar]3d64s2, of which the 3d and 4s electrons are relatively close in energy, and thus a number of electrons can be ionized.[12]
Iron forms compounds mainly in the oxidation states +2 (iron(II), «ferrous») and +3 (iron(III), «ferric»). Iron also occurs in higher oxidation states, e.g., the purple potassium ferrate (K2FeO4), which contains iron in its +6 oxidation state. The anion [FeO4]– with iron in its +7 oxidation state, along with an iron(V)-peroxo isomer, has been detected by infrared spectroscopy at 4 K after cocondensation of laser-ablated Fe atoms with a mixture of O2/Ar.[54] Iron(IV) is a common intermediate in many biochemical oxidation reactions.[55][56] Numerous organoiron compounds contain formal oxidation states of +1, 0, −1, or even −2. The oxidation states and other bonding properties are often assessed using the technique of Mössbauer spectroscopy.[57] Many mixed valence compounds contain both iron(II) and iron(III) centers, such as magnetite and Prussian blue (Fe4(Fe[CN]6)3).[56] The latter is used as the traditional «blue» in blueprints.[58]
Iron is the first of the transition metals that cannot reach its group oxidation state of +8, although its heavier congeners ruthenium and osmium can, with ruthenium having more difficulty than osmium.[6] Ruthenium exhibits an aqueous cationic chemistry in its low oxidation states similar to that of iron, but osmium does not, favoring high oxidation states in which it forms anionic complexes.[6] In the second half of the 3d transition series, vertical similarities down the groups compete with the horizontal similarities of iron with its neighbors cobalt and nickel in the periodic table, which are also ferromagnetic at room temperature and share similar chemistry. As such, iron, cobalt, and nickel are sometimes grouped together as the iron triad.[53]
Unlike many other metals, iron does not form amalgams with mercury. As a result, mercury is traded in standardized 76 pound flasks (34 kg) made of iron.[59]
Iron is by far the most reactive element in its group; it is pyrophoric when finely divided and dissolves easily in dilute acids, giving Fe2+. However, it does not react with concentrated nitric acid and other oxidizing acids due to the formation of an impervious oxide layer, which can nevertheless react with hydrochloric acid.[6] High purity iron, called electrolytic iron, is considered to be resistant to rust, due to its oxide layer.
Binary compounds
Oxides and sulfides
Ferrous or iron(II) oxide,
FeO
Ferric or iron(III) oxide
Fe2O3
Ferrosoferric or iron(II,III) oxide
Fe3O4
Iron forms various oxide and hydroxide compounds; the most common are iron(II,III) oxide (Fe3O4), and iron(III) oxide (Fe2O3). Iron(II) oxide also exists, though it is unstable at room temperature. Despite their names, they are actually all non-stoichiometric compounds whose compositions may vary.[60] These oxides are the principal ores for the production of iron (see bloomery and blast furnace). They are also used in the production of ferrites, useful magnetic storage media in computers, and pigments. The best known sulfide is iron pyrite (FeS2), also known as fool’s gold owing to its golden luster.[56] It is not an iron(IV) compound, but is actually an iron(II) polysulfide containing Fe2+ and S2−
2 ions in a distorted sodium chloride structure.[60]
Halides
Hydrated iron(III) chloride (ferric chloride)
The binary ferrous and ferric halides are well-known. The ferrous halides typically arise from treating iron metal with the corresponding hydrohalic acid to give the corresponding hydrated salts.[56]
- Fe + 2 HX → FeX2 + H2 (X = F, Cl, Br, I)
Iron reacts with fluorine, chlorine, and bromine to give the corresponding ferric halides, ferric chloride being the most common.[61]
- 2 Fe + 3 X2 → 2 FeX3 (X = F, Cl, Br)
Ferric iodide is an exception, being thermodynamically unstable due to the oxidizing power of Fe3+ and the high reducing power of I−:[61]
- 2 I− + 2 Fe3+ → I2 + 2 Fe2+ (E0 = +0.23 V)
Ferric iodide, a black solid, is not stable in ordinary conditions, but can be prepared through the reaction of iron pentacarbonyl with iodine and carbon monoxide in the presence of hexane and light at the temperature of −20 °C, with oxygen and water excluded.[61]Complexes of ferric iodide with some soft bases are known to be stable compounds.[62][63]
Solution chemistry
Comparison of colors of solutions of ferrate (left) and permanganate (right)
The standard reduction potentials in acidic aqueous solution for some common iron ions are given below:[6]
| [Fe(H2O)6]2+ + 2 e− | ⇌ Fe | E0 = −0.447 V |
| [Fe(H2O)6]3+ + e− | ⇌ [Fe(H2O)6]2+ | E0 = +0.77 V |
| FeO2− 4 + 8 H3O+ + 3 e− |
⇌ [Fe(H2O)6]3+ + 6 H2O | E0 = +2.20 V |
The red-purple tetrahedral ferrate(VI) anion is such a strong oxidizing agent that it oxidizes ammonia to nitrogen (N2) and water to oxygen[61]
- 4 FeO2−
4 + 34 H
2O → 4 [Fe(H2O)6]3+ + 20 OH−
+ 3 O2
The pale-violet hexaquo complex [Fe(H2O)6]3+ is an acid such that above above pH 0 it is fully hydrolyzed:[64]
| [Fe(H2O)6]3+ | ⇌ [Fe(H2O)5(OH)]2+ + H+ | K = 10−3.05 mol dm−3 |
| [Fe(H2O)5(OH)]2+ | ⇌ [Fe(H2O)4(OH)2]+ + H+ | K = 10−3.26 mol dm−3 |
| 2[Fe(H2O)6]3+ | ⇌ [Fe(H2O)4(OH)]4+2 + 2H+ + 2H2O | K = 10−2.91 mol dm−3 |
As pH rises above 0 the above yellow hydrolyzed species form and as it rises above 2–3, reddish-brown hydrous iron(III) oxide precipitates out of solution. Although Fe3+ has a d5 configuration, its absorption spectrum is not like that of Mn2+ with its weak, spin-forbidden d–d bands, because Fe3+ has higher positive charge and is more polarizing, lowering the energy of its ligand-to-metal charge transfer absorptions. Thus, all the above complexes are rather strongly colored, with the single exception of the hexaquo ion – and even that has a spectrum dominated by charge transfer in the near ultraviolet region.[64] On the other hand, the pale green iron(II) hexaquo ion [Fe(H2O)6]2+ does not undergo appreciable hydrolysis. Carbon dioxide is not evolved when carbonate anions are added, which instead results in white iron(II) carbonate being precipitated out. In excess carbon dioxide this forms the slightly soluble bicarbonate, which occurs commonly in groundwater, but it oxidises quickly in air to form iron(III) oxide that accounts for the brown deposits present in a sizeable number of streams.[65]
Coordination compounds
Due to its electronic structure, iron has a very large coordination and organometallic chemistry.
Many coordination compounds of iron are known. A typical six-coordinate anion is hexachloroferrate(III), [FeCl6]3−, found in the mixed salt tetrakis(methylammonium) hexachloroferrate(III) chloride.[66][67] Complexes with multiple bidentate ligands have geometric isomers. For example, the trans-chlorohydridobis(bis-1,2-(diphenylphosphino)ethane)iron(II) complex is used as a starting material for compounds with the Fe(dppe)2 moiety.[68][69] The ferrioxalate ion with three oxalate ligands (shown at right) displays helical chirality with its two non-superposable geometries labelled Λ (lambda) for the left-handed screw axis and Δ (delta) for the right-handed screw axis, in line with IUPAC conventions.[64] Potassium ferrioxalate is used in chemical actinometry and along with its sodium salt undergoes photoreduction applied in old-style photographic processes. The dihydrate of iron(II) oxalate has a polymeric structure with co-planar oxalate ions bridging between iron centres with the water of crystallisation located forming the caps of each octahedron, as illustrated below.[70]
Crystal structure of iron(II) oxalate dihydrate, showing iron (gray), oxygen (red), carbon (black), and hydrogen (white) atoms.
Blood-red positive thiocyanate test for iron(III)
Iron(III) complexes are quite similar to those of chromium(III) with the exception of iron(III)’s preference for O-donor instead of N-donor ligands. The latter tend to be rather more unstable than iron(II) complexes and often dissociate in water. Many Fe–O complexes show intense colors and are used as tests for phenols or enols. For example, in the ferric chloride test, used to determine the presence of phenols, iron(III) chloride reacts with a phenol to form a deep violet complex:[64]
- 3 ArOH + FeCl3 → Fe(OAr)3 + 3 HCl (Ar = aryl)
Among the halide and pseudohalide complexes, fluoro complexes of iron(III) are the most stable, with the colorless [FeF5(H2O)]2− being the most stable in aqueous solution. Chloro complexes are less stable and favor tetrahedral coordination as in [FeCl4]−; [FeBr4]− and [FeI4]− are reduced easily to iron(II). Thiocyanate is a common test for the presence of iron(III) as it forms the blood-red [Fe(SCN)(H2O)5]2+. Like manganese(II), most iron(III) complexes are high-spin, the exceptions being those with ligands that are high in the spectrochemical series such as cyanide. An example of a low-spin iron(III) complex is [Fe(CN)6]3−. Iron shows a great variety of electronic spin states, including every possible spin quantum number value for a d-block element from 0 (diamagnetic) to 5⁄2 (5 unpaired electrons). This value is always half the number of unpaired electrons. Complexes with zero to two unpaired electrons are considered low-spin and those with four or five are considered high-spin.[60]
Iron(II) complexes are less stable than iron(III) complexes but the preference for O-donor ligands is less marked, so that for example [Fe(NH3)6]2+ is known while [Fe(NH3)6]3+ is not. They have a tendency to be oxidized to iron(III) but this can be moderated by low pH and the specific ligands used.[65]
Organometallic compounds
Organoiron chemistry is the study of organometallic compounds of iron, where carbon atoms are covalently bound to the metal atom. They are many and varied, including cyanide complexes, carbonyl complexes, sandwich and half-sandwich compounds.
Prussian blue or «ferric ferrocyanide», Fe4[Fe(CN)6]3, is an old and well-known iron-cyanide complex, extensively used as pigment and in several other applications. Its formation can be used as a simple wet chemistry test to distinguish between aqueous solutions of Fe2+ and Fe3+ as they react (respectively) with potassium ferricyanide and potassium ferrocyanide to form Prussian blue.[56]
Another old example of an organoiron compound is iron pentacarbonyl, Fe(CO)5, in which a neutral iron atom is bound to the carbon atoms of five carbon monoxide molecules. The compound can be used to make carbonyl iron powder, a highly reactive form of metallic iron. Thermolysis of iron pentacarbonyl gives triiron dodecacarbonyl, Fe3(CO)12, a complex with a cluster of three iron atoms at its core. Collman’s reagent, disodium tetracarbonylferrate, is a useful reagent for organic chemistry; it contains iron in the −2 oxidation state. Cyclopentadienyliron dicarbonyl dimer contains iron in the rare +1 oxidation state.[71]
Structural formula of ferrocene and a powdered sample
A landmark in this field was the discovery in 1951 of the remarkably stable sandwich compound ferrocene Fe(C5H5)2, by Pauson and Kealy[72] and independently by Miller and colleagues,[73] whose surprising molecular structure was determined only a year later by Woodward and Wilkinson[74] and Fischer.[75]
Ferrocene is still one of the most important tools and models in this class.[76]
Iron-centered organometallic species are used as catalysts. The Knölker complex, for example, is a transfer hydrogenation catalyst for ketones.[77]
Industrial uses
The iron compounds produced on the largest scale in industry are iron(II) sulfate (FeSO4·7H2O) and iron(III) chloride (FeCl3). The former is one of the most readily available sources of iron(II), but is less stable to aerial oxidation than Mohr’s salt ((NH4)2Fe(SO4)2·6H2O). Iron(II) compounds tend to be oxidized to iron(III) compounds in the air.[56]
History
Development of iron metallurgy
Iron is one of the elements undoubtedly known to the ancient world.[78] It has been worked, or wrought, for millennia. However, iron artefacts of great age are much rarer than objects made of gold or silver due to the ease with which iron corrodes.[79] The technology developed slowly, and even after the discovery of smelting it took many centuries for iron to replace bronze as the metal of choice for tools and weapons.
Meteoritic iron
Beads made from meteoric iron in 3500 BC or earlier were found in Gerzeh, Egypt by G.A. Wainwright.[80] The beads contain 7.5% nickel, which is a signature of meteoric origin since iron found in the Earth’s crust generally has only minuscule nickel impurities.
Meteoric iron was highly regarded due to its origin in the heavens and was often used to forge weapons and tools.[80] For example, a dagger made of meteoric iron was found in the tomb of Tutankhamun, containing similar proportions of iron, cobalt, and nickel to a meteorite discovered in the area, deposited by an ancient meteor shower.[81][82][83] Items that were likely made of iron by Egyptians date from 3000 to 2500 BC.[79]
Meteoritic iron is comparably soft and ductile and easily cold forged but may get brittle when heated because of the nickel content.[84]
Wrought iron
The symbol for Mars has been used since antiquity to represent iron.
The iron pillar of Delhi is an example of the iron extraction and processing methodologies of early India.
The first iron production started in the Middle Bronze Age, but it took several centuries before iron displaced bronze. Samples of smelted iron from Asmar, Mesopotamia and Tall Chagar Bazaar in northern Syria were made sometime between 3000 and 2700 BC.[85] The Hittites established an empire in north-central Anatolia around 1600 BC. They appear to be the first to understand the production of iron from its ores and regard it highly in their society.[86] The Hittites began to smelt iron between 1500 and 1200 BC and the practice spread to the rest of the Near East after their empire fell in 1180 BC.[85] The subsequent period is called the Iron Age.
Artifacts of smelted iron are found in India dating from 1800 to 1200 BC,[87] and in the Levant from about 1500 BC (suggesting smelting in Anatolia or the Caucasus).[88][89] Alleged references (compare history of metallurgy in South Asia) to iron in the Indian Vedas have been used for claims of a very early usage of iron in India respectively to date the texts as such. The rigveda term ayas (metal) refers to copper, while iron which is called as śyāma ayas, literally «black copper», first is mentioned in the post-rigvedic Atharvaveda.[90]
Some archaeological evidence suggests iron was smelted in Zimbabwe and southeast Africa as early as the eighth century BC.[91] Iron working was introduced to Greece in the late 11th century BC, from which it spread quickly throughout Europe.[92]
Iron sickle from Ancient Greece.
The spread of ironworking in Central and Western Europe is associated with Celtic expansion. According to Pliny the Elder, iron use was common in the Roman era.[80] In the lands of what is now considered China, iron appears approximately 700–500 BC.[93] Iron smelting may have been introduced into China through Central Asia.[94] The earliest evidence of the use of a blast furnace in China dates to the 1st century AD,[95] and cupola furnaces were used as early as the Warring States period (403–221 BC).[96] Usage of the blast and cupola furnace remained widespread during the Tang and Song dynasties.[97]
During the Industrial Revolution in Britain, Henry Cort began refining iron from pig iron to wrought iron (or bar iron) using innovative production systems. In 1783 he patented the puddling process for refining iron ore. It was later improved by others, including Joseph Hall.[98]
Cast iron
Cast iron was first produced in China during 5th century BC,[99] but was hardly in Europe until the medieval period.[100][101] The earliest cast iron artifacts were discovered by archaeologists in what is now modern Luhe County, Jiangsu in China. Cast iron was used in ancient China for warfare, agriculture, and architecture.[102] During the medieval period, means were found in Europe of producing wrought iron from cast iron (in this context known as pig iron) using finery forges. For all these processes, charcoal was required as fuel.[103]
Medieval blast furnaces were about 10 feet (3.0 m) tall and made of fireproof brick; forced air was usually provided by hand-operated bellows.[101] Modern blast furnaces have grown much bigger, with hearths fourteen meters in diameter that allow them to produce thousands of tons of iron each day, but essentially operate in much the same way as they did during medieval times.[103]
In 1709, Abraham Darby I established a coke-fired blast furnace to produce cast iron, replacing charcoal, although continuing to use blast furnaces. The ensuing availability of inexpensive iron was one of the factors leading to the Industrial Revolution. Toward the end of the 18th century, cast iron began to replace wrought iron for certain purposes, because it was cheaper. Carbon content in iron was not implicated as the reason for the differences in properties of wrought iron, cast iron, and steel until the 18th century.[85]
Since iron was becoming cheaper and more plentiful, it also became a major structural material following the building of the innovative first iron bridge in 1778. This bridge still stands today as a monument to the role iron played in the Industrial Revolution. Following this, iron was used in rails, boats, ships, aqueducts, and buildings, as well as in iron cylinders in steam engines.[103] Railways have been central to the formation of modernity and ideas of progress[104] and various languages refer to railways as iron road (e.g. French chemin de fer, German Eisenbahn, Turkish demiryolu, Russian железная дорога, Chinese, Japanese, and Korean 鐵道, Vietnamese đường sắt).
Steel
Steel (with smaller carbon content than pig iron but more than wrought iron) was first produced in antiquity by using a bloomery. Blacksmiths in Luristan in western Persia were making good steel by 1000 BC.[85] Then improved versions, Wootz steel by India and Damascus steel were developed around 300 BC and AD 500 respectively. These methods were specialized, and so steel did not become a major commodity until the 1850s.[105]
New methods of producing it by carburizing bars of iron in the cementation process were devised in the 17th century. In the Industrial Revolution, new methods of producing bar iron without charcoal were devised and these were later applied to produce steel. In the late 1850s, Henry Bessemer invented a new steelmaking process, involving blowing air through molten pig iron, to produce mild steel. This made steel much more economical, thereby leading to wrought iron no longer being produced in large quantities.[106]
Foundations of modern chemistry
In 1774, Antoine Lavoisier used the reaction of water steam with metallic iron inside an incandescent iron tube to produce hydrogen in his experiments leading to the demonstration of the conservation of mass, which was instrumental in changing chemistry from a qualitative science to a quantitative one.[107]
Symbolic role
«Gold gab ich für Eisen» – «I gave gold for iron». German-American brooch from WWI.
Iron plays a certain role in mythology and has found various usage as a metaphor and in folklore. The Greek poet Hesiod’s Works and Days (lines 109–201) lists different ages of man named after metals like gold, silver, bronze and iron to account for successive ages of humanity.[108] The Iron Age was closely related with Rome, and in Ovid’s Metamorphoses
The Virtues, in despair, quit the earth; and the depravity of man becomes universal and complete. Hard steel succeeded then.
An example of the importance of iron’s symbolic role may be found in the German Campaign of 1813. Frederick William III commissioned then the first Iron Cross as military decoration. Berlin iron jewellery reached its peak production between 1813 and 1815, when the Prussian royal family urged citizens to donate gold and silver jewellery for military funding. The inscription Gold gab ich für Eisen (I gave gold for iron) was used as well in later war efforts.[109]
Production of metallic iron
Iron furnace in Columbus, Ohio, 1922
Laboratory routes
For a few limited purposes when it is needed, pure iron is produced in the laboratory in small quantities by reducing the pure oxide or hydroxide with hydrogen, or forming iron pentacarbonyl and heating it to 250 °C so that it decomposes to form pure iron powder.[41] Another method is electrolysis of ferrous chloride onto an iron cathode.[110]
Main industrial route
| Country | Iron ore | Pig iron | Direct iron | Steel |
|---|---|---|---|---|
| 1,114.9 | 549.4 | 573.6 | ||
| 393.9 | 4.4 | 5.2 | ||
| 305.0 | 25.1 | 0.011 | 26.5 | |
| 66.9 | 87.5 | |||
| 257.4 | 38.2 | 23.4 | 63.5 | |
| 92.1 | 43.9 | 4.7 | 60.0 | |
| 65.8 | 25.7 | 29.9 | ||
| 0.1 | 27.3 | 48.6 | ||
| 0.4 | 20.1 | 0.38 | 32.7 | |
| World | 1,594.9 | 914.0 | 64.5 | 1,232.4 |
Nowadays, the industrial production of iron or steel consists of two main stages. In the first stage, iron ore is reduced with coke in a blast furnace, and the molten metal is separated from gross impurities such as silicate minerals. This stage yields an alloy—pig iron—that contains relatively large amounts of carbon. In the second stage, the amount of carbon in the pig iron is lowered by oxidation to yield wrought iron, steel, or cast iron.[112] Other metals can be added at this stage to form alloy steels.
17th century Chinese illustration of workers at a blast furnace, making wrought iron from pig iron[113]
How iron was extracted in the 19th century
Blast furnace processing
The blast furnace is loaded with iron ores, usually hematite Fe2O3 or magnetite Fe3O4, along with coke (coal that has been separately baked to remove volatile components) and flux (limestone or dolomite). «Blasts» of air pre-heated to 900 °C (sometimes with oxygen enrichment) is blown through the mixture, in sufficient amount to turn the carbon into carbon monoxide:[112]
This reaction raises the temperature to about 2000 °C. The carbon monoxide reduces the iron ore to metallic iron[112]
Some iron in the high-temperature lower region of the furnace reacts directly with the coke:[112]
The flux removes silicaceous minerals in the ore, which would otherwise clog the furnace: The heat of the furnace decomposes the carbonates to calcium oxide, which reacts with any excess silica to form a slag composed of calcium silicate CaSiO3 or other products. At the furnace’s temperature, the metal and the slag are both molten. They collect at the bottom as two immiscible liquid layers (with the slag on top), that are then easily separated.[112] The slag can be used as a material in road construction or to improve mineral-poor soils for agriculture.[101]
Steelmaking thus remains one of the largest industrial contributors of CO2 emissions in the world.[114]
This heap of iron ore pellets will be used in steel production.
Steelmaking
A pot of molten iron being used to make steel
The pig iron produced by the blast furnace process contains up to 4–5% carbon (by mass), with small amounts of other impurities like sulfur, magnesium, phosphorus, and manganese. This high level of carbon makes it relatively weak and brittle. Reducing the amount of carbon to 0.002–2.1% produces steel, which may be up to 1000 times harder than pure iron. A great variety of steel articles can then be made by cold working, hot rolling, forging, machining, etc. Removing the impurities from pig iron, but leaving 2–4% carbon, results in cast iron, which is cast by foundries into articles such as stoves, pipes, radiators, lamp-posts, and rails.[112]
Steel products often undergo various heat treatments after they are forged to shape. Annealing consists of heating them to 700–800 °C for several hours and then gradual cooling. It makes the steel softer and more workable.[115]
Direct iron reduction
Owing to environmental concerns, alternative methods of processing iron have been developed. «Direct iron reduction» reduces iron ore to a ferrous lump called «sponge» iron or «direct» iron that is suitable for steelmaking.[101] Two main reactions comprise the direct reduction process:
Natural gas is partially oxidized (with heat and a catalyst):[101]
Iron ore is then treated with these gases in a furnace, producing solid sponge iron:[101]
Silica is removed by adding a limestone flux as described above.[101]
Thermite process
Ignition of a mixture of aluminium powder and iron oxide yields metallic iron via the thermite reaction:
Alternatively pig iron may be made into steel (with up to about 2% carbon) or wrought iron (commercially pure iron). Various processes have been used for this, including finery forges, puddling furnaces, Bessemer converters, open hearth furnaces, basic oxygen furnaces, and electric arc furnaces. In all cases, the objective is to oxidize some or all of the carbon, together with other impurities. On the other hand, other metals may be added to make alloy steels.[103]
Applications
As structural material
Iron is the most widely used of all the metals, accounting for over 90% of worldwide metal production. Its low cost and high strength often make it the material of choice to withstand stress or transmit forces, such as the construction of machinery and machine tools, rails, automobiles, ship hulls, concrete reinforcing bars, and the load-carrying framework of buildings. Since pure iron is quite soft, it is most commonly combined with alloying elements to make steel.[116]
Mechanical properties
| Material | TS (MPa) |
BH (Brinell) |
|---|---|---|
| Iron whiskers | 11000 | |
| Ausformed (hardened) steel |
2930 | 850–1200 |
| Martensitic steel | 2070 | 600 |
| Bainitic steel | 1380 | 400 |
| Pearlitic steel | 1200 | 350 |
| Cold-worked iron | 690 | 200 |
| Small-grain iron | 340 | 100 |
| Carbon-containing iron | 140 | 40 |
| Pure, single-crystal iron | 10 | 3 |
The mechanical properties of iron and its alloys are extremely relevant to their structural applications. Those properties can be evaluated in various ways, including the Brinell test, the Rockwell test and the Vickers hardness test.
The properties of pure iron are often used to calibrate measurements or to compare tests.[118][119] However, the mechanical properties of iron are significantly affected by the sample’s purity: pure, single crystals of iron are actually softer than aluminium,[117] and the purest industrially produced iron (99.99%) has a hardness of 20–30 Brinell.[120] The pure iron (99.9%~99.999%), especially called electrolytic iron, is industrially produced by electrolytic refining.
An increase in the carbon content will cause a significant increase in the hardness and tensile strength of iron. Maximum hardness of 65 Rc is achieved with a 0.6% carbon content, although the alloy has low tensile strength.[121] Because of the softness of iron, it is much easier to work with than its heavier congeners ruthenium and osmium.[12]
Iron-carbon phase diagram
Types of steels and alloys
α-Iron is a fairly soft metal that can dissolve only a small concentration of carbon (no more than 0.021% by mass at 910 °C).[122] Austenite (γ-iron) is similarly soft and metallic but can dissolve considerably more carbon (as much as 2.04% by mass at 1146 °C). This form of iron is used in the type of stainless steel used for making cutlery, and hospital and food-service equipment.[16]
Commercially available iron is classified based on purity and the abundance of additives. Pig iron has 3.5–4.5% carbon[123] and contains varying amounts of contaminants such as sulfur, silicon and phosphorus. Pig iron is not a saleable product, but rather an intermediate step in the production of cast iron and steel. The reduction of contaminants in pig iron that negatively affect material properties, such as sulfur and phosphorus, yields cast iron containing 2–4% carbon, 1–6% silicon, and small amounts of manganese.[112] Pig iron has a melting point in the range of 1420–1470 K, which is lower than either of its two main components, and makes it the first product to be melted when carbon and iron are heated together.[6] Its mechanical properties vary greatly and depend on the form the carbon takes in the alloy.[12]
«White» cast irons contain their carbon in the form of cementite, or iron carbide (Fe3C).[12] This hard, brittle compound dominates the mechanical properties of white cast irons, rendering them hard, but unresistant to shock. The broken surface of a white cast iron is full of fine facets of the broken iron carbide, a very pale, silvery, shiny material, hence the appellation. Cooling a mixture of iron with 0.8% carbon slowly below 723 °C to room temperature results in separate, alternating layers of cementite and α-iron, which is soft and malleable and is called pearlite for its appearance. Rapid cooling, on the other hand, does not allow time for this separation and creates hard and brittle martensite. The steel can then be tempered by reheating to a temperature in between, changing the proportions of pearlite and martensite. The end product below 0.8% carbon content is a pearlite-αFe mixture, and that above 0.8% carbon content is a pearlite-cementite mixture.[12]
In gray iron the carbon exists as separate, fine flakes of graphite, and also renders the material brittle due to the sharp edged flakes of graphite that produce stress concentration sites within the material.[124] A newer variant of gray iron, referred to as ductile iron, is specially treated with trace amounts of magnesium to alter the shape of graphite to spheroids, or nodules, reducing the stress concentrations and vastly increasing the toughness and strength of the material.[124]
Wrought iron contains less than 0.25% carbon but large amounts of slag that give it a fibrous characteristic.[123] It is a tough, malleable product, but not as fusible as pig iron. If honed to an edge, it loses it quickly. Wrought iron is characterized by the presence of fine fibers of slag entrapped within the metal. Wrought iron is more corrosion resistant than steel. It has been almost completely replaced by mild steel for traditional «wrought iron» products and blacksmithing.
Mild steel corrodes more readily than wrought iron, but is cheaper and more widely available. Carbon steel contains 2.0% carbon or less,[125] with small amounts of manganese, sulfur, phosphorus, and silicon. Alloy steels contain varying amounts of carbon as well as other metals, such as chromium, vanadium, molybdenum, nickel, tungsten, etc. Their alloy content raises their cost, and so they are usually only employed for specialist uses. One common alloy steel, though, is stainless steel. Recent developments in ferrous metallurgy have produced a growing range of microalloyed steels, also termed ‘HSLA’ or high-strength, low alloy steels, containing tiny additions to produce high strengths and often spectacular toughness at minimal cost.[125][126][127]
Alloys with high purity elemental makeups (such as alloys of electrolytic iron) have specifically enhanced properties such as ductility, tensile strength, toughness, fatigue strength, heat resistance, and corrosion resistance.
Apart from traditional applications, iron is also used for protection from ionizing radiation. Although it is lighter than another traditional protection material, lead, it is much stronger mechanically. The attenuation of radiation as a function of energy is shown in the graph.[128]
The main disadvantage of iron and steel is that pure iron, and most of its alloys, suffer badly from rust if not protected in some way, a cost amounting to over 1% of the world’s economy.[129] Painting, galvanization, passivation, plastic coating and bluing are all used to protect iron from rust by excluding water and oxygen or by cathodic protection. The mechanism of the rusting of iron is as follows:[129]
- Cathode: 3 O2 + 6 H2O + 12 e− → 12 OH−
- Anode: 4 Fe → 4 Fe2+ + 8 e−; 4 Fe2+ → 4 Fe3+ + 4 e−
- Overall: 4 Fe + 3 O2 + 6 H2O → 4 Fe3+ + 12 OH− → 4 Fe(OH)3 or 4 FeO(OH) + 4 H2O
The electrolyte is usually iron(II) sulfate in urban areas (formed when atmospheric sulfur dioxide attacks iron), and salt particles in the atmosphere in seaside areas.[129]
Catalysts and reagents
Because Fe is inexpensive and nontoxic, much effort has been devoted to the development of Fe-based catalysts and reagents. Iron is however less common as a catalyst in commercial processes than more expensive metals.[130] In biology, Fe-containing enzymes are pervasive.[131]
Iron catalysts are traditionally used in the Haber–Bosch process for the production of ammonia and the Fischer–Tropsch process for conversion of carbon monoxide to hydrocarbons for fuels and lubricants.[132] Powdered iron in an acidic medium is used in the Bechamp reduction, the conversion of nitrobenzene to aniline.[133]
Iron compounds
Iron(III) oxide mixed with aluminium powder can be ignited to create a thermite reaction, used in welding large iron parts (like rails) and purifying ores. Iron(III) oxide and oxyhydroxide are used as reddish and ocher pigments.
Iron(III) chloride finds use in water purification and sewage treatment, in the dyeing of cloth, as a coloring agent in paints, as an additive in animal feed, and as an etchant for copper in the manufacture of printed circuit boards.[134] It can also be dissolved in alcohol to form tincture of iron, which is used as a medicine to stop bleeding in canaries.[135]
Iron(II) sulfate is used as a precursor to other iron compounds. It is also used to reduce chromate in cement. It is used to fortify foods and treat iron deficiency anemia. Iron(III) sulfate is used in settling minute sewage particles in tank water. Iron(II) chloride is used as a reducing flocculating agent, in the formation of iron complexes and magnetic iron oxides, and as a reducing agent in organic synthesis.[134]
Sodium nitroprusside is a drug used as a vasodilator. It is on the World Health Organization’s List of Essential Medicines.[136]
Biological and pathological role
Iron is required for life.[5][137][138] The iron–sulfur clusters are pervasive and include nitrogenase, the enzymes responsible for biological nitrogen fixation. Iron-containing proteins participate in transport, storage and used of oxygen.[5] Iron proteins are involved in electron transfer.[139]
Simplified structure of Heme b; in the protein additional ligand(s) are attached to Fe.
Examples of iron-containing proteins in higher organisms include hemoglobin, cytochrome (see high-valent iron), and catalase.[5][140] The average adult human contains about 0.005% body weight of iron, or about four grams, of which three quarters is in hemoglobin – a level that remains constant despite only about one milligram of iron being absorbed each day,[139] because the human body recycles its hemoglobin for the iron content.[141]
Microbial growth may be assisted by oxidation of iron(II) or by reduction of iron (III).[142]
Biochemistry
Iron acquisition poses a problem for aerobic organisms because ferric iron is poorly soluble near neutral pH. Thus, these organisms have developed means to absorb iron as complexes, sometimes taking up ferrous iron before oxidising it back to ferric iron.[5] In particular, bacteria have evolved very high-affinity sequestering agents called siderophores.[143][144][145]
After uptake in human cells, iron storage is precisely regulated.[5][146] A major component of this regulation is the protein transferrin, which binds iron ions absorbed from the duodenum and carries it in the blood to cells.[5][147] Transferrin contains Fe3+ in the middle of a distorted octahedron, bonded to one nitrogen, three oxygens and a chelating carbonate anion that traps the Fe3+ ion: it has such a high stability constant that it is very effective at taking up Fe3+ ions even from the most stable complexes. At the bone marrow, transferrin is reduced from Fe3+ and Fe2+ and stored as ferritin to be incorporated into hemoglobin.[139]
The most commonly known and studied bioinorganic iron compounds (biological iron molecules) are the heme proteins: examples are hemoglobin, myoglobin, and cytochrome P450.[5] These compounds participate in transporting gases, building enzymes, and transferring electrons.[139] Metalloproteins are a group of proteins with metal ion cofactors. Some examples of iron metalloproteins are ferritin and rubredoxin.[139] Many enzymes vital to life contain iron, such as catalase,[148] lipoxygenases,[149] and IRE-BP.[150]
Hemoglobin is an oxygen carrier that occurs in red blood cells and contributes their color, transporting oxygen in the arteries from the lungs to the muscles where it is transferred to myoglobin, which stores it until it is needed for the metabolic oxidation of glucose, generating energy.[5] Here the hemoglobin binds to carbon dioxide, produced when glucose is oxidized, which is transported through the veins by hemoglobin (predominantly as bicarbonate anions) back to the lungs where it is exhaled.[139] In hemoglobin, the iron is in one of four heme groups and has six possible coordination sites; four are occupied by nitrogen atoms in a porphyrin ring, the fifth by an imidazole nitrogen in a histidine residue of one of the protein chains attached to the heme group, and the sixth is reserved for the oxygen molecule it can reversibly bind to.[139] When hemoglobin is not attached to oxygen (and is then called deoxyhemoglobin), the Fe2+ ion at the center of the heme group (in the hydrophobic protein interior) is in a high-spin configuration. It is thus too large to fit inside the porphyrin ring, which bends instead into a dome with the Fe2+ ion about 55 picometers above it. In this configuration, the sixth coordination site reserved for the oxygen is blocked by another histidine residue.[139]
When deoxyhemoglobin picks up an oxygen molecule, this histidine residue moves away and returns once the oxygen is securely attached to form a hydrogen bond with it. This results in the Fe2+ ion switching to a low-spin configuration, resulting in a 20% decrease in ionic radius so that now it can fit into the porphyrin ring, which becomes planar.[139] (Additionally, this hydrogen bonding results in the tilting of the oxygen molecule, resulting in a Fe–O–O bond angle of around 120° that avoids the formation of Fe–O–Fe or Fe–O2–Fe bridges that would lead to electron transfer, the oxidation of Fe2+ to Fe3+, and the destruction of hemoglobin.) This results in a movement of all the protein chains that leads to the other subunits of hemoglobin changing shape to a form with larger oxygen affinity. Thus, when deoxyhemoglobin takes up oxygen, its affinity for more oxygen increases, and vice versa.[139] Myoglobin, on the other hand, contains only one heme group and hence this cooperative effect cannot occur. Thus, while hemoglobin is almost saturated with oxygen in the high partial pressures of oxygen found in the lungs, its affinity for oxygen is much lower than that of myoglobin, which oxygenates even at low partial pressures of oxygen found in muscle tissue.[139] As described by the Bohr effect (named after Christian Bohr, the father of Niels Bohr), the oxygen affinity of hemoglobin diminishes in the presence of carbon dioxide.[139]
Carbon monoxide and phosphorus trifluoride are poisonous to humans because they bind to hemoglobin similarly to oxygen, but with much more strength, so that oxygen can no longer be transported throughout the body. Hemoglobin bound to carbon monoxide is known as carboxyhemoglobin. This effect also plays a minor role in the toxicity of cyanide, but there the major effect is by far its interference with the proper functioning of the electron transport protein cytochrome a.[139] The cytochrome proteins also involve heme groups and are involved in the metabolic oxidation of glucose by oxygen. The sixth coordination site is then occupied by either another imidazole nitrogen or a methionine sulfur, so that these proteins are largely inert to oxygen – with the exception of cytochrome a, which bonds directly to oxygen and thus is very easily poisoned by cyanide.[139] Here, the electron transfer takes place as the iron remains in low spin but changes between the +2 and +3 oxidation states. Since the reduction potential of each step is slightly greater than the previous one, the energy is released step-by-step and can thus be stored in adenosine triphosphate. Cytochrome a is slightly distinct, as it occurs at the mitochondrial membrane, binds directly to oxygen, and transports protons as well as electrons, as follows:[139]
- 4 Cytc2+ + O2 + 8H+
inside → 4 Cytc3+ + 2 H2O + 4H+
outside
Although the heme proteins are the most important class of iron-containing proteins, the iron–sulfur proteins are also very important, being involved in electron transfer, which is possible since iron can exist stably in either the +2 or +3 oxidation states. These have one, two, four, or eight iron atoms that are each approximately tetrahedrally coordinated to four sulfur atoms; because of this tetrahedral coordination, they always have high-spin iron. The simplest of such compounds is rubredoxin, which has only one iron atom coordinated to four sulfur atoms from cysteine residues in the surrounding peptide chains. Another important class of iron–sulfur proteins is the ferredoxins, which have multiple iron atoms. Transferrin does not belong to either of these classes.[139]
The ability of sea mussels to maintain their grip on rocks in the ocean is facilitated by their use of organometallic iron-based bonds in their protein-rich cuticles. Based on synthetic replicas, the presence of iron in these structures increased elastic modulus 770 times, tensile strength 58 times, and toughness 92 times. The amount of stress required to permanently damage them increased 76 times.[152]
Nutrition
Diet
Iron is pervasive, but particularly rich sources of dietary iron include red meat, oysters, beans, poultry, fish, leaf vegetables, watercress, tofu, and blackstrap molasses.[5] Bread and breakfast cereals are sometimes specifically fortified with iron.[5][153]
Iron provided by dietary supplements is often found as iron(II) fumarate, although iron(II) sulfate is cheaper and is absorbed equally well.[134] Elemental iron, or reduced iron, despite being absorbed at only one-third to two-thirds the efficiency (relative to iron sulfate),[154] is often added to foods such as breakfast cereals or enriched wheat flour. Iron is most available to the body when chelated to amino acids[155] and is also available for use as a common iron supplement. Glycine, the least expensive amino acid, is most often used to produce iron glycinate supplements.[156]
Dietary recommendations
The U.S. Institute of Medicine (IOM) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for iron in 2001.[5] The current EAR for iron for women ages 14–18 is 7.9 mg/day, 8.1 for ages 19–50 and 5.0 thereafter (post menopause). For men the EAR is 6.0 mg/day for ages 19 and up. The RDA is 15.0 mg/day for women ages 15–18, 18.0 for 19–50 and 8.0 thereafter. For men, 8.0 mg/day for ages 19 and up. RDAs are higher than EARs so as to identify amounts that will cover people with higher than average requirements. RDA for pregnancy is 27 mg/day and, for lactation, 9 mg/day.[5] For children ages 1–3 years 7 mg/day, 10 for ages 4–8 and 8 for ages 9–13. As for safety, the IOM also sets Tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. In the case of iron the UL is set at 45 mg/day. Collectively the EARs, RDAs and ULs are referred to as Dietary Reference Intakes.[157]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women the PRI is 13 mg/day ages 15–17 years, 16 mg/day for women ages 18 and up who are premenopausal and 11 mg/day postmenopausal. For pregnancy and lactation, 16 mg/day. For men the PRI is 11 mg/day ages 15 and older. For children ages 1 to 14 the PRI increases from 7 to 11 mg/day. The PRIs are higher than the U.S. RDAs, with the exception of pregnancy.[158] The EFSA reviewed the same safety question did not establish a UL.[159]
Infants may require iron supplements if they are bottle-fed cow’s milk.[160] Frequent blood donors are at risk of low iron levels and are often advised to supplement their iron intake.[161]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For iron labeling purposes 100% of the Daily Value was 18 mg, and as of May 27, 2016 remained unchanged at 18 mg.[162][163] A table of the old and new adult daily values is provided at Reference Daily Intake.
Deficiency
Iron deficiency is the most common nutritional deficiency in the world.[5][164][165][166] When loss of iron is not adequately compensated by adequate dietary iron intake, a state of latent iron deficiency occurs, which over time leads to iron-deficiency anemia if left untreated, which is characterised by an insufficient number of red blood cells and an insufficient amount of hemoglobin.[167] Children, pre-menopausal women (women of child-bearing age), and people with poor diet are most susceptible to the disease. Most cases of iron-deficiency anemia are mild, but if not treated can cause problems like fast or irregular heartbeat, complications during pregnancy, and delayed growth in infants and children.[168]
Excess
Iron uptake is tightly regulated by the human body, which has no regulated physiological means of excreting iron. Only small amounts of iron are lost daily due to mucosal and skin epithelial cell sloughing, so control of iron levels is primarily accomplished by regulating uptake.[169] Regulation of iron uptake is impaired in some people as a result of a genetic defect that maps to the HLA-H gene region on chromosome 6 and leads to abnormally low levels of hepcidin, a key regulator of the entry of iron into the circulatory system in mammals.[170] In these people, excessive iron intake can result in iron overload disorders, known medically as hemochromatosis.[5] Many people have an undiagnosed genetic susceptibility to iron overload, and are not aware of a family history of the problem. For this reason, people should not take iron supplements unless they suffer from iron deficiency and have consulted a doctor. Hemochromatosis is estimated to be the cause of 0.3 to 0.8% of all metabolic diseases of Caucasians.[171]
Overdoses of ingested iron can cause excessive levels of free iron in the blood. High blood levels of free ferrous iron react with peroxides to produce highly reactive free radicals that can damage DNA, proteins, lipids, and other cellular components. Iron toxicity occurs when the cell contains free iron, which generally occurs when iron levels exceed the availability of transferrin to bind the iron. Damage to the cells of the gastrointestinal tract can also prevent them from regulating iron absorption, leading to further increases in blood levels. Iron typically damages cells in the heart, liver and elsewhere, causing adverse effects that include coma, metabolic acidosis, shock, liver failure, coagulopathy, long-term organ damage, and even death.[172] Humans experience iron toxicity when the iron exceeds 20 milligrams for every kilogram of body mass; 60 milligrams per kilogram is considered a lethal dose.[173] Overconsumption of iron, often the result of children eating large quantities of ferrous sulfate tablets intended for adult consumption, is one of the most common toxicological causes of death in children under six.[173] The Dietary Reference Intake (DRI) sets the Tolerable Upper Intake Level (UL) for adults at 45 mg/day. For children under fourteen years old the UL is 40 mg/day.[174]
The medical management of iron toxicity is complicated, and can include use of a specific chelating agent called deferoxamine to bind and expel excess iron from the body.[172][175][176]
ADHD
Some research has suggested that low thalamic iron levels may play a role in the pathophysiology of ADHD.[177] Some researchers have found that iron supplementation can be effective especially in the inattentive subtype of the disorder.[178] One study also showed that iron may be able to decrease the risk of cardiovascular events during treatment with ADHD drugs.[179]
Some researchers in the 2000s suggested a link between low levels of iron in the blood and ADHD. A 2012 study found no such correlation.[180]
Cancer
The role of iron in cancer defense can be described as a «double-edged sword» because of its pervasive presence in non-pathological processes.[181] People having chemotherapy may develop iron deficiency and anemia, for which intravenous iron therapy is used to restore iron levels.[182] Iron overload, which may occur from high consumption of red meat,[5] may initiate tumor growth and increase susceptibility to cancer onset,[182] particularly for colorectal cancer.[5]
Marine systems
Iron plays an essential role in marine systems and can act as a limiting nutrient for planktonic activity.[183] Because of this, too much of a decrease in iron may lead to a decrease in growth rates in phytoplanktonic organisms such as diatoms.[184] Iron can also be oxidized by marine microbes under conditions that are high in iron and low in oxygen.[185]
Iron can enter marine systems through adjoining rivers and directly from the atmosphere. Once iron enters the ocean, it can be distributed throughout the water column through ocean mixing and through recycling on the cellular level.[186] In the arctic, sea ice plays a major role in the store and distribution of iron in the ocean, depleting oceanic iron as it freezes in the winter and releasing it back into the water when thawing occurs in the summer.[187] The iron cycle can fluctuate the forms of iron from aqueous to particle forms altering the availability of iron to primary producers.[188] Increased light and warmth increases the amount of iron that is in forms that are usable by primary producers.[189]
See also
- El Mutún in Bolivia, where 10% of the world’s accessible iron ore is located
- Iron and steel industry
- Iron cycle
- Iron nanoparticle
- Iron–platinum nanoparticle
- Iron fertilization – proposed fertilization of oceans to stimulate phytoplankton growth
- Iron-oxidizing bacteria
- List of countries by iron production
- Pelletising – process of creation of iron ore pellets
- Rustproof iron
- Steel
References
- ^ «Standard Atomic Weights: Iron». CIAAW. 1993.
- ^ Ram, R. S.; Bernath, P. F. (2003). «Fourier transform emission spectroscopy of the g4Δ–a4Δ system of FeCl». Journal of Molecular Spectroscopy. 221 (2): 261. Bibcode:2003JMoSp.221..261R. doi:10.1016/S0022-2852(03)00225-X.
- ^ Demazeau, G.; Buffat, B.; Pouchard, M.; Hagenmuller, P. (1982). «Recent developments in the field of high oxidation states of transition elements in oxides stabilization of six-coordinated Iron(V)». Zeitschrift für anorganische und allgemeine Chemie. 491: 60–66. doi:10.1002/zaac.19824910109.
- ^ Lu, J.; Jian, J.; Huang, W.; Lin, H.; Li, J; Zhou, M. (2016). «Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4−«. Physical Chemistry Chemical Physics. 18 (45): 31125–31131. Bibcode:2016PCCP…1831125L. doi:10.1039/C6CP06753K. PMID 27812577.
- ^ a b c d e f g h i j k l m n o p q «Iron». Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, Oregon. April 2016. Retrieved 6 March 2018.
- ^ a b c d e f g h Greenwood & Earnshaw 1997, pp. 1075–79.
- ^ Hirose, K., Tateno, S. (2010). «The Structure of Iron in Earth’s Inner Core». Science. American Association for the Advancement of Science. 330 (6002): 359–361. Bibcode:2010Sci…330..359T. doi:10.1126/science.1194662. PMID 20947762. S2CID 206528628.
- ^ Chamati, Gaminchev (2014). «Dynamic stability of Fe under high pressure». Journal of Physics. IOP Publishing. 558 (1): 012013. Bibcode:2014JPhCS.558a2013G. doi:10.1088/1742-6596/558/1/012013.
- ^ Boehler, Reinhard (2000). «High-pressure experiments and the phase diagram of lower mantle and core materials». Reviews of Geophysics. American Geophysical Union. 38 (2): 221–45. Bibcode:2000RvGeo..38..221B. doi:10.1029/1998RG000053. S2CID 33458168.
- ^ Stixrude, Lars; Wasserman, Evgeny; Cohen, Ronald E. (10 November 1997). «Composition and temperature of Earth’s inner core». Journal of Geophysical Research: Solid Earth. 102 (B11): 24729–39. Bibcode:1997JGR…10224729S. doi:10.1029/97JB02125.
- ^ Greenwood & Earnshaw 1997, p. 1116.
- ^ a b c d e f Greenwood & Earnshaw 1997, pp. 1074–75.
- ^ Boehler, Reinhard; Ross, M. (2007). «Properties of Rocks and Minerals_High-Pressure Melting». Mineral Physics. Treatise on Geophysics. Vol. 2. Elsevier. pp. 527–41. doi:10.1016/B978-044452748-6.00047-X. ISBN 9780444527486.
- ^ Steinmetz, Charles (1917). «fig. 42». Theory and Calculation of Electric Circuits. McGraw-Hill.
- ^ a b Cullity; C. D. Graham (2008). Introduction to Magnetic Materials, 2nd. New York: Wiley–IEEE. p. 116. ISBN 978-0-471-47741-9.
- ^ a b Bramfitt, B.L.; Benscoter, Arlan O. (2002). «The Iron Carbon Phase Diagram». Metallographer’s guide: practice and procedures for irons and steels. ASM International. pp. 24–28. ISBN 978-0-87170-748-2.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), «The NUBASE evaluation of nuclear and decay properties», Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729….3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ Rugel, G.; Faestermann, T.; Knie, K.; Korschinek, G.; Poutivtsev, M.; Schumann, D.; Kivel, N.; Günther-Leopold, I.; Weinreich, R.; Wohlmuther, M. (2009). «New Measurement of the 60Fe Half-Life». Physical Review Letters. 103 (7): 072502. Bibcode:2009PhRvL.103g2502R. doi:10.1103/PhysRevLett.103.072502. PMID 19792637.
- ^ Dauphas, N.; Rouxel, O. (2006). «Mass spectrometry and natural variations of iron isotopes» (PDF). Mass Spectrometry Reviews. 25 (4): 515–50. Bibcode:2006MSRv…25..515D. doi:10.1002/mas.20078. PMID 16463281. Archived from the original (PDF) on 10 June 2010.
- ^ Mostefaoui, S.; Lugmair, G.W.; Hoppe, P.; El Goresy, A. (2004). «Evidence for live 60Fe in meteorites». New Astronomy Reviews. 48 (1–4): 155–59. Bibcode:2004NewAR..48..155M. doi:10.1016/j.newar.2003.11.022.
- ^ Fewell, M. P. (1995). «The atomic nuclide with the highest mean binding energy». American Journal of Physics. 63 (7): 653. Bibcode:1995AmJPh..63..653F. doi:10.1119/1.17828.
- ^ a b c Greenwood & Earnshaw 1997, p. 12.
- ^ Woosley, S.; Janka, T. (2006). «The physics of core collapse supernovae». Nature Physics. 1 (3): 147–54. arXiv:astro-ph/0601261. Bibcode:2005NatPh…1..147W. doi:10.1038/nphys172. S2CID 118974639.
- ^ McDonald, I.; Sloan, G. C.; Zijlstra, A. A.; Matsunaga, N.; Matsuura, M.; Kraemer, K. E.; Bernard-Salas, J.; Markwick, A. J. (2010). «Rusty Old Stars: A Source of the Missing Interstellar Iron?». The Astrophysical Journal Letters. 717 (2): L92–L97. arXiv:1005.3489. Bibcode:2010ApJ…717L..92M. doi:10.1088/2041-8205/717/2/L92. S2CID 14437704.
- ^ Bautista, Manuel A.; Pradhan, Anil K. (1995). «Iron and Nickel Abundances in H~II Regions and Supernova Remnants». Bulletin of the American Astronomical Society. 27: 865. Bibcode:1995AAS…186.3707B.
- ^ Dyson, Freeman J. (1979). «Time without end: Physics and biology in an open universe». Reviews of Modern Physics. 51 (3): 447–60. Bibcode:1979RvMP…51..447D. doi:10.1103/RevModPhys.51.447.
- ^ Aron, Jacob. «Supernova space bullets could have seeded Earth’s iron core». New Scientist. Retrieved 2 October 2020.
- ^ Croswell, Ken. «Iron in the Fire: The Little-Star Supernovae That Could». Scientific American. Retrieved 3 January 2021.
- ^ Buchwald, V F (1992). «On the Use of Iron by the Eskimos in Greenland». Materials Characterization. 29 (2): 139–176. doi:10.1016/1044-5803(92)90112-U.
- ^ Emiliani, Cesare (1992). Planet earth: cosmology, geology, and the evolution of life and environment. Cambridge University Press. p. 152. Bibcode:1992pecg.book…..E. ISBN 978-0-521-40949-0.
- ^ Pernet-Fisher, J.; Day, J.M.D.; Howarth, G.H.; Ryabov, V.V.; Taylor, L.A. (2017). «Atmospheric outgassing and native-iron formation during carbonaceous sediment–basalt melt interactions». Earth and Planetary Science Letters. 460: 201–212. Bibcode:2017E&PSL.460..201P. doi:10.1016/j.epsl.2016.12.022.
- ^ Stark, Anne M. (20 September 2007) Researchers locate mantle’s spin transition zone, leading to clues about earth’s structure. Lawrence Livermore National Laboratory
- ^ Ferropericlase. Mindat.org
- ^ Murakami, M.; Ohishi Y.; Hirao N.; Hirose K. (2012). «A perovskitic lower mantle inferred from high-pressure, high-temperature sound velocity data». Nature. 485 (7396): 90–94. Bibcode:2012Natur.485…90M. doi:10.1038/nature11004. PMID 22552097. S2CID 4387193.
- ^ Sharp, T. (27 November 2014). «Bridgmanite – named at last». Science. 346 (6213): 1057–58. Bibcode:2014Sci…346.1057S. doi:10.1126/science.1261887. PMID 25430755. S2CID 206563252.
- ^ Kong, L. T.; Li, J. F.; Shi, Q. W.; Huang, H. J.; Zhao, K. (6 March 2012). «Dynamical stability of iron under high-temperature and high-pressure conditions». EPL. 97 (5): 56004p1–56004p5. Bibcode:2012EL…..9756004K. doi:10.1209/0295-5075/97/56004. S2CID 121861429.
- ^ Gaminchev, K. G.; Chamati, H. (3 December 2014). «Dynamic stability of Fe under high pressure». J. Phys. 558 (1): 012013(1–7). Bibcode:2014JPhCS.558a2013G. doi:10.1088/1742-6596/558/1/012013.
- ^ Morgan, John W. & Anders, Edward (1980). «Chemical composition of Earth, Venus, and Mercury». Proc. Natl. Acad. Sci. 77 (12): 6973–77. Bibcode:1980PNAS…77.6973M. doi:10.1073/pnas.77.12.6973. PMC 350422. PMID 16592930.
- ^ «Pyrrhotite». Mindat.org. Retrieved 7 July 2009.
- ^ Klein, Cornelis and Cornelius S. Hurlbut, Jr. (1985) Manual of Mineralogy, Wiley, 20th ed, pp. 278–79 ISBN 0-471-80580-7
- ^ a b Greenwood & Earnshaw 1997, p. 1071.
- ^ Lyons, T. W.; Reinhard, C. T. (2009). «Early Earth: Oxygen for heavy-metal fans». Nature. 461 (7261): 179–181. Bibcode:2009Natur.461..179L. doi:10.1038/461179a. PMID 19741692. S2CID 205049360.
- ^ Cloud, P. (1973). «Paleoecological Significance of the Banded Iron-Formation». Economic Geology. 68 (7): 1135–43. doi:10.2113/gsecongeo.68.7.1135.
- ^ Dickinson, Robert E. (1964). Germany: A regional and economic geography (2nd ed.). London: Methuen.
- ^ Naturwerksteine in Baden-Württemberg. Landesamt für Geologie, Rohstoffe und Bergbau, Baden-Württemberg
- ^ «Tales From The Riverbank». Minerva Stone Conservation. Archived from the original on 28 September 2015. Retrieved 22 September 2015.
- ^ Klingelhöfer, G.; Morris, R. V.; Souza, P. A.; Rodionov, D.; Schröder, C. (2007). «Two earth years of Mössbauer studies of the surface of Mars with MIMOS II». Hyperfine Interactions. 170 (1–3): 169–77. Bibcode:2006HyInt.170..169K. doi:10.1007/s10751-007-9508-5. S2CID 98227499.
- ^ Winderlich, R.; Peter, W. (1954). Lehrbuch der Chemie für Höhere Lehranstalten : Einheitsausgabe für Unter- und Oberstufe (in German). Wiesbaden. p. 75. ISBN 978-3-663-04370-6. OCLC 913701506.
- ^ Bertau, Martin (2013). Industrielle Anorganische Chemie (in German). Weinheim: Wiley-VCH. p. 696. ISBN 978-3-527-64956-3. OCLC 855858511.
- ^ Metal Stocks in Society: Scientific synthesis, 2010, International Resource Panel, UNEP
- ^ Stoll, Heather (17 February 2020). «30 years of the iron hypothesis of ice ages». Nature. Springer Science and Business Media LLC. 578 (7795): 370–371. doi:10.1038/d41586-020-00393-x. ISSN 0028-0836.
- ^ Greenwood & Earnshaw 1997, p. 905.
- ^ a b Greenwood & Earnshaw 1997, p. 1070.
- ^ Lu, Jun-Bo; Jian, Jiwen; Huang, Wei; Lin, Hailu; Li, Jun; Zhou, Mingfei (16 November 2016). «Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4−«. Phys. Chem. Chem. Phys. 18 (45): 31125–31131. Bibcode:2016PCCP…1831125L. doi:10.1039/c6cp06753k. PMID 27812577.
- ^ Nam, Wonwoo (2007). «High-Valent Iron(IV)–Oxo Complexes of Heme and Non-Heme Ligands in Oxygenation Reactions» (PDF). Accounts of Chemical Research. 40 (7): 522–531. doi:10.1021/ar700027f. PMID 17469792. Archived from the original (PDF) on 15 June 2021. Retrieved 22 February 2022.
- ^ a b c d e f Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). «Iron». Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 1125–46. ISBN 3-11-007511-3.
- ^ Reiff, William Michael; Long, Gary J. (1984). «Mössbauer Spectroscopy and the Coordination Chemistry of Iron». Mössbauer spectroscopy applied to inorganic chemistry. Springer. pp. 245–83. ISBN 978-0-306-41647-7.
- ^ Ware, Mike (1999). «An introduction in monochrome». Cyanotype: the history, science and art of photographic printing in Prussian blue. NMSI Trading Ltd. pp. 11–19. ISBN 978-1-900747-07-3.
- ^ Gmelin, Leopold (1852). «Mercury and Iron». Hand-book of chemistry. Vol. 6. Cavendish Society. pp. 128–29.
- ^ a b c Greenwood & Earnshaw 1997, p. 1079.
- ^ a b c d Greenwood & Earnshaw 1997, pp. 1082–84.
- ^ Siegfried Pohl, Ulrich Bierbach, Wolfgang Saak; «FeI3SC(NMe2)2, a Neutral Thiourea Complex of Iron(III) Iodide», Angewandte Chemie International Edition in English (1989) 28 (6), 776-777. https://doi.org/10.1002/anie.198907761
- ^ Nicholas A. Barnes, Stephen M.Godfrey, Nicholas Ho, Charles A.McAuliffe, Robin G.Pritchard; «Facile synthesis of a rare example of an iron(III) iodide complex, [FeI3(AsMe3)2], from the reaction of Me3AsI2 with unactivated iron powder», Polyhedron (2013) 55, 67-72. https://doi.org/10.1016/j.poly.2013.02.066
- ^ a b c d Greenwood & Earnshaw 1997, pp. 1088–91.
- ^ a b Greenwood & Earnshaw 1997, pp. 1091–97.
- ^ Clausen, C.A.; Good, M.L. (1968). «Stabilization of the hexachloroferrate(III) anion by the methylammonium cation». Inorganic Chemistry. 7 (12): 2662–63. doi:10.1021/ic50070a047.
- ^ James, B.D.; Bakalova, M.; Lieseganga, J.; Reiff, W.M.; Hockless, D.C.R.; Skelton, B.W.; White, A.H. (1996). «The hexachloroferrate(III) anion stabilized in hydrogen bonded packing arrangements. A comparison of the X-ray crystal structures and low temperature magnetism of tetrakis(methylammonium) hexachloroferrate(III) chloride (I) and tetrakis(hexamethylenediammonium) hexachloroferrate(III) tetrachloroferrate(III) tetrachloride (II)«. Inorganica Chimica Acta. 247 (2): 169–74. doi:10.1016/0020-1693(95)04955-X.
- ^ Giannoccaro, P.; Sacco, A. (1977). Bis[ethylenebis(diphenylphosphine)]-Hydridoiron Complexes. Inorg. Synth. Inorganic Syntheses. Vol. 17. pp. 69–72. doi:10.1002/9780470132487.ch19. ISBN 978-0-470-13248-7.
- ^ Lee, J.; Jung, G.; Lee, S.W. (1998). «Structure of trans-chlorohydridobis(diphenylphosphinoethane)iron(II)». Bull. Korean Chem. Soc. 19 (2): 267–69. doi:10.1007/BF02698412. S2CID 35665289.
- ^ Echigo, Takuya; Kimata, Mitsuyoshi (2008). «Single-crystal X-ray diffraction and spectroscopic studies on humboldtine and lindbergite: weak Jahn–Teller effect of Fe2+ ion». Phys. Chem. Minerals. 35 (8): 467–75. Bibcode:2008PCM….35..467E. doi:10.1007/s00269-008-0241-7. S2CID 98739882.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 1282–86. ISBN 978-0-08-022057-4..
- ^ Kealy, T.J.; Pauson, P.L. (1951). «A New Type of Organo-Iron Compound». Nature. 168 (4285): 1039–40. Bibcode:1951Natur.168.1039K. doi:10.1038/1681039b0. S2CID 4181383.
- ^ Miller, S. A.; Tebboth, J. A.; Tremaine, J. F. (1952). «114. Dicyclopentadienyliron». J. Chem. Soc.: 632–635. doi:10.1039/JR9520000632.
- ^ Wilkinson, G.; Rosenblum, M.; Whiting, M. C.; Woodward, R. B. (1952). «The Structure of Iron Bis-Cyclopentadienyl». J. Am. Chem. Soc. 74 (8): 2125–2126. doi:10.1021/ja01128a527.
- ^ Okuda, Jun (28 December 2016). «Ferrocene – 65 Years After». European Journal of Inorganic Chemistry. 2017 (2): 217–219. doi:10.1002/ejic.201601323. ISSN 1434-1948.
- ^ Greenwood & Earnshaw 1997, p. 1104.
- ^ Bullock, R.M. (11 September 2007). «An Iron Catalyst for Ketone Hydrogenations under Mild Conditions». Angew. Chem. Int. Ed. 46 (39): 7360–63. doi:10.1002/anie.200703053. PMID 17847139.
- ^ Weeks 1968, p. 4.
- ^ a b Weeks 1968, p. 29.
- ^ a b c Weeks 1968, p. 31.
- ^ Bjorkman, Judith Kingston (1973). «Meteors and Meteorites in the ancient Near East». Meteoritics. 8 (2): 91–132. Bibcode:1973Metic…8…91B. doi:10.1111/j.1945-5100.1973.tb00146.x.
- ^ Comelli, Daniela; d’Orazio, Massimo; Folco, Luigi; El-Halwagy, Mahmud; Frizzi, Tommaso; Alberti, Roberto; Capogrosso, Valentina; Elnaggar, Abdelrazek; Hassan, Hala; Nevin, Austin; Porcelli, Franco; Rashed, Mohamed G; Valentini, Gianluca (2016). «The meteoritic origin of Tutankhamun’s iron dagger blade». Meteoritics & Planetary Science. 51 (7): 1301–09. Bibcode:2016M&PS…51.1301C. doi:10.1111/maps.12664.
- ^ Walsh, Declan (2 June 2016). «King Tut’s Dagger Made of ‘Iron From the Sky,’ Researchers Say». The New York Times. Archived from the original on 3 January 2022. Retrieved 4 June 2016.
the blade’s composition of iron, nickel and cobalt was an approximate match for a meteorite that landed in northern Egypt. The result «strongly suggests an extraterrestrial origin»
- ^ Ure, Andrew (1843). Technisches wörterbuch oder Handbuch der Gewerbskunde … : Bearb. nach Dr. Andrew Ure’s Dictionary of arts, manufactures and mines (in German). G. Haase. p. 492.
- ^ a b c d Weeks 1968, p. 32.
- ^ McNutt, Paula (1990 1). The Forging of Israel: Iron Technology, Symbolism and Tradition in Ancient Society. A&C Black.
- ^ Tewari, Rakesh. «The origins of Iron Working in India: New evidence from the Central Ganga plain and the Eastern Vindhyas» (PDF). State Archaeological Department. Retrieved 23 May 2010.
- ^ Photos, E. (1989). «The Question of Meteoritic versus Smelted Nickel-Rich Iron: Archaeological Evidence and Experimental Results». World Archaeology. Taylor & Francis, Ltd. 20 (3): 403–21. doi:10.1080/00438243.1989.9980081. JSTOR 124562.
- ^ Muhly, James D. (2003). «Metalworking/Mining in the Levant». In Lake, Richard Winona (ed.). Near Eastern Archaeology IN: Eisenbrauns. Vol. 180. pp. 174–83.
- ^ Witzel, Michael (2001), «Autochthonous Aryans? The Evidence from Old Indian and Iranian Texts», in Electronic Journal of Vedic Studies (EJVS) 7-3, pp. 1–93
- ^ Weeks, p. 33, quoting Cline, Walter (1937) «Mining and Metallurgy in Negro Africa,» George Banta Publishing Co., Menasha, Wis., pp. 17–23.
- ^ Riederer, Josef; Wartke, Ralf-B. (2009) «Iron», Cancik, Hubert; Schneider, Helmuth (eds.): Brill’s New Pauly, Brill.
- ^ Sawyer, Ralph D. and Sawyer, Mei-chün (1993). The Seven Military Classics of Ancient China. Boulder: Westview. ISBN 0-465-00304-4. p. 10.
- ^ Pigott, Vincent C. (1999). The Archaeometallurgy of the Asian Old World. Philadelphia: University of Pennsylvania Museum of Archaeology and Anthropology. ISBN 0-924171-34-0, p. 8.
- ^ Golas, Peter J. (1999). Science and Civilisation in China: Volume 5, Chemistry and Chemical Technology, Part 13, Mining. Cambridge University Press. p. 152. ISBN 978-0-521-58000-7.
earliest blast furnace discovered in China from about the first century AD
- ^ Pigott, Vincent C. (1999). The Archaeometallurgy of the Asian Old World. Philadelphia: University of Pennsylvania Museum of Archaeology and Anthropology. ISBN 0-924171-34-0, p. 191.
- ^ The Coming of the Ages of Steel. Brill Archive. 1961. p. 54.
- ^ Mott, R.A (2014). «Dry and Wet Puddling». Transactions of the Newcomen Society. 49: 156–57. doi:10.1179/tns.1977.011.
- ^ Wagner, Donald B. (2003). «Chinese blast furnaces from the 10th to the 14th century» (PDF). Historical Metallurgy. 37 (1): 25–37. Archived from the original (PDF) on 7 January 2018. Retrieved 7 January 2018. originally published in Wagner, Donald B. (2001). «Chinese blast furnaces from the 10th to the 14th century». West Asian Science, Technology, and Medicine. 18: 41–74. doi:10.1163/26669323-01801008.
- ^ Giannichedda, Enrico (2007): «Metal production in Late Antiquity», in Technology in Transition AD 300–650 Lavan, L.; Zanini, E. and Sarantis, A.(eds.), Brill, Leiden; ISBN 90-04-16549-5, p. 200.
- ^ a b c d e f g Biddle, Verne; Parker, Gregory. Chemistry, Precision and Design. A Beka Book, Inc.
- ^ Wagner, Donald B. (1993). Iron and Steel in Ancient China. Brill. pp. 335–340. ISBN 978-90-04-09632-5.
- ^ a b c d Greenwood & Earnshaw 1997, p. 1072.
- ^ Schivelbusch, G. (1986) The Railway Journey: Industrialization and Perception of Time and Space in the 19th Century. Oxford: Berg.
- ^ Spoerl, Joseph S. A Brief History of Iron and Steel Production Archived 2 June 2010 at the Wayback Machine. Saint Anselm College
- ^ Enghag, Per (8 January 2008). Encyclopedia of the Elements: Technical Data – History – Processing – Applications. pp. 190–91. ISBN 978-3-527-61234-5.
- ^ Whitaker, Robert D (1975). «An historical note on the conservation of mass». Journal of Chemical Education. 52 (10): 658. Bibcode:1975JChEd..52..658W. doi:10.1021/ed052p658.
- ^ Fontenrose, Joseph (1974). «Work, Justice, and Hesiod’s Five Ages». Classical Philology. 69 (1): 1–16. doi:10.1086/366027. JSTOR 268960. S2CID 161808359.
- ^ Schmidt, Eva (1981) Der preußische Eisenkunstguss. (Art of Prussian cast iron) Technik, Geschichte, Werke, Künstler. Verlag Mann, Berlin, ISBN 3-7861-1130-8
- ^ Lux, H. (1963) «Metallic Iron» in Handbook of Preparative Inorganic Chemistry, 2nd Ed. G. Brauer (ed.), Academic Press, NY. Vol. 2. pp. 1490–91.
- ^ Steel Statistical Yearbook 2010. World Steel Association
- ^ a b c d e f g Greenwood & Earnshaw 1997, p. 1073.
- ^ Song Yingxing (1637): The Tiangong Kaiwu encyclopedia.
- ^ Wang, Peng; Ryberg, Morten; Yang, Yi; Feng, Kuishuang; Kara, Sami; Hauschild, Michael; Chen, Wei-Qiang (6 April 2021). «Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts». Nature Communications. 12 (1): 2066. Bibcode:2021NatCo..12.2066W. doi:10.1038/s41467-021-22245-6. ISSN 2041-1723. PMC 8024266. PMID 33824307.
- ^ Verhoeven, J.D. (1975) Fundamentals of Physical Metallurgy, Wiley, New York, p. 326
- ^ Greenwood & Earnshaw 1997, pp. 1070–71.
- ^ a b Kohl, Walter H. (1995). Handbook of materials and techniques for vacuum devices. Springer. pp. 164–67. ISBN 1-56396-387-6.
- ^ a b Kuhn, Howard; Medlin, Dana; et al., eds. (2000). ASM Handbook – Mechanical Testing and Evaluation (PDF). Vol. 8. ASM International. p. 275. ISBN 0-87170-389-0. Archived from the original (PDF) on 9 February 2019. Retrieved 22 February 2022.
- ^ «Hardness Conversion Chart». Maryland Metrics. Archived from the original on 18 June 2015. Retrieved 23 May 2010.
- ^ Takaji, Kusakawa; Toshikatsu, Otani (1964). «Properties of Various Pure Irons: Study on pure iron I». Tetsu-to-Hagane. 50 (1): 42–47. doi:10.2355/tetsutohagane1955.50.1_42.
- ^ Raghavan, V. (2004). Materials Science and Engineering. PHI Learning Pvt. Ltd. p. 218. ISBN 81-203-2455-2.
- ^ Martin, John Wilson (2007). Concise encyclopedia of the structure of materials. Elsevier. p. 183. ISBN 978-0-08-045127-5.
- ^ a b Camp, James McIntyre; Francis, Charles Blaine (1920). The Making, Shaping and Treating of Steel. Pittsburgh: Carnegie Steel Company. pp. 173–74. ISBN 1-147-64423-3.
- ^ a b Smith, William F.; Hashemi, Javad (2006), Foundations of Materials Science and Engineering (4th ed.), McGraw-Hill, p. 431, ISBN 0-07-295358-6.
- ^ a b «Classification of Carbon and Low-Alloy Steels». Archived from the original on 2 January 2011. Retrieved 5 January 2008.
- ^ HSLA Steel, 15 November 2002, archived from the original on 30 December 2009, retrieved 11 October 2008.
- ^ Oberg, E.; et al. (1996), «Machinery’s Handbook», New York: Industrial Press (25th ed.), Industrial Press Inc: 440–42, Bibcode:1984msh..book…..R
- ^ Rokni, Sayed H.; Cossairt, J. Donald; Liu, James C. (January 2008). «Radiation Shielding at High-Energy Electron and Proton Accelerators» (PDF). Retrieved 6 August 2016.
- ^ a b c Greenwood & Earnshaw 1997, p. 1076.
- ^ Fürstner, Alois (2016). «Iron Catalysis in Organic Synthesis: A Critical Assessment of What It Takes to Make This Base Metal a Multitasking Champion». ACS Central Science. 2 (11): 778–789. doi:10.1021/acscentsci.6b00272. PMC 5140022. PMID 27981231.
- ^ Bullock, R. Morris; et al. (2020). «Using nature’s blueprint to expand catalysis with Earth-abundant metals». Science. 369 (6505): eabc3183. doi:10.1126/science.abc3183. PMC 7875315. PMID 32792370.
- ^ Kolasinski, Kurt W. (2002). «Where are Heterogenous Reactions Important». Surface science: foundations of catalysis and nanoscience. John Wiley and Sons. pp. 15–16. ISBN 978-0-471-49244-3.
- ^ McKetta, John J. (1989). «Nitrobenzene and Nitrotoluene». Encyclopedia of Chemical Processing and Design: Volume 31 – Natural Gas Liquids and Natural Gasoline to Offshore Process Piping: High Performance Alloys. CRC Press. pp. 166–67. ISBN 978-0-8247-2481-8.
- ^ a b c Wildermuth, Egon; Stark, Hans; Friedrich, Gabriele; Ebenhöch, Franz Ludwig; Kühborth, Brigitte; Silver, Jack; Rituper, Rafael (2000). «Iron Compounds». Ullmann’s Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a14_591. ISBN 3-527-30673-0.
- ^ Stroud, Robert (1933). Diseases of Canaries. Canary Publishers Company. p. 203. ISBN 978-1-4465-4656-7.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Dlouhy, Adrienne C.; Outten, Caryn E. (2013). Banci, Lucia (ed.). Metallomics and the Cell. Metal Ions in Life Sciences. Vol. 12. Springer. pp. 241–78. doi:10.1007/978-94-007-5561-1_8. ISBN 978-94-007-5560-4. PMC 3924584. PMID 23595675. electronic-book ISBN 978-94-007-5561-1
- ^
Yee, Gereon M.; Tolman, William B. (2015). Peter M.H. Kroneck; Martha E. Sosa Torres (eds.). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. Vol. 15. Springer. pp. 131–204. doi:10.1007/978-3-319-12415-5_5. PMID 25707468. - ^ a b c d e f g h i j k l m n o p Greenwood & Earnshaw 1997, pp. 1098–104.
- ^ Lippard, S.J.; Berg, J.M. (1994). Principles of Bioinorganic Chemistry. Mill Valley: University Science Books. ISBN 0-935702-73-3.
- ^ Kikuchi, G.; Yoshida, T.; Noguchi, M. (2005). «Heme oxygenase and heme degradation». Biochemical and Biophysical Research Communications. 338 (1): 558–67. doi:10.1016/j.bbrc.2005.08.020. PMID 16115609.
- ^
Uebe, René; Schüler, Dirk; «The Formation of Iron Biominerals «, pp 159-184 in «Metals, Microbes and Minerals: The Biogeochemical Side of Life» (2021) pp xiv + 341. Walter de Gruyter, Berlin. Editors Kroneck, Peter M.H. and Sosa Torres, Martha. DOI 10.1515/9783110589771-006 - ^ Neilands, J.B. (1995). «Siderophores: structure and function of microbial iron transport compounds». The Journal of Biological Chemistry. 270 (45): 26723–26. doi:10.1074/jbc.270.45.26723. PMID 7592901.
- ^ Neilands, J.B. (1981). «Microbial Iron Compounds». Annual Review of Biochemistry. 50 (1): 715–31. doi:10.1146/annurev.bi.50.070181.003435. PMID 6455965.
- ^ Boukhalfa, Hakim; Crumbliss, Alvin L. (2002). «Chemical aspects of siderophore mediated iron transport». BioMetals. 15 (4): 325–39. doi:10.1023/A:1020218608266. PMID 12405526. S2CID 19697776.
- ^ Nanami, M.; Ookawara, T.; Otaki, Y.; Ito, K.; Moriguchi, R.; Miyagawa, K.; Hasuike, Y.; Izumi, M.; Eguchi, H.; Suzuki, K.; Nakanishi, T. (2005). «Tumor necrosis factor-α-induced iron sequestration and oxidative stress in human endothelial cells». Arteriosclerosis, Thrombosis, and Vascular Biology. 25 (12): 2495–501. doi:10.1161/01.ATV.0000190610.63878.20. PMID 16224057.
- ^ Rouault, Tracey A. (2003). «How Mammals Acquire and Distribute Iron Needed for Oxygen-Based Metabolism». PLOS Biology. 1 (3): e9. doi:10.1371/journal.pbio.0000079. PMC 300689. PMID 14691550.
- ^ Boon EM, Downs A, Marcey D. «Proposed Mechanism of Catalase». Catalase: H2O2: H2O2 Oxidoreductase: Catalase Structural Tutorial. Retrieved 11 February 2007.
- ^ Boyington JC, Gaffney BJ, Amzel LM (1993). «The three-dimensional structure of an arachidonic acid 15-lipoxygenase». Science. 260 (5113): 1482–86. Bibcode:1993Sci…260.1482B. doi:10.1126/science.8502991. PMID 8502991.
- ^ Gray, N.K.; Hentze, M.W. (August 1994). «Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs». EMBO J. 13 (16): 3882–91. doi:10.1002/j.1460-2075.1994.tb06699.x. PMC 395301. PMID 8070415.
- ^ Gregory B. Vásquez; Xinhua Ji; Clara Fronticelli; Gary L. Gilliland (1998). «Human Carboxyhemoglobin at 2.2 Å Resolution: Structure and Solvent Comparisons of R-State, R2-State and T-State Hemoglobins». Acta Crystallogr. D. 54 (3): 355–66. doi:10.1107/S0907444997012250. PMID 9761903.
- ^ Sanderson, K (2017). «Mussels’ iron grip inspires strong and stretchy polymer». Chemical & Engineering News. American Chemical Society. 95 (44): 8. doi:10.1021/cen-09544-notw3. Retrieved 2 November 2017.
- ^ Food Standards Agency – Eat well, be well – Iron deficiency Archived 8 August 2006 at the Wayback Machine. Eatwell.gov.uk (5 March 2012). Retrieved on 27 June 2012.
- ^ Hoppe, M.; Hulthén, L.; Hallberg, L. (2005). «The relative bioavailability in humans of elemental iron powders for use in food fortification». European Journal of Nutrition. 45 (1): 37–44. doi:10.1007/s00394-005-0560-0. PMID 15864409. S2CID 42983904.
- ^ Pineda, O.; Ashmead, H. D. (2001). «Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate». Nutrition. 17 (5): 381–4. doi:10.1016/S0899-9007(01)00519-6. PMID 11377130.
- ^ Ashmead, H. DeWayne (1989). Conversations on Chelation and Mineral Nutrition. Keats Publishing. ISBN 0-87983-501-X.
- ^ Institute of Medicine (US) Panel on Micronutrients (2001). «Iron» (PDF). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Iron. National Academy Press. pp. 290–393. ISBN 0-309-07279-4. PMID 25057538. Archived from the original (PDF) on 9 September 2017. Retrieved 9 March 2017.
- ^ «Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies» (PDF). European Food Safety Authority. 2017.
- ^ «Tolerable Upper Intake Levels For Vitamins And Minerals» (PDF). European Food Safety Authority. 2006.
- ^ «Iron Deficiency Anemia». MediResource. Retrieved 17 December 2008.
- ^ Milman, N. (1996). «Serum ferritin in Danes: studies of iron status from infancy to old age, during blood donation and pregnancy». International Journal of Hematology. 63 (2): 103–35. doi:10.1016/0925-5710(95)00426-2. PMID 8867722.
- ^ «Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982» (PDF).
- ^ «Daily Value Reference of the Dietary Supplement Label Database (DSLD)». Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- ^ Centers for Disease Control and Prevention (2002). «Iron deficiency – United States, 1999–2000». MMWR. 51 (40): 897–99. PMID 12418542.
- ^ Hider, Robert C.; Kong, Xiaole (2013). «Chapter 8. Iron: Effect of Overload and Deficiency». In Astrid Sigel, Helmut Sigel and Roland K.O. Sigel (ed.). Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. Vol. 13. Springer. pp. 229–94. doi:10.1007/978-94-007-7500-8_8. PMID 24470094.
- ^ Dlouhy, Adrienne C.; Outten, Caryn E. (2013). «Chapter 8.4 Iron Uptake, Trafficking and Storage». In Banci, Lucia (ed.). Metallomics and the Cell. Metal Ions in Life Sciences. Vol. 12. Springer. pp. 241–78. doi:10.1007/978-94-007-5561-1_8. ISBN 978-94-007-5560-4. PMC 3924584. PMID 23595675. electronic-book ISBN 978-94-007-5561-1
- ^ CDC Centers for Disease Control and Prevention (3 April 1998). «Recommendations to Prevent and Control Iron Deficiency in the United States». Morbidity and Mortality Weekly Report. 47 (RR3): 1. Retrieved 12 August 2014.
- ^ Centers for Disease Control and Prevention. «Iron and Iron Deficiency». Retrieved 12 August 2014.
- ^ Ramzi S. Cotran; Vinay Kumar; Tucker Collins; Stanley Leonard Robbins (1999). Robbins pathologic basis of disease. Saunders. ISBN 978-0-7216-7335-6. Retrieved 27 June 2012.
- ^ Ganz T (August 2003). «Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation». Blood. 102 (3): 783–8. doi:10.1182/blood-2003-03-0672. PMID 12663437. S2CID 28909635.
- ^ Durupt, S.; Durieu, I.; Nové-Josserand, R.; Bencharif, L.; Rousset, H.; Vital Durand, D. (2000). «Hereditary hemochromatosis». Rev Méd Interne. 21 (11): 961–71. doi:10.1016/S0248-8663(00)00252-6. PMID 11109593.
- ^ a b Cheney, K.; Gumbiner, C.; Benson, B.; Tenenbein, M. (1995). «Survival after a severe iron poisoning treated with intermittent infusions of deferoxamine». J Toxicol Clin Toxicol. 33 (1): 61–66. doi:10.3109/15563659509020217. PMID 7837315.
- ^ a b «Toxicity, Iron». Medscape. Retrieved 23 May 2010.
- ^ Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals (PDF), Food and Nutrition Board, Institute of Medicine, National Academies, 2004, archived from the original (PDF) on 14 March 2013, retrieved 9 June 2009
- ^ Tenenbein, M. (1996). «Benefits of parenteral deferoxamine for acute iron poisoning». J Toxicol Clin Toxicol. 34 (5): 485–89. doi:10.3109/15563659609028005. PMID 8800185.
- ^ Wu H, Wu T, Xu X, Wang J, Wang J (May 2011). «Iron toxicity in mice with collagenase-induced intracerebral hemorrhage». J Cereb Blood Flow Metab. 31 (5): 1243–50. doi:10.1038/jcbfm.2010.209. PMC 3099628. PMID 21102602.
- ^ Robberecht, Harry; et al. (2020). «Magnesium, Iron, Zinc, Copper and Selenium Status in Attention-Deficit/Hyperactivity Disorder (ADHD)». Molecules. 25 (19): 4440. doi:10.3390/molecules25194440. PMC 7583976. PMID 32992575.
- ^ Soto-Insuga, V; et al. (2013). «[Role of iron in the treatment of attention deficit-hyperactivity disorder]». An Pediatr (Barc). 79 (4): 230–235. doi:10.1016/j.anpedi.2013.02.008. PMID 23582950.
- ^ Parisi, Pasquale; Villa, Maria Pia; Donfrancesco, Renato; Miano, Silvia; Paolino, Maria Chiara; Cortese, Samuele (August 2012). «Could treatment of iron deficiency both improve ADHD and reduce cardiovascular risk during treatment with ADHD drugs?». Medical Hypotheses. 79 (2): 246–249. doi:10.1016/j.mehy.2012.04.049. PMID 22632845.
- ^ Donfrancesco, Renato; Parisi, Pasquale; Vanacore, Nicola; Martines, Francesca; Sargentini, Vittorio; Cortese, Samuele (May 2013). «Iron and ADHD: Time to Move Beyond Serum Ferritin Levels». Journal of Attention Disorders. 17 (4): 347–357. doi:10.1177/1087054711430712. ISSN 1087-0547. PMID 22290693. S2CID 22445593.
- ^ Thévenod, Frank (2018). «Chapter 15. Iron and Its Role in Cancer Defense: A Double-Edged Sword». In Sigel, Astrid; Sigel, Helmut; Freisinger, Eva; Sigel, Roland K. O. (eds.). Metallo-Drugs: Development and Action of Anticancer Agents. Metal Ions in Life Sciences. Vol. 18. Berlin: de Gruyter GmbH. pp. 437–67. doi:10.1515/9783110470734-021. PMID 29394034.
- ^ a b Beguin, Y; Aapro, M; Ludwig, H; Mizzen, L; Osterborg, A (2014). «Epidemiological and nonclinical studies investigating effects of iron in carcinogenesis—a critical review». Critical Reviews in Oncology/Hematology. 89 (1): 1–15. doi:10.1016/j.critrevonc.2013.10.008. PMID 24275533.
- ^ Morel, F.M.M., Hudson, R.J.M., & Price, N.M. (1991). Limitation of productivity by trace metals in the sea. Limnology and Oceanography, 36(8), 1742-1755. doi:10.4319/lo.1991.36.8.1742
- ^ Brezezinski, M.A., Baines, S.B., Balch, W.M., Beucher, C.P., Chai, F., Dugdale, R.C., Krause, J.W., Landry, M.R., Marchi, A., Measures, C.I., Nelson, D.M., Parker, A.E., Poulton, A.J., Selph, K.E., Strutton, P.G., Taylor, A.G., & Twining, B.S.(2011). Co-limitation of diatoms by iron and silicic acid in the equatorial Pacific. Deep-Sea Research Part II: Topical Studies in Oceanography, 58(3-4), 493-511. doi:10.1016/j.dsr2.2010.08.005
- ^ Field, E. K., Kato, S., Findlay, A. J., MacDonald, D. J., Chiu, B. K., Luther, G. W., & Chan, C. S. (2016). Planktonic marine iron oxidizers drive iron mineralization under low-oxygen conditions. Geobiology, 14(5), 499-508. doi:10.1111/gbi.12189
- ^ Wells, M.L., Price, N.M., & Bruland, K.W. (1995). Iron chemistry in seawater and its relationship to phytoplankton: a workshop report. Marine Chemistry, 48(2), 157-182. doi:10.1016/0304-4203(94)00055-I
- ^ Lannuzel, D., Vancoppenolle, M., van der Merwe, P., de Jong, J., Meiners, K.M., Grotti, M., Nishioska, J., & Schoemann. (2016). Iron in sea ice: Review and new insights. Elementa: Science of the Anthropocene, 4 000130.
doi:10.12952/journal.elementa.000130 - ^ Raiswell, R. 2011. Iron Transport from the Continents to the Open Ocean: The Aging–Rejuvenation Cycle. Elements, 7(2), 101–106. doi:10.2113/gselements.7.2.101
- ^ Tagliabue, A., Bopp, L., Aumont,O., & Arrigo, K.R. (2009). Influence of light and temperature on the marine iron cycle: From theoretical to global modeling. Global Biogeochemical Cycles, 23.
doi:10.1029/2008GB003214
Bibliography
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Weeks, Mary Elvira; Leichester, Henry M. (1968). «Elements known to the ancients». Discovery of the elements. Easton, PA: Journal of Chemical Education. pp. 29–40. ISBN 0-7661-3872-0. LCCN 68-15217.
Further reading
- H.R. Schubert, History of the British Iron and Steel Industry … to 1775 AD (Routledge, London, 1957)
- R.F. Tylecote, History of Metallurgy (Institute of Materials, London 1992).
- R.F. Tylecote, «Iron in the Industrial Revolution» in J. Day and R.F. Tylecote, The Industrial Revolution in Metals (Institute of Materials 1991), 200–60.
External links
Wikiquote has quotations related to Iron.
Look up iron in Wiktionary, the free dictionary.
Wikimedia Commons has media related to Iron.
- It’s Elemental – Iron
- Iron at The Periodic Table of Videos (University of Nottingham)
- Metallurgy for the non-Metallurgist
- Iron by J.B. Calvert
Железо в таблице менделеева занимает 26 место, в 4 периоде.
| Символ | Fe |
| Номер | 26 |
| Атомный вес | 55.8450000 |
| Латинское название | Ferrum |
| Русское название | Железо |
Как самостоятельно построить электронную конфигурацию? Ответ здесь
Электронная схема железа
Fe: 1s2 2s2 2p6 3s2 3p6 4s2 3d6
Короткая запись:
Fe: [Ar]4s2 3d6
Одинаковую электронную конфигурацию имеют
атом железа и
Mn-1, Co+1, Ni+2
Порядок заполнения оболочек атома железа (Fe) электронами:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d →
5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
На подуровне ‘s’ может находиться до 2 электронов, на ‘s’ — до 6, на
‘d’ — до 10 и на ‘f’ до 14
Железо имеет 26 электронов,
заполним электронные оболочки в описанном выше порядке:
2 электрона на 1s-подуровне
2 электрона на 2s-подуровне
6 электронов на 2p-подуровне
2 электрона на 3s-подуровне
6 электронов на 3p-подуровне
2 электрона на 4s-подуровне
6 электронов на 3d-подуровне
Степень окисления железа
Атомы железа в соединениях имеют степени окисления 6, 5, 4, 3, 2, 1, 0, -1, -2.
Степень окисления — это условный заряд атома в соединении: связь в молекуле
между атомами основана на разделении электронов, таким образом, если у атома виртуально увеличивается
заряд, то степень окисления отрицательная (электроны несут отрицательный заряд), если заряд уменьшается,
то степень окисления положительная.
Ионы железа
Валентность Fe
Атомы железа в соединениях проявляют валентность VI, V, IV, III, II, I.
Валентность железа характеризует способность атома Fe к образованию хмических связей.
Валентность следует из строения электронной оболочки атома, электроны, участвующие в образовании
химических соединений называются валентными электронами. Более обширное определение валентности это:
Число химических связей, которыми данный атом соединён с другими атомами
Валентность не имеет знака.
Квантовые числа Fe
Квантовые числа определяются последним электроном в конфигурации,
для атома Fe эти числа имеют значение N = 3, L = 2, Ml = 3, Ms = -½
Видео заполнения электронной конфигурации (gif):
Результат:
Энергия ионизации
Чем ближе электрон к центру атома — тем больше энергии необходимо, что бы его оторвать.
Энергия, затрачиваемая на отрыв электрона от атома называется энергией ионизации и обозначается Eo.
Если не указано иное, то энергия ионизации — это энергия отрыва первого электрона, также существуют энергии
ионизации для каждого последующего электрона.
Энергия ионизации Fe:
Eo = 763 кДж/моль
— Что такое ион читайте в статье.
Перейти к другим элементам таблицы менделеева
Где Fe в таблице менделеева?
Таблица Менделеева
Скачать таблицу менделеева в хорошем качестве
Железо (Fe от лат. Ferrum) — элемент восьмой группы (по старой классификации — побочной подгруппы восьмой группы) четвёртого периода периодической системы химических элементов Д. И. Менделеева с атомным номером 26. Один из самых распространённых в земной коре металлов: второе место после алюминия.
Простое вещество железо — ковкий металл серебристо-белого цвета с высокой химической реакционной способностью: железо быстро корродирует при высоких температурах или при высокой влажности на воздухе. В чистом кислороде железо горит, а в мелкодисперсном состоянии самовозгорается и на воздухе.
Собственно железом обычно называют его сплавы с малым содержанием примесей (до 0,8 %), которые сохраняют мягкость и пластичность чистого металла. Но на практике чаще применяются сплавы железа с углеродом: сталь (до 2,14 вес. % углерода) и чугун (более 2,14 вес. % углерода), а также нержавеющая (легированная) сталь с добавками легирующих металлов (хром, марганец, никель и др.). Совокупность специфических свойств железа и его сплавов делают его «металлом № 1» по важности для человека.
В природе железо редко встречается в чистом виде, чаще всего оно встречается в составе железо-никелевых метеоритов. Распространённость железа в земной коре — 4,65 % (4-е место после O, Si, Al). Считается также, что железо составляет бо́льшую часть земного ядра.
|
|
|
| Название, символ, номер | Железо / Ferrum (Fe), 26 |
|---|---|
| Атомная масса (молярная масса) |
55,845(2) а. е. м. (г/моль) |
| Электронная конфигурация | [Ar] 3d6 4s2 |
| Радиус атома | 126 пм |
| Ковалентный радиус | 117 пм |
| Радиус иона | (+3e) 64 (+2e) 74 пм |
| Электроотрицательность | 1,83 (шкала Полинга) |
| Электродный потенциал | Fe←Fe3+ −0,04 В Fe←Fe2+ −0,44 В |
| Степени окисления | 6, 3, 2, 0 |
| Энергия ионизации (первый электрон) |
759,1 (7,87) кДж/моль (эВ) |
| Плотность (при н. у.) | 7,874 г/см³ |
| Температура плавления | 1812 K (1538,85 °C) |
| Температура кипения | 3134 K (2861 °C) |
| Уд. теплота плавления | 247,1 кДж/кг 13,8 кДж/моль |
| Уд. теплота испарения | ~6088 кДж/кг ~340 кДж/моль |
| Молярная теплоёмкость | 25,14 Дж/(K·моль) |
| Молярный объём | 7,1 см³/моль |
| Структура решётки | кубическая объёмноцентрированная |
| Параметры решётки | 2,866 Å |
| Температура Дебая | 460 K |
| Теплопроводность | (300 K) 80,4 Вт/(м·К) |
| Номер CAS | 7439-89-6 |
История
Основная статья: История железа
Железо как инструментальный материал известно с древнейших времён. Самые древние изделия из железа, найденные при археологических раскопках, датируются 4-м тысячелетием до н. э. и относятся к древнешумерской и древнеегипетской цивилизациям. Это изготовленные из метеоритного железа, то есть сплава железа и никеля (содержание последнего колеблется от 5 до 30 %), украшения из египетских гробниц (около 3800 года до н. э.) и кинжал из шумерского города Ура (около 3100 года до н. э.). От небесного происхождения метеоритного железа происходит, видимо, одно из названий железа в греческом и латинском языках: «сидер» (что значит «звёздный»).
Первыми освоили метод выплавки железа хатты. На это указывает древнейшее (2-е тысячелетие до н. э.) упоминание железа в текстах хеттов, основавших свою империю на территории хаттов (современной Анатолии в Турции). Так, в тексте хеттского царя Анитты (около 1800 года до н. э.) говорится:
Когда на город Пурусханду в поход я пошёл, человек из города Пурусханды ко мне поклониться пришёл (…?) и он мне 1 железный трон и 1 железный скипетр (?) в знак покорности (?) преподнёс…
— Гиоргадзе Г. Г. «Текст Анитты» и некоторые вопросы ранней истории хеттов // Вестник древней истории. 1965. № 4.
В древности мастерами железных изделий слыли халибы. В легенде об аргонавтах (их поход в Колхиду состоялся примерно за 50 лет до троянской войны) рассказывается, что царь Колхиды Эет дал Ясону железный плуг, чтобы он вспахал поле Ареса, и описываются его подданные халиберы:
Они не пашут землю, не сажают плодовые деревья, не пасут стада на тучных лугах; они добывают руду и железо из необработанной земли и выменивают на них продукты питания. День не начинается для них без тяжкого труда, в темноте ночи и густом дыму проводят они, работая весь день…
Аристотель описал их способ получения стали: «халибы несколько раз промывали речной песок их страны — тем самым выделяя чёрный шлих (тяжёлая фракция, состоящая в основном из магнетита и гематита), и плавили в печах; полученный таким образом металл имел серебристый цвет и был нержавеющим».
В качестве сырья для выплавки стали использовались магнетитовые пески, которые часто встречаются по всему побережью Чёрного моря: эти магнетитовые пески состоят из смеси мелких зёрен магнетита, титано-магнетита или ильменита, и обломков других пород, так что выплавляемая халибами сталь была легированной, и имела превосходные свойства. Такой своеобразный способ получения железа говорит о том, что халибы лишь распространили железо как технологический материал, но их способ не мог быть методом повсеместного промышленного производства железных изделий. Однако их производство послужило толчком для дальнейшего развития металлургии железа.
Климент Александрийский в своём энциклопедическом труде «Строматы» упоминает, что по греческим преданиям железо (видимо, выплавка его из руды) было открыто на горе Иде — так называлась горная цепь возле Трои (в Илиаде она упоминается как гора Ида, с которой Зевс наблюдал за битвой греков с троянцами). Произошло это через 73 года после Девкалионова потопа, а этот потоп, согласно Паросской хронике, был в 1528 году до н. э., то есть метод выплавки железа из руды был открыт примерно в 1455 году до н. э. Однако из описания Климента не ясно, говорит ли он именно об этой горе в Передней Азии (Ида Фригийская у Вергилия), или же о горе Ида на острове Крит, о которой римский поэт Вергилий в Энеиде пишет как о прародине троянцев:
Остров Юпитера, Крета, лежит средь широкого моря,
Нашего племени там колыбель, где высится Ида…
Более вероятно, что Климент Александрийский говорит именно о фригийской Иде возле Трои, так как там были найдены древние железные копи и очаги железоделательного производства. Первое письменное свидетельство о железе имеется в глиняных табличках архива египетских фараонов Аменхотепа III и Эхнатона, и относится к тому же времени (1450—1400 год до н. э.). Там упоминается о выделке железа на юге Закавказья, которое греки называли Колхидой (и возможно, что слово «kolhidos» может быть модификацией слова «halibos») — а именно, что царь страны Митанни и властитель Армении и Южного Закавказья послал египетскому фараону Аменхотепу II «вместе с 318 наложницами кинжалы и кольца из хорошего железа». Такие же подарки фараонам дарили и хетты.
В самой глубокой древности железо ценилось дороже золота, и по описанию Страбона, у африканских племён за 1 фунт железа давали 10 фунтов золота, а по исследованиям историка Г. Арешяна стоимости меди, серебра, золота и железа у древних хеттов были в соотношении 1 : 160 : 1280 : 6400. В те времена железо использовалось как ювелирный металл, из него делали троны и другие регалии царской власти: например, в библейской книге Второзаконие 3,11 описан «одр железный» рефаимского царя Ога.
В гробнице Тутанхамона (около 1350 года до н. э.) было найдено девятнадцать предметов из железа, включая кинжал из железа в золотой оправе — возможно, подаренный хеттами в дипломатических целях. Но хетты не стремились к широкому распространению железа и его технологий, что видно и из дошедшей до нас переписки египетского фараона Тутанхамона и его тестя Хаттусиля — царя хеттов. Фараон просит прислать побольше железа, а царь хеттов уклончиво отвечает, что запасы железа иссякли, а кузнецы заняты на сельскохозяйственных работах, поэтому он не может выполнить просьбу царственного зятя, и посылает только один кинжал из «хорошего железа» (то есть стали). Как видно, хетты старались использовать свои знания для достижения военных преимуществ, и не давали другим возможности сравняться с ними. Видимо, поэтому железные изделия получили широкое распространение только после Троянской войны и падения державы хеттов, когда благодаря торговой активности греков технология железа стала известной многим, и были открыты новые месторождения железа и рудники. Так на смену «Бронзовому» веку настал век «Железный».
По описаниям Гомера, хотя во время Троянской войны (примерно 1250 год до н. э.) оружие было в основном из меди и бронзы, но железо уже было хорошо известно и пользовалось большим спросом, хотя больше как драгоценный металл. Например, в 23-й песне «Илиады» Гомер рассказывает, что Ахилл наградил диском из железной крицы победителя в соревновании по метанию диска. Это железо ахейцы добывали у троянцев и сопредельных народов (Илиада 7,473), в том числе у халибов, которые воевали на стороне троянцев:
Прочие мужи ахейские меной вино покупали,
Те за звенящую медь, за седое железо меняли,
Те за воловые кожи или волов круторогих,
Те за своих полонёных. И пир уготовлен весёлый…
Возможно, железо было одной из причин, побудивших греков-ахейцев двинуться в Малую Азию, где они узнали секреты его производства. А раскопки в Афинах показали, что уже около 1100 года до н. э. и позднее уже широко были распространены железные мечи, копья, топоры, и даже железные гвозди. В библейской книге Иисуса Навина 17,16 (ср. Судей 14,4) описывается, что филистимляне (библейские «PILISTIM», а это были протогреческие племена, родственные позднейшим эллинам, в основном пеласги) имели множество железных колесниц, то есть в это время железо уже стало широко применяться в больших количествах.
Гомер в «Илиаде» и «Одиссее» называет железо «многотрудный металл», и описывает закалку орудий:
Расторопный ковач, изготовив топор иль секиру,
В воду металл, раскаливши его, чтоб двойную
Он крепость имел, погружает…
Гомер называет железо многотрудным, потому что в древности основным методом его получения был сыродутный процесс: перемежающиеся слои железной руды и древесного угля прокаливались в специальных печах (горнах — от древнего «Horn» — рог, труба, первоначально это была просто труба, вырытая в земле, обычно горизонтально в склоне оврага). В горне окислы железа восстанавливаются до металла раскалённым углём, который отбирает кислород, окисляясь до окиси углерода, и в результате такого прокаливания руды с углём получалось тестообразное кричное (губчатое) железо. Крицу очищали от шлаков ковкой, выдавливая примеси сильными ударами молота. Первые горны имели сравнительно низкую температуру — заметно меньше температуры плавления чугуна, поэтому железо получалось сравнительно малоуглеродистым. Чтобы получить крепкую сталь, приходилось много раз прокаливать и проковывать железную крицу с углём, при этом поверхностный слой металла дополнительно насыщался углеродом и упрочнялся. Так получалось «хорошее железо» — и хотя это требовало больших трудов, изделия, полученные таким способом, были существенно более крепкими и твёрдыми, чем бронзовые.
В дальнейшем научились делать более эффективные печи (в русском языке — домна, домница) для производства стали, и применили меха для подачи воздуха в горн. Уже римляне умели доводить температуру в печи до плавления стали (около 1400 °C, а чистое железо плавится при 1535 °C). При этом образуется чугун с температурой плавления 1100—1200 °C, очень хрупкий в твёрдом состоянии (даже не поддающийся ковке) и не обладающий упругостью стали. Первоначально его считали вредным побочным продуктом (англ. pig iron, по-русски, свинское железо, чушки, откуда, собственно, и происходит слово чугун), но потом обнаружилось, что при повторной переплавке в печи с усиленным продуванием через него воздуха чугун превращается в сталь хорошего качества, так как лишний углерод выгорает. Такой двухстадийный процесс производства стали из чугуна оказался более простым и выгодным, чем кричный, и этот принцип используется без особых изменений многие века, оставаясь и до наших дней основным способом производства железных материалов.
Происхождение названия
Праславянское *želězo (белор. жалеза, укр. залізо, ст.‑слав. желѣзо, болг. желязо, сербохорв. жељезо, польск. żelazo, чеш. železo, словен. železo) имеет ясные параллели в балтийских языках (лит. geležis, латыш. dzelzs). Слово является однокоренным словам «железа» и «желвак»; и имеет смысл «округлый камень, окатыш, блямба».
Имеется несколько версий дальнейшей этимологии этого балтославянского слова.
Одна из них связывает праслав. *želězo с греческим словом χαλκός, что означало железо и медь, согласно другой версии *želězo родственно словам *žely «черепаха» и *glazъ «скала», с общей семой «камень». Третья версия предполагает древнее заимствование из неизвестного языка.
Романские языки (итал. ferro, фр. fer, исп. hierro, порт. ferro, рум. fier) продолжают лат. ferrum. Латинское ferrum (< *ferzom), возможно, заимствовано из какого-то восточного языка, скорее всего, из финикийского. Ср. ивр. barzel, шумерск. barzal, ассирийск. parzilla. Отсюда же, вероятно, баскское burdina.
Германские языки заимствовали название железа (готск. eisarn, англ. iron, нем. Eisen, нидерл. ijzer, дат. jern, швед. järn) из кельтских.
Пракельтское слово *isarno- (> др.-ирл. iarn, др.-брет. hoiarn), вероятно, восходит к пра-и.е. *h1esh2r-no- «кровавый» с семантическим развитием «кровавый» > «красный» > «железо». Согласно другой гипотезе данное слово восходит к пра-и.е. *(H)ish2ro- «сильный, святой, обладающий сверхъестественной силой».
Древнегреческое слово σίδηρος, возможно, было заимствовано из того же источника, что и славянское, германское и балтийское слова для серебра.
Название природного карбоната железа (сидерита) происходит от лат. sidereus — звёздный; действительно, первое железо, попавшее в руки людям, было метеоритного происхождения. Возможно, это совпадение не случайно. В частности, древнегреческое слово сидерос (σίδηρος) для железа и латинское sidus, означающее «звезда», вероятно, имеют общее происхождение.
Изотопы
Основная статья: Изотопы железа
Природное железо состоит из четырёх стабильных изотопов: 54Fe (изотопная распространённость 5,845 %), 56Fe (91,754 %), 57Fe (2,119 %) и 58Fe (0,282 %). Так же известно более 20 нестабильных изотопов железа с массовыми числами от 45 до 72, наиболее устойчивые из которых — 60Fe (период полураспада по уточнённым в 2009 году данным составляет 2,6 миллиона лет), 55Fe (2,737 года), 59Fe (44,495 суток) и 52Fe (8,275 часа); остальные изотопы имеют период полураспада менее 10 минут.
Изотоп железа 56Fe относится к наиболее стабильным ядрам: все следующие элементы могут уменьшить энергию связи на нуклон путём распада, а все предыдущие элементы, в принципе, могли бы уменьшить энергию связи на нуклон за счёт синтеза. Полагают, что железом оканчивается ряд синтеза элементов в ядрах нормальных звёзд (см. Железная звезда), а все последующие элементы могут образоваться только в результате взрывов сверхновых.
Геохимия железа
Гидротермальный источник с железистой водой. Оксиды железа окрашивают воду в бурый цвет
Железо — один из самых распространённых элементов в Солнечной системе, особенно на планетах земной группы, в частности, на Земле. Значительная часть железа планет земной группы находится в ядрах планет, где его содержание, по оценкам, около 90 %. Содержание железа в земной коре составляет 5 %, а в мантии около 12 %. Из металлов железо уступает по распространённости в коре только алюминию. При этом в ядре находится около 86 % всего железа, а в мантии 14 %. Содержание железа значительно повышается в изверженных породах основного состава, где оно связано с пироксеном, амфиболом, оливином и биотитом. В промышленных концентрациях железо накапливается в течение почти всех экзогенных и эндогенных процессов, происходящих в земной коре. В морской воде железо содержится в очень малых количествах 0,002—0,02 мг/л. В речной воде его концентрация значительно выше — 2 мг/л.
Геохимические свойства железа
Распространение железа в пересчёте на 106 атомов кремния.
Важнейшая геохимическая особенность железа — наличие у него нескольких степеней окисления. Железо в нейтральной форме — металлическое — слагает ядро Земли, возможно, присутствует в мантии и очень редко встречается в земной коре. Закисное железо FeO — основная форма нахождения железа в мантии и земной коре. Окисное железо Fe2O3 характерно для самых верхних, наиболее окисленных, частей земной коры, в частности, осадочных пород.
По кристаллохимическим свойствам ион Fe2+ близок к ионам Mg2+ и Ca2+ — другим главным элементам, составляющим значительную часть всех земных пород. В силу кристаллохимического сходства железо замещает магний и, частично, кальций во многих силикатах. При этом содержание железа в минералах переменного состава обычно увеличивается с уменьшением температуры.
Минералы железа
В земной коре железо распространено достаточно широко — на его долю приходится около 4,1 % массы земной коры (4-е место среди всех элементов, 2-е среди металлов). В мантии и земной коре железо сосредоточено главным образом в силикатах, при этом его содержание значительно в основных и ультраосновных породах, и мало — в кислых и средних породах.
Известно большое число руд и минералов, содержащих железо. Наибольшее практическое значение имеют красный железняк (гематит, Fe2O3; содержит до 70 % Fe), магнитный железняк (магнетит, FeO · Fe2O3 или Fe3O4; содержит 72,4 % Fe), бурый железняк или лимонит (гётит и гидрогётит, соответственно FeOOH и FeOOH·nH2O). Гётит и гидрогётит чаще всего встречаются в корах выветривания, образуя так называемые «железные шляпы», мощность которых достигает несколько сотен метров. Также они могут иметь осадочное происхождение, выпадая из коллоидных растворов в озёрах или прибрежных зонах морей. При этом образуются оолитовые, или бобовые, железные руды. В них часто встречается вивианит Fe3(PO4)2·8H2O, образующий чёрные удлинённые кристаллы и радиально-лучистые агрегаты.
В природе также широко распространены сульфиды железа — пирит FeS2 (серный или железный колчедан) и пирротин. Они не являются железной рудой — пирит используют для получения серной кислоты, а пирротин часто содержит никель и кобальт.
По запасам железных руд Россия занимает первое место в мире.
Содержание железа в морской воде — 1⋅10−5—1⋅10−8 %.
Другие часто встречающиеся минералы железа:
- Сидерит — FeCO3 — содержит примерно 35 % железа. Обладает желтовато-белым (с серым или коричневым оттенком в случае загрязнения) цветом. Плотность равна 3 г/см³ и твёрдость 3,5—4,5 по шкале Мооса.
- Марказит — FeS2 — содержит 46,6 % железа. Встречается в виде жёлтых, как латунь, бипирамидальных ромбических кристаллов с плотностью 4,6—4,9 г/см³ и твёрдостью 5—6 по шкале Мооса.
- Лёллингит — FeAs2 — содержит 27,2 % железа и встречается в виде серебристо-белых бипирамидальных ромбических кристаллов. Плотность равна 7—7,4 г/см³, твёрдость 5—5,5 по шкале Мооса.
- Миспикель — FeAsS — содержит 34,3 % железа. Встречается в виде белых моноклинных призм с плотностью 5,6—6,2 г/см³ и твёрдостью 5,5—6 по шкале Мооса.
- Мелантерит — FeSO4·7H2O — реже встречается в природе и представляет собой зелёные (или серые из-за примесей) моноклинные кристаллы, обладающие стеклянным блеском, хрупкие. Плотность равна 1,8—1,9 г/см³.
- Вивианит — Fe3(PO4)2·8H2O — встречается в виде сине-серых или зелёно-серых моноклинных кристаллов с плотностью 2,95 г/см³ и твёрдостью 1,5—2 по шкале Мооса.
Помимо вышеописанных минералов железа, существуют, например:
|
|
|
|
Основные месторождения
По данным Геологической службы США (оценка 2011 г.), мировые разведанные запасы железной руды составляют порядка 178 млрд тонн. Основные месторождения железа находятся в Бразилии (1 место), Австралии, США, Канаде, Швеции, Венесуэле, Либерии, Украине, Франции, Индии. В России железо добывается на Курской магнитной аномалии (КМА), Кольском полуострове, в Карелии и в Сибири. Значительную роль в последнее время приобретают донные океанские месторождения, в которых железо совместно с марганцем и другими ценными металлами находится в конкрециях.
Получение
В промышленности железо получают из железной руды, в основном из гематита (Fe2O3) и магнетита (FeO·Fe2O3).
Существуют различные способы извлечения железа из руд. Наиболее распространённым является доменный процесс.
Первый этап производства — восстановление железа углеродом в доменной печи при температуре 2000 °C. В доменной печи углерод в виде кокса, железная руда в виде агломерата или окатышей и флюс (например, известняк) подаются сверху, а снизу их встречает поток нагнетаемого горячего воздуха.
В печи углерод в виде кокса окисляется до монооксида углерода. Данный оксид образуется при горении в недостатке кислорода:
- 2C + O2 ⟶ 2CO↑
В свою очередь, монооксид углерода восстанавливает железо из руды. Чтобы данная реакция шла быстрее, нагретый угарный газ пропускают через оксид железа(III):
- 3CO + Fe2O3 ⟶ 2Fe + 3CO2↑
Флюс добавляется для избавления от нежелательных примесей (в первую очередь от силикатов; например, кварц) в добываемой руде. Типичный флюс содержит известняк (карбонат кальция) и доломит (карбонат магния). Для устранения других примесей используют другие флюсы.
Действие флюса (в данном случае карбонат кальция) заключается в том, что при его нагревании он разлагается до его оксида:
- CaCO3 →1000∘C CaO + CO2↑
Оксид кальция соединяется с диоксидом кремния, образуя шлак — метасиликат кальция:
- CaO + SiO2 →>1000∘C CaSiO3
Шлак, в отличие от диоксида кремния, плавится в печи. Более лёгкий, чем железо, шлак плавает на поверхности — это свойство позволяет разделять шлак от металла. Шлак затем может использоваться при строительстве и сельском хозяйстве. Расплав железа, полученный в доменной печи, содержит довольно много углерода (чугун). Кроме таких случаев, когда чугун используется непосредственно, он требует дальнейшей переработки.
Излишки углерода и другие примеси (сера, фосфор) удаляют из чугуна окислением в мартеновских печах или в конвертерах. Электрические печи используются и для выплавки легированных сталей.
Кроме доменного процесса, распространён процесс прямого получения железа. В этом случае предварительно измельчённую руду смешивают с особой глиной, формируя окатыши. Окатыши обжигают, и обрабатывают в шахтной печи горячими продуктами конверсии метана, которые содержат водород. Водород легко восстанавливает железо:
- Fe2O3 + 3H2 →1000∘C 2Fe + 3H2O,
при этом не происходит загрязнения железа такими примесями, как сера и фосфор, которые являются обычными примесями в каменном угле. Железо получается в твёрдом виде, и в дальнейшем переплавляется в электрических печах.
Химически чистое железо получается электролизом растворов его солей.
Физические свойства
Железо — типичный металл, в свободном состоянии — серебристо-белого цвета с сероватым оттенком. Чистый металл пластичен, различные примеси (в частности — углерод) повышают его твёрдость и хрупкость. Обладает ярко выраженными магнитными свойствами. Часто выделяют так называемую «триаду железа» — группу трёх металлов (железо Fe, кобальт Co, никель Ni), обладающих схожими физическими свойствами, атомными радиусами и значениями электроотрицательности.
Для железа характерен полиморфизм, оно имеет четыре кристаллические модификации:
- до 769 °C существует α-Fe (феррит) с объёмноцентрированной кубической решёткой и свойствами ферромагнетика (769 °C ≈ 1043 K — точка Кюри для железа);
- в температурном интервале 769—917 °C существует β-Fe, который отличается от α-Fe только параметрами объёмно-центрированной кубической решётки и магнитными свойствами парамагнетика;
- в температурном интервале 917—1394 °C существует γ-Fe (аустенит) с гранецентрированной кубической решёткой;
- выше 1394 °C устойчиво δ-Fe с объёмно-центрированной кубической решёткой.
Металловедение не выделяет β-Fe как отдельную фазу, и рассматривает её как разновидность α-Fe. При нагреве железа или стали выше точки Кюри (769 °C ≈ 1043 K) тепловое движение ионов расстраивает ориентацию спиновых магнитных моментов электронов, ферромагнетик становится парамагнетиком — происходит фазовый переход второго рода, но фазового перехода первого рода с изменением основных физических параметров кристаллов не происходит.
Для чистого железа при нормальном давлении, с точки зрения металловедения, существуют следующие устойчивые модификации:
- от абсолютного нуля до 910 °C устойчива α-модификация с объёмноцентрированной кубической (ОЦК) кристаллической решёткой;
- от 910 до 1400 °C устойчива γ-модификация с гранецентрированной кубической (ГЦК) кристаллической решёткой;
- от 1400 до 1539 °C устойчива δ-модификация с объёмно-центрированной кубической (ОЦК) кристаллической решёткой.
Наличие в стали углерода и легирующих элементов существенным образом изменяет температуры фазовых переходов (см. фазовую диаграмму железо—углерод). Твёрдый раствор углерода в α- и δ-железе называется ферритом. Иногда различают высокотемпературный δ-феррит и низкотемпературный α-феррит (или просто феррит), хотя их атомные структуры одинаковы. Твёрдый раствор углерода в γ-железе называется аустенитом.
- В области высоких давлений (свыше 13 ГПа, 128,3 тыс. атм.) возникает модификация ε-железа с гексагональной плотноупакованной (ГПУ) решёткой.
Явление полиморфизма чрезвычайно важно для металлургии стали. Именно благодаря α—γ переходам кристаллической решётки происходит термообработка стали. Без этого явления железо как основа стали не получило бы такого широкого применения.
Железо относится к умеренно тугоплавким металлам. В ряду стандартных электродных потенциалов железо стоит до водорода и легко реагирует с разбавленными кислотами. Таким образом, железо относится к металлам средней активности.
Температура плавления железа 1539 °C, температура кипения — 2862 °C.
Химические свойства
Характерные степени окисления
| Степень окисления | Оксид | Гидроксид | Характер | Примечания |
|---|---|---|---|---|
| +2 | FeO | Fe(OH)2 | Слабоосновный | Слабый восстановитель |
| +3 | Fe2O3 | Fe(OH)3 | Очень слабое основание, иногда — амфотерный | Слабый окислитель |
| +6 | Не получен | <H2FeO4>* | Кислотный | Сильный окислитель |
* Кислота в свободном виде не существует — получены только её соли.
Диаграмма Пурбе для Fe-H2O
Для железа характерны степени окисления — +2 и +3.
Степени окисления +2 соответствует чёрный оксид FeO и зелёный гидроксид Fe(OH)2. Они имеют основный характер. В солях Fe(+2) присутствует в виде катиона. Fe(+2) — слабый восстановитель.
Степени окисления +3 соответствуют красно-коричневый оксид Fe2O3 и коричневый гидроксид Fe(OH)3. Они носят амфотерный характер, хотя и кислотные, и основные свойства у них выражены слабо. Так, ионы Fe3+ нацело гидролизуются даже в кислой среде. Fe(OH)3 растворяется (и то не полностью), только в концентрированных щелочах. Fe2O3 реагирует со щелочами только при сплавлении, давая ферриты (формальные соли не существующей в свободном виде кислоты HFeO2):
-
- Fe2O3 + 2NaOH → 2NaFeO2 + H2O
Железо (+3) чаще всего проявляет слабые окислительные свойства.
Степени окисления +2 и +3 легко переходят между собой при изменении окислительно-восстановительных условий.
Кроме того, существует оксид Fe3O4, формальная степень окисления железа в котором +8/3. Однако этот оксид можно также рассматривать как феррит железа (II) Fe+2(Fe+3O2)2.
Также существует степень окисления +6. Соответствующего оксида и гидроксида в свободном виде не существует, но получены соли — ферраты (например, K2FeO4). Железо (+6) находится в них в виде аниона. Ферраты являются сильными окислителями.
Известны также степени окисления: −2 (тетракарбонилферрат натрия), −1, 0 (пентакарбонил железа), +1, +4, +5.
Свойства простого вещества
При хранении на воздухе при температуре до 200 °C железо постепенно покрывается плотной плёнкой оксида, препятствующей дальнейшему окислению металла. Во влажном воздухе железо покрывается рыхлым слоем ржавчины, который не препятствует доступу кислорода и влаги к металлу и его разрушению. Ржавчина не имеет постоянного химического состава, приближённо её химическую формулу можно записать как Fe2O3·xH2O.
Взаимодействует с кислотами.
- С соляной кислотой:
-
- Fe + 2HCl → FeCl2 + H2↑
- С разбавленной серной кислотой:
-
- Fe + H2SO4 → FeSO4 + H2↑
- Концентрированные азотная и серная кислоты пассивируют железо. C концентрированной серной кислотой взаимодействует только при нагревании:
-
- 2Fe + 6H2SO4 →to Fe2(SO4)3 + 3SO2↑ + 6H2O
- Взаимодействие с кислородом.
-
- Железо горит в кислороде, нагретое горит на воздухе, пирофорное — на воздухе без нагревания:
-
- 3Fe + 2O2 →150−600oC Fe3O4
- Пропускание кислорода или воздуха через расплавленное железо:
-
- 2Fe + O2 →to 2FeO
- Взаимодействие с порошком серы при нагревании:
-
- Fe + S →to FeS
- Взаимодействие с галогенами при нагревании.
-
- Горение в хлоре:
-
- 2Fe + 3Cl2 →to 2FeCl3
- При повышенном давлении паров брома:
-
- 2Fe + 3Br2 →p 2FeBr3
- Взаимодействие с йодом:
-
- 3Fe + 4I2 → Fe3I8
- Взаимодействие с неметаллами.
-
- С азотом при нагревании:
-
- 6Fe + N2 →to 2Fe3N
- С фосфором при нагревании:
-
- Fe + P →to FeP
- 2Fe + P →to Fe2P
- 3Fe + P →to Fe3P
- С углеродом:
-
- 3Fe + C → Fe3C
- С кремнием:
-
- Fe + Si → FeSi
- Взаимодействие раскалённого железа с водяным паром:
-
- 3Fe + 4H2O →to Fe3O4 + 4H2↑
- Железо восстанавливает металлы, которые в ряду активности стоят правее него, из растворов солей:
-
- Fe + CuSO4 → FeSO4 + Cu
- Железо восстанавливает соединения железа(III):
-
- Fe + 2FeCl3 → 3FeCl2
При повышенном давлении металлическое железо реагирует с оксидом углерода(II) CO, причём образуется жидкий, при обычных условиях легко летучий пентакарбонил железа Fe(CO)5. Известны также карбонилы железа составов Fe2(CO)9 и Fe3(CO)12. Карбонилы железа служат исходными веществами при синтезе железоорганических соединений, в том числе и ферроцена состава (η5-C5H5)2Fe.
Чистое металлическое железо устойчиво в воде и в разбавленных растворах щелочей. Железо не растворяется в холодных концентрированных серной и азотной кислотах из-за пассивации поверхности металла прочной оксидной плёнкой. Горячая концентрированная серная кислота, являясь более сильным окислителем, взаимодействует с железом.
Соединения железа (II)
Оксид железа(II) FeO обладает основными свойствами, ему отвечает основание Fe(OH)2. Соли железа (II) обладают светло-зелёным цветом. При их хранении, особенно во влажном воздухе, они коричневеют за счёт окисления до железа (III). Такой же процесс протекает при хранении водных растворов солей железа(II):
-
- 4FeCl2 + O2 + 2H2O → 4Fe(OH)Cl2
Из солей железа(II) в водных растворах устойчива соль Мора — двойной сульфат аммония и железа(II) (NH4)2Fe(SO4)2·6H2O.
Реактивом на ионы Fe2+ в растворе может служить гексацианоферрат(III) калия K3[Fe(CN)6] (красная кровяная соль). При взаимодействии ионов Fe2+ и [Fe(CN)6]3− выпадает осадок гексацианоферрата (III) калия-железа (II) (турнбулева синь):
-
- K3[Fe(CN)6] + Fe2+ → KFeII[FeIII(CN)6]↓ + 2K+,
который внутримолекулярно перегруппировывается в гексацианоферрат (II) калия-железа (III) (берлинская лазурь):
-
- KFeII[FeIII(CN)6] → KFeIII[FeII(CN)6]
Для количественного определения железа (II) в растворе используют фенантролин Phen, образующий с железом (II) красный комплекс FePhen3 (максимум светопоглощения — 520 нм) в широком диапазоне рН (4-9).
Соединения железа (III)
Оксид железа(III) Fe2O3 слабо амфотерен, ему отвечает ещё более слабое, чем Fe(OH)2, основание Fe(OH)3, которое реагирует с кислотами:
-
- 2Fe(OH)3 + 3H2SO4 → Fe2(SO4)3 + 6H2O
Соли Fe3+ склонны к образованию кристаллогидратов. В них ион Fe3+, как правило, окружён шестью молекулами воды. Такие соли имеют розовый или фиолетовый цвет.
Ион Fe3+ полностью гидролизуется даже в кислой среде. При pH>4 этот ион практически полностью осаждается в виде Fe(OH)3:
-
- Fe3+ + 3H2O → Fe(OH)3↓ + 3H+
При частичном гидролизе иона Fe3+ образуются многоядерные оксо- и гидроксокатионы, из-за чего растворы приобретают коричневый цвет.
Кислотные свойства гидроксида железа(III) Fe(OH)3 выражены очень слабо. Он способен реагировать только с концентрированными растворами щелочей:
-
- Fe(OH)3 + 3KOH → K3[Fe(OH)6]
Образующиеся при этом гидроксокомплексы железа(III) устойчивы только в сильно щелочных растворах. При разбавлении растворов водой они разрушаются, причём в осадок выпадает Fe(OH)3.
При сплавлении со щелочами и оксидами других металлов Fe2O3 образует разнообразные ферриты:
-
- Fe2O3 + 2NaOH → 2NaFeO2 + H2O
Соединения железа(III) в растворах восстанавливаются металлическим железом:
-
- Fe + 2FeCl3 → 3FeCl2
Железо(III) способно образовывать двойные сульфаты с однозарядными катионами типа квасцов, например, KFe(SO4)2 — железокалиевые квасцы, (NH4)Fe(SO4)2 — железоаммонийные квасцы и т. д.
Для качественного обнаружения в растворе соединений железа(III) используют качественную реакцию ионов Fe3+ с неорганическими тиоцианатами SCN−. При этом образуется смесь ярко-красных роданидных комплексов железа [Fe(SCN)]2+, [Fe(SCN)2]+, Fe(SCN)3, [Fe(SCN)4]—. Состав смеси (а значит, и интенсивность её окраски) зависит от различных факторов, поэтому для точного качественного определения железа этот метод неприменим.
Другим качественным реактивом на ионы Fe3+ служит гексацианоферрат(II) калия K4[Fe(CN)6] (жёлтая кровяная соль). При взаимодействии ионов Fe3+ и [Fe(CN)6]4− выпадает ярко-синий осадок гексацианоферрата (II) калия-железа (III) (берлинская лазурь):
-
- K4[Fe(CN)6] + FeCl3 → KFeIII[FeII(CN)6]↓ + 3KCl
Количественно ионы Fe3+ определяют по образованию красных (в слабокислой среде) или жёлтых (в слабощелочной среде) комплексов с сульфосалициловой кислотой. Эта реакция требует грамотного подбора буферов, так как некоторые анионы (в частности, ацетат) образуют с железом и сульфосалициловой кислотой смешанные комплексы со своими оптическими характеристиками.
Соединения железа (VI)
Ферраты — соли не существующей в свободном виде железной кислоты H2FeO4. Это соединения фиолетового цвета, по окислительным свойствам напоминающие перманганаты, а по растворимости — сульфаты. Получают ферраты при действии газообразного хлора или озона на взвесь Fe(OH)3 в щёлочи:
-
- 2Fe(OH)3 + 3Cl2 + 10KOH → 2K2FeO4 + 6KCl + 8H2O
Ферраты также можно получить электролизом 30%-ного раствора щёлочи на железном аноде:
-
- Fe + 2KOH + 2H2O → K2FeO4 + 3H2↑
Ферраты — сильные окислители. В кислой среде разлагаются с выделением кислорода:
-
- 4FeO42− + 20H+ → 4Fe3+ + 3O2↑ + 10H2O
Окислительные свойства ферратов используют для обеззараживания воды.
Соединения железа VII и VIII
Известна степень окисления VII в анионе [FeO4]—.
Имеются сообщения об электрохимическом получении соединений железа(VIII), однако независимых работ, подтверждающих эти результаты, нет.
Применение
Железо — один из самых используемых металлов, на него приходится до 95 % мирового металлургического производства.
- Железо является основным компонентом сталей и чугунов — важнейших конструкционных материалов.
- Железо может входить в состав сплавов на основе других металлов — например, никелевых.
- Магнитная окись железа (магнетит) — важный материал в производстве устройств долговременной компьютерной памяти: жёстких дисков, дискет и т. п.
- Ультрадисперсный порошок магнетита используется во многих чёрно-белых лазерных принтерах в смеси с полимерными гранулами в качестве тонера. Здесь одновременно используется чёрный цвет магнетита и его способность прилипать к намагниченному валику переноса.
- Уникальные ферромагнитные свойства ряда сплавов на основе железа способствуют их широкому применению в электротехнике для магнитопроводов трансформаторов и электродвигателей.
- Хлорид железа(III) (хлорное железо) используется в радиолюбительской практике для травления печатных плат.
- Семиводный сульфат железа (железный купорос) в смеси с медным купоросом используется для борьбы с вредными грибками в садоводстве и строительстве.
- Железо применяется в качестве анода в железо-никелевых аккумуляторах, железо-воздушных аккумуляторах.
- Водные растворы хлоридов двухвалентного и трёхвалентного железа, а также его сульфатов используются в качестве коагулянтов в процессах очистки природных и сточных вод на водоподготовке промышленных предприятий.
Биологическое значение железа
В живых организмах железо является важным микроэлементом, катализирующим процессы обмена кислородом (дыхания). Основным внутриклеточным депо железа является глобулярный белковый комплекс — ферритин. Недостаток железа проявляется как болезнь организма: хлороз у растений и анемия у животных.
Обычно железо входит в ферменты в виде комплекса, называемого гемом. В частности, этот комплекс присутствует в гемоглобине — важнейшем белке, обеспечивающем транспорт кислорода с кровью ко всем органам человека и животных. И именно он окрашивает кровь в характерный красный цвет.
Комплексы железа, отличные от гема, встречаются, например, в ферменте метан-моноксигеназе, окисляющем метан в метанол, в важном ферменте рибонуклеотид-редуктазе, который участвует в синтезе ДНК. Неорганические соединения железа встречаются в некоторых бактериях, иногда используется ими для связывания азота воздуха.
Железо в организме человека
В организме взрослого человека содержится около 3—4 граммов железа (около 0,02 %), из которых только около 3,5 мг находится в плазме крови. Гемоглобин имеет примерно 68 % железа всего организма, ферритин — 27 %, миоглобин — 4 %, трансферрин — 0,1 %. Источниками железа при биосинтезе железосодержащих белков служат железо, поступающее из пищи, и железо, освобождающееся при постоянном распаде эритроцитов в гепатоцитах (клетках печени) и клетках селезёнки.
Суточная потребность человека в железе, по российским данным, следующая: дети — от 4 до 18 мг, взрослые мужчины — 10 мг, взрослые женщины — 18 мг, беременные женщины во второй половине беременности — 33 мг. У женщин детородного возраста потребность в железе выше ввиду регулярной кровопотери во время менструаций.
Национальная Академия медицины США различает среднюю потребность в железе и рекомендованное потребление железа, последняя норма разработана с тем, чтоб обеспечивать среднюю потребность для не менее 97 % в каждой группе населения. Расчет средней потребности в железе зависит от усваиваемости железа, нижеприведённая таблица основана на предположении о потреблении 10 % железа из животных продуктов (средняя усваиваемость 25 %) и 90 % железа из растительных продуктов (средняя усваиваемость 16,8 %), с общей усваиваемостью 18 %. Поскольку рацион детей до года сильно отличается от взрослого, норма для них основана на предполагаемой усваиваемости 10 %
| Пол | Возраст | Рекомендованное потребление железа (по Национальной Академии медицины США), мг/сутки |
|---|---|---|
| Младенцы | до 6 месяцев | 0,27 |
| Младенцы | 7—12 месяцев | 11 |
| Дети | 1—3 года | 7 |
| Дети | 4—8 лет | 10 |
| Подростки | 9—13 лет | 8 |
| Юноши | 14—18 лет | 11 |
| Девушки | 14—18 лет | 15 |
| Мужчины | 19 лет и старше | 8 |
| Женщины | 19—50 лет | 18 |
| Женщины | 50 лет и старше | 8 |
В организм животных и человека железо поступает с пищей. Наиболее богаты им печень и мясо, в меньшей степени яйца, бобовые, семена тыквы и кунжута, цельнозерновые крупы, а также некоторые виды зелени — тимьян, петрушка, полевой салат. Долгое время список железосодержащих продуктов возглавлял шпинат, ошибочно внесённый из-за опечатки в результатах анализа (был потерян «лишний» ноль после запятой).
Железо в питании подразделяют на гемовое, или гемное (из мяса и других животных источников) и негемовое (из растительной пищи). В гемсодержащих белках железо находится в составе гема. В негемовых железосодержащих белках железо непосредственно связывается с белком. К таким белкам относят трансферрин, ферритин, окислительные ферменты рибонуклеотидредуктазу и ксантиноксидазу, железофлавопротеины NADH-дегидрогеназа и сукцинат-дегидрогеназа. Описанные белки, содержащие негемовое железо, относятся к классу ферредоксинов, наиболее изученные из которых содержатся в хлоропластах зелёных растений и окисляются при переносе электрона в процессе фотосинтеза, а также бактериальные ферредоксины (например анаэробной бактерии Clostridium pasteurianum), участвующие в аэробном или анаэробном переносе электрона. Человеческий ферредоксин-1 участвует в гидроксилировании и расщеплении стероидных гормонов и холестерола в системе микросомальных (эндоплазматического ретикулума гепатоцитов) ферментов цитохрома Р450, а также в синтезе гормонов щитовидной железы. Сердцевина ферредоксина состоит из молекул двух- или четырёх-валентной серы и четырёхвалентного железа и имеет общую формулу вида FenSmHp (например Fe2S2), она соединена с белковыми остовами через аминокислоту цистеин.Гемовое железо усваивается наиболее эффективно (от 15 до 35 %). На усвоение негемового железа (даже в животной пище его порядка 60 %) влияют многочисленные факторы. Заметно улучшают усвоение железа потребляемые вместе с пищей аскорбиновая кислота или мясной белок. Препятствуют усвоению железа яйца, кальций, но главным образом антипитательные вещества — фитиновая кислота, оксалаты, танины и кофеин.
К примеру, из-за высокого уровня фитиновых соединений усвоение железа из бобовых находится в районе 0,84-0,91 %. Согласно одному из американских исследований, потребление с железосодержащей пищей богатого танинами чая снижает усвоение микроэлемента на 62 %, кофе — на 35 %, а потребление апельсинового сока (с высоким содержанием аскорбиновой кислоты) увеличивает его на 85 %. В то же время данные из Китая указывают на то, что даже очень высокое потребление чая в целом не сказывается на содержании железа в крови.
Дефицит железа
Основная статья: Дефицит железа
При недостатке железа костный мозг продуцирует меньше эритроцитов, а клетки крови сокращаются в размерах.
При сбалансированной диете железа, поступающего с пищей, как правило, вполне достаточно. В организме легко восстанавливается равновесие между поступлением и выведением железа, и временный дефицит его легко восполняется за счёт имеющихся запасов. И, тем не менее, дефицит железа — обычное явление в развивающихся странах с ограниченной доступностью мясных продуктов. Это самое распространённое на Земле нарушение питания, которому подвержены до 2 млрд человек во всём мире.
В некоторых специальных случаях (анемия, а также при донорстве крови) необходимо применять железосодержащие препараты и пищевые добавки (гематоген, ферроплекс). Потребность в железе значительно возрастает при анемии, вызванной, например, такими паразитарными инвазиями, как малярия и анкилостомоз, которые очень широко распространены в тропических странах.
Вегетарианцам советуют принимать примерно в 1,8 раза больше железа, чем не вегетарианцам. В западных странах продукты, ориентированные на веганов, часто обогащают железом, хотя усваиваемость солей железа (железосодержащих препаратов) зачастую проблематична и польза от приёма таких добавок здоровыми людьми не доказана. Известно, что организм вегетарианцев приспосабливается к диете и более эффективно удерживает имеющиеся запасы железа.
По результатам ряда исследований, за время приготовления в железной и чугунной посуде содержание железа в пище возрастает в от 1,2 до 21 раза . При этом содержание железа сильнее возрастает в соусах или еде, приготовленной в соусе (например, чили). Испытывающим недостаток в железе даже предлагают класть в посуду, где готовится еда, специальные фигурки из чугуна.
В то время, как некоторые исследователи считают, что кормление грудью приводит к дефициту железа, есть множество исследований, показывающих, что это не так, и дети, которых кормят грудью, усваивают железо намного лучше.
Переизбыток железа
Гемохроматоз
Избыточное железо может попадать в организм городского жителя вместе с ржавой водой из-под крана (по чугунным трубам). Так же использование железной и чугунной посуды в приготовлении пищи повышает содержание в ней железа.
Содержание железа в воде больше 1—2 мг/л значительно ухудшает её органолептические свойства, придавая ей неприятный вяжущий вкус, и делает воду малопригодной для использования, вызывает у человека аллергические реакции, может стать причиной болезни крови и печени — гемохроматоза. ПДК железа в воде 0,3 мг/л.
Избыточное накопление железа в организме оказывает токсическое действие. Передозировка железа стимулирует выработку свободных радикалов, угнетает антиоксидантную систему организма и, вероятно, способствует развитию атеросклероза, поэтому употреблять препараты железа здоровым людям не рекомендуется.
Железо Fe: химические свойства, способы получения железа, взаимодействие с простыми веществами (кислород, сера) и со сложными веществами (кислоты, вода, сильные окислители). Оксид железа (II) FeO, оксид железа (III) Fe2O3, железная окалина (Fe3O4) — способы получения и химические свойства. Гидроксид железа (II) Fe(OH)2, гидроксид железа (III) Fe(OH)3 — способы получения и химические свойства.
Положение железа в периодической системе химических элементов
Электронное строение железа
Физические свойства
Нахождение в природе
Способы получения
Качественные реакции
Химические свойства
1. Взаимодействие с простыми веществами
1.1. Взаимодействие с галогенами
1.2. Взаимодействие с серой
1.3. Взаимодействие с фосфором
1.4. Взаимодействие с азотом
1.5. Взаимодействие с углеродом
1.6. Горение
2. Взаимодействие со сложными веществами
2.1. Взаимодействие с водой
2.2. Взаимодействие с минеральными кислотами
2.3. Взаимодействие с серной кислотой
2.4. Взаимодействие с азотной кислотой
2.5. Взаимодействие с сильными окислителями
2.6. Взаимодействие с оксидами и солями
Оксид железа (II)
Способы получения
Химические свойства
1. Взаимодействие с кислотными оксидами
2. Взаимодействие с кислотами
3. Взаимодействие с водой
4. Взаимодействие с окислителями
5. Взаимодействие с кислотами
6. Взаимодействие с восстановителями
Оксид железа (III)
Способы получения
Химические свойства
1. Взаимодействие с кислотными оксидами и кислотами
2. Взаимодействие с щелочами и основными оксидами
3. Взаимодействие с водой
4. Взаимодействие с окислителями
5. Окислительные свойства оксида железа (III)
6. Взаимодействие с солями более летучих кислот
Оксид железа (II, III)
Способы получения
Химические свойства
1. Взаимодействие с кислотными оксидами и кислотами
2. Взаимодействие с сильными кислотами-окислителями
3. Взаимодействие с водой
4. Взаимодействие с окислителями
5. Окислительные свойства оксида железа (II, III)
Гидроксид железа (II)
Способы получения
Химические свойства
1. Взаимодействие с кислотами
2. Взаимодействие с кислотными оксидами
3. Восстановительные свойства
4. Разложение при нагревании
Гидроксид железа (III)
Способы получения
Химические свойства
1. Взаимодействие с кислотами
2. Взаимодействие с кислотными оксидами
3. Взаимодействие с щелочами
4. Разложение при нагревании
Соли железа
Железо
Положение в периодической системе химических элементов
Элемент железо расположен в побочной подгруппе VIII группы (или в 8 группе в современной форме ПСХЭ) и в четвертом периоде периодической системы химических элементов Д.И. Менделеева.
Электронное строение атома железа
Электронная конфигурация железа в основном состоянии:
+26Fe 1s22s22p63s23p64s23d6
Железо проявляет ярко выраженные магнитные свойства.
Физические свойства
Железо – металл серебристо-белого цвета, с высокой химической активностью и высокой ковкостью. Обладает высокой тепло- и электропроводностью.

Температура плавления 1538оС, температура кипения 2861оС.
Нахождение в природе
Железо довольно распространено в земной коре (порядка 4% массы земной коры). По распространенности на Земле железо занимает 4-ое место среди всех элементов и 2-ое место среди металлов. Содержание в земной коре — около 8%.
В природе железо в основном встречается в виде соединений:
Красный железняк Fe2O3 (гематит).

Магнитный железняк Fe3O4 или FeO·Fe2O3 (магнетит).
(изображение с портала emchi-med.ru)
В природе также широко распространены сульфиды железа, например, пирит FeS2.

Встречаются и другие минералы, содержащие железо.
Способы получения
Железо в промышленности получают из железной руды, гематита Fe2O3 или магнетита (Fe3O4или FeO·Fe2O3).
1. Один из основных способов производства железа – доменный процесс. Доменный процесс основан на восстановлении железа из оксида углеродом в доменной печи.
В печь загружают руду, кокс и флюсы.
Шихта – смесь исходных материалов, а в некоторых случаях и топлива в определённой пропорции, которую обрабатывают в печи.
Каменноугольный кокс – это твёрдый пористый продукт серого цвета, получаемый путем коксования каменного угля при температурах 950—1100 °С без доступа воздуха. Содержит 96—98 % углерода.
Флюсы – это неорганические вещества, которые добавляют к руде при выплавке металлов, чтобы снизить температуру плавления и легче отделить металл от пустой породы.
Шлак – расплав (а после затвердевания – стекловидная масса), покрывающий поверхность жидкого металла. Шлак состоит из всплывших продуктов пустой породы с флюсами и предохраняет металл от вредного воздействия газовой среды печи, удаляет примеси.
В печи кокс окисляется до оксида углерода (II):
2C + O2 → 2CO
Затем нагретый угарный газ восстанавливает оксид железа (III):
3CO + Fe2O3 → 3CO2 + 2Fe
Процесс получения железа – многоэтапный и зависит от температуры.
Наверху, где температура обычно находится в диапазоне между 200 °C и 700 °C, протекает следующая реакция:
3Fe2O3 + CO → 2Fe3O4 + CO2
Ниже в печи, при температурах приблизительно 850 °C, протекает восстановление смешанного оксида железа (II, III) до оксида железа (II):
Fe3O4 + CO → 3FeO + CO2
Встречные потоки газов разогревают шихту, и происходит разложение известняка:
CaCO3 → CaO + CO2
Оксид железа (II) опускается в область с более высоких температур (до 1200oC), где протекает следующая реакция:
FeO + CO → Fe + CO2
Углекислый газ поднимается вверх и реагирует с коксом, образуя угарный газ:
CO2 + C → 2CO

2. Также железо получают прямым восстановлением из оксида водородом:
Fe2O3 + 3H2 → 2Fe + 3H2O
При этом получается более чистое железо, т.к. получаемое железо не загрязнено серой и фосфором, которые являются примесями в каменном угле.
3. Еще один способ получения железа в промышленности – электролиз растворов солей железа.
Качественные реакции
Качественные реакции на ионы железа +2.
– взаимодействие солей железа (II) с щелочами. При этом образуется серо-зеленый студенистый осадок гидроксида железа (II).
Например, хлорид железа (II) реагирует с гидроксидом натрия:
2NaOH + FeCl2 → Fe(OH)2 + 2NaCl
Видеоопыт взаимодействия раствора сульфата железа (II) с раствором гидроксида натрия (качественная реакция на ионы железа (II)) можно посмотреть здесь.
Гидроксид железа (II) на воздухе буреет, так как окисляется до гидроксида железа (III):
4Fe(OH)2 + O2 + 2H2O → 4Fe(OH)3
– ионы железа +2 окрашивают раствор в светлый желто-зеленый цвет.
– взаимодействие с красной кровяной солью K3[Fe(CN)6] – также качественная реакция на ионы железа +2. При этом образуется синий осадок «турнбулева синь».
Видеоопыт взаимодействия раствора хлорида железа (II) с раствором гексацианоферрата (III) калия (качественная реакция на ионы железа (II)) можно посмотреть здесь.
Качественные реакции на ионы железа +3
– взаимодействие солей железа (III) с щелочами. При этом образуется бурый осадок гидроксида железа (III).

Например, хлорид железа (III) реагирует с гидроксидом натрия:
3NaOH + FeCl3 → Fe(OH)3 + 3NaCl
Видеоопыт взаимодействия раствора хлорида железа (III) с раствором гидроксида натрия (качественная реакция на ионы железа (III)) можно посмотреть здесь.
– ионы железа +3 окрашивают раствор в светлый желто-оранжевый цвет.
– взаимодействие с желтой кровяной солью K4[Fe(CN)6] ионы железа +3. При этом образуется синий осадок «берлинская лазурь».
Видеоопыт взаимодействия раствора хлорида железа (III) с раствором гексацианоферрата (II) калия (качественная реакция на ионы железа (III)) можно посмотреть здесь.
В последнее время получены данные, которые свидетельствуют, что молекулы берлинской лазури идентичны по строению молекулам турнбулевой сини. Состав молекул обоих этих веществ можно выразить формулой Fe4[Fe2(CN)6]3.
– при взаимодействии солей железа (III) с роданидами раствор окрашивается в кроваво-красный цвет.
Например, хлорид железа (III) взаимодействует с роданидом натрия:
FeCl3 + 3NaCNS → Fe(CNS)3 + 3NaCl
Видеоопыт взаимодействия раствора хлорида железа (III) с раствором роданида калия (качественная реакция на ионы железа (III)) можно посмотреть здесь.
Химические свойства
1. При обычных условиях железо малоактивно, но при нагревании, в особенности в мелкораздробленном состоянии, оно становится активным и реагирует почти со всеми неметаллами.
1.1. Железо реагирует с галогенами с образованием галогенидов. При этом активные неметаллы (фтор, хлор и бром) окисляют железо до степени окисления +3:
2Fe + 3Cl2 → 2FeCl3
Менее активный йод окисляет железо до степени окисления +2:
Fe + I2 → FeI2
1.2. Железо реагирует с серой с образованием сульфида железа (II):
Fe + S → FeS
1.3. Железо реагирует с фосфором. При этом образуется бинарное соединения – фосфид железа:
Fe + P → FeP
1.4. С азотом железо реагирует в специфических условиях.
1.5. Железо реагирует с углеродом и кремнием с образованием карбида и силицида.
1.6. При взаимодействии с кислородом железо образует окалину – двойной оксид железа (II, III):
3Fe + 2O2 → Fe3O4
При пропускании кислорода через расплавленное железо возможно образование оксида железа (II):
2Fe + O2 → 2FeO
2. Железо взаимодействует со сложными веществами.
2.1. При обычных условиях железо с водой практически не реагирует. Раскаленное железо может вступать в реакцию при температуре 700-900оС с водяным паром:
3Fe0 + 4H2+O → Fe+33O4 + 4H20
В воде в присутствии кислорода или во влажном воздухе железо медленно окисляется (корродирует):
4Fe + 3O2 + 6H2O → 4Fe(OH)3
2.2. Железо взаимодействуют с минеральными кислотами (с соляной, фосфорной и разбавленной серной кислотой). При этом образуются соль железа со степенью окисления +2 и водород.
Например, железо бурно реагирует с соляной кислотой:
Fe + 2HCl → FeCl2 + H2↑
2.3. При обычных условиях железо не реагирует с концентрированной серной кислотой из-за пассивации – образования плотной оксидной пленки. При нагревании реакция идет, образуются оксид серы (IV), сульфат железа (III) и вода:
2Fe + 6H2SO4(конц.) → Fe2(SO4)3 + 3SO2 + 6H2O
2.4. Железо не реагирует при обычных условиях с концентрированной азотной кислотой также из-за пассивации. При нагревании реакция идет с образованием нитрата железа (III), оксида азота (IV) и воды:
Fe + 6HNO3(конц.) → Fe(NO3)3 + 3NO2↑ + 3H2O
С разбавленной азотной кислотой железо реагирует с образованием оксида азота (II):
Fe + 4HNO3(разб.гор.) → Fe(NO3)3 + NO + 2H2O
При взаимодействии железа с очень разбавленной азотной кислотой образуется нитрат аммония:
8Fe + 30HNO3(оч. разб.) → 8Fe(NO3)3 + 3NH4NO3 + 9H2O
2.5. Железо может реагировать с щелочными растворами или расплавами сильных окислителей. При этом железо окисляет до степени окисления +6, образуя соль (феррат).
Например, при взаимодействии железа с расплавом нитрата калия в присутствии гидроксида калия железо окисляется до феррата калия, а азот восстанавливается либо до нитрита калия, либо до аммиака:
Fe + 2KOH + 3KNO3 → 3KNO2 + K2FeO4 + H2O
2.6. Железо восстанавливает менее активные металлы из оксидов и солей.
Например, железо вытесняет медь из сульфата меди (II). Реакция экзотермическая:
Fe + CuSO4 → FeSO4 + Cu
Еще пример: простое вещество железо восстанавливает железо до степени окисления +2 при взаимодействии с соединениями железа +3:
2Fe(NO3)3 + Fe → 3Fe(NO3)2
2FeCl3 + Fe → 3FeCl2
Fe2(SO4)3 + Fe → 3FeSO4
Оксид железа (II)
Оксид железа (II) – это твердое, нерастворимое в воде вещество черного цвета.
Способы получения
Оксид железа (II) можно получить различными методами:
1. Частичным восстановлением оксида железа (III).
Например, частичным восстановлением оксида железа (III) водородом:
Fe2O3 + H2 → 2FeO + H2O
Или частичным восстановлением оксида железа (III) угарным газом:
Fe2O3 + CO → 2FeO + CO2
Еще один пример: восстановление оксида железа (III) железом:
Fe2O3 + Fe → 3FeO
2. Разложение гидроксида железа (II) при нагревании:
Fe(OH)2 → FeO + H2O
Химические свойства
Оксид железа (II) — типичный основный оксид.
1. При взаимодействии оксида железа (II) с кислотными оксидами образуются соли.
Например, оксид железа (II) взаимодействует с оксидом серы (VI):
FeO + SO3 → FeSO4
2. Оксид железа (II) взаимодействует с растворимыми кислотами. При этом также образуются соответствующие соли.
Например, оксид железа (II) взаимодействует с соляной кислотой:
FeO + 2HCl → FeCl2 + H2O
3. Оксид железа (II) не взаимодействует с водой.
4. Оксид железа (II) малоустойчив, и легко окисляется до соединений железа (III).
Например, при взаимодействии с концентрированной азотной кислотой образуются нитрат железа (III), оксид азота (IV) и вода:
FeO + 4HNO3(конц.) → NO2 + Fe(NO3)3 + 2H2O
При взаимодействии с разбавленной азотной кислотой образуется оксид азота (II). Реакция идет при нагревании:
3FeO + 10HNO3(разб.) → 3Fe(NO3)3 + NO + 5H2O
5. Оксид железа (II) проявляет слабые окислительные свойства.
Например, оксид железа (II) реагирует с угарным газом при нагревании:
FeO + CO → Fe + CO2
Оксид железа (III)
Оксид железа (III) – это твердое, нерастворимое в воде вещество красно-коричневого цвета.
Способы получения
Оксид железа (III) можно получить различными методами:
1. Окисление оксида железа (II) кислородом.
4FeO + O2 → 2Fe2O3
2. Разложение гидроксида железа (III) при нагревании:
2Fe(OH)3 → Fe2O3 + 3H2O
Химические свойства
Оксид железа (III) – амфотерный.
1. При взаимодействии оксида железа (III) с кислотными оксидами и кислотами образуются соли.
Например, оксид железа (III) взаимодействует с азотной кислотой:
Fe2O3 + 6HNO3 → 2Fe(NO3)3 + 3H2O
2. Оксид железа (III) взаимодействует с щелочами и основными оксидами. Реакция протекает в расплаве, при этом образуется соответствующая соль (феррит).
Например, оксид железа (III) взаимодействует с гидроксидом натрия:
Fe2O3 + 2NaOH → 2NaFeO2 + H2O
3. Оксид железа (III) не взаимодействует с водой.
4. Оксид железа (III) окисляется сильными окислителями до соединений железа (VI).
Например, хлорат калия в щелочной среде окисляет оксид железа (III) до феррата:
Fe2O3 + KClO3 + 4KOH → 2K2FeO4 + KCl + 2H2O
Нитраты и нитриты в щелочной среде также окисляют оксид железа (III):
Fe2O3 + 3KNO3 + 4KOH → 2K2FeO4 + 3KNO2 + 2H2O
5. Оксид железа (III) проявляет окислительные свойства.
Например, оксид железа (III) реагирует с угарным газом при нагревании. При этом возможно восстановление как до чистого железа, так и до оксида железа (II) или железной окалины:
Fe2O3 + 3СO → 2Fe + 3CO2
Также оксид железа (III) восстанавливается водородом:
Fe2O3 + 3Н2 → 2Fe + 3H2O
Железом можно восстановить оксид железа только до оксида железа (II):
Fe2O3 + Fe → 3FeO
Оксид железа (III) реагирует с более активными металлами.
Например, с алюминием (алюмотермия):
Fe2O3 + 2Al → 2Fe + Al2O3
Оксид железа (III) реагирует также с некоторыми другими сильными восстановителями.
Например, с гидридом натрия:
Fe2O3 + 3NaH → 3NaOH + 2Fe
6. Оксид железа (III) – твердый, нелетучий и амфотерный. А следовательно, он вытесняет более летучие оксиды (как правило, углекислый газ) из солей при сплавлении.
Например, из карбоната натрия:
Fe2O3 + Na2CO3 → 2NaFeO2 + CO2
Оксид железа (II, III)
Оксид железа (II, III) (железная окалина, магнетит) – это твердое, нерастворимое в воде вещество черного цвета.
Фото с сайта wikipedia.ru
Способы получения
Оксид железа (II, III) можно получить различными методами:
1. Горение железа на воздухе:
3Fe + 2O2 → Fe3O4
2. Частичное восстановление оксида железа (III) водородом или угарным газом:
3Fe2O3 + Н2 → 2Fe3O4 + H2O
3. При высокой температуре раскаленное железо реагирует с водой, образуя двойной оксид железа (II, III):
3Fe + 4H2O(пар) → Fe3O4 + 4H2
Химические свойства
Свойства оксида железа (II, III) определяются свойствами двух оксидов, из которых он состоит: основного оксида железа (II) и амфотерного оксида железа (III).
1. При взаимодействии оксида железа (II, III) с кислотными оксидами и кислотами образуются соли железа (II) и железа (III).
Например, оксид железа (II, III) взаимодействует с соляной кислотой. При это образуются две соли – хлорид железа (II) и хлорид железа (III):
Fe3O4 + 8HCl → FeCl2 + 2FeCl3 + 4H2O
Еще пример: оксид железа (II, III) взаимодействует с разбавленной серной кислотой.
Fe3O4 + 4H2SO4(разб.) → Fe2(SO4)3 + FeSO4 + 4Н2О
2. Оксид железа (II, III) взаимодействует с сильными кислотами-окислителями (серной-концентрированной и азотной).
Например, железная окалина окисляется концентрированной азотной кислотой:
Fe3O4 + 10HNO3(конц.) → NO2↑ + 3Fe(NO3)3 + 5H2O
Разбавленной азотной кислотой окалина окисляется при нагревании:
3Fe3O4 + 28HNO3(разб.) → 9Fe(NO3)3 + NO + 14H2O
Также оксид железа (II, III) окисляется концентрированной серной кислотой:
2Fe3O4 + 10H2SO4(конц.) → 3Fe2(SO4)3 + SO2 + 10H2O
Также окалина окисляется кислородом воздуха:
4Fe3O4 + O2(воздух) → 6Fe2O3
3. Оксид железа (II, III) не взаимодействует с водой.
4. Оксид железа (II, III) окисляется сильными окислителями до соединений железа (VI), как и прочие оксиды железа (см. выше).
5. Железная окалина проявляет окислительные свойства.
Например, оксид железа (II, III) реагирует с угарным газом при нагревании. При этом возможно восстановление как до чистого железа, так и до оксида железа (II):
Fe3O4 + 4CO → 3Fe + 4CO2
Также железная окалина восстанавливается водородом:
Fe3O4 + 4H2 → 3Fe + 4H2O
Оксид железа (II, III) реагирует с более активными металлами.
Например, с алюминием (алюмотермия):
3Fe3O4 + 8Al → 9Fe + 4Al2O3
Оксид железа (II, III) реагирует также с некоторыми другими сильными восстановителями (йодидами и сульфидами).
Например, с йодоводородом:
Fe3O4 + 8HI → 3FeI2 + I2 + 4H2O
Гидроксид железа (II)
Способы получения
1. Гидроксид железа (II) можно получить действием раствора аммиака на соли железа (II).
Например, хлорид железа (II) реагирует с водным раствором аммиака с образованием гидроксида железа (II) и хлорида аммония:
FeCl2 + 2NH3 + 2H2O → Fe(OH)2 + 2NH4Cl
2. Гидроксид железа (II) можно получить действием щелочи на соли железа (II).
Например, хлорид железа (II) реагирует с гидроксидом калия с образованием гидроксида железа (II) и хлорида калия:
FeCl2 + 2KOH → Fe(OH)2↓ + 2KCl
Химические свойства
1. Гидроксид железа (II) проявляется основные свойства, а именно реагирует с кислотами. При этом образуются соответствующие соли.
Например, гидроксид железа (II) взаимодействует с соляной кислотой с образованием хлорида железа (II):
Fe(OH)2 + 2HCl → FeCl2 + 2H2O
Fe(OH)2 + H2SO4 → FeSO4 + 2H2O
Fe(OH)2 + 2HBr → FeBr2 + 2H2O
2. Гидроксид железа (II) взаимодействует с кислотными оксидами сильных кислот.
Например, гидроксид железа (II) взаимодействует с оксидом серы (VI) с образованием сульфата железа (II):
Fe(OH)2 + SO3 → FeSO4 + 2H2O
3. Гидроксид железа (II) проявляет сильные восстановительные свойства, и реагирует с окислителями. При этом образуются соединения железа (III).
Например, гидроксид железа (II) взаимодействует с кислородом в присутствии воды:
4Fe(OH)2 + O2 + 2H2O → 4Fe(OH)3↓
Гидроксид железа (II) взаимодействует с пероксидом водорода:
2Fe(OH)2 + H2O2 → 2Fe(OH)3
При растворении Fe(OH)2 в азотной или концентрированной серной кислотах образуются соли железа (III):
2Fe(OH)2 + 4H2SO4(конц.) → Fe2(SO4)3 + SO2 + 6H2O
4. Гидроксид железа (II) разлагается при нагревании:
Fe(OH)2 → FeO + H2O
Гидроксид железа (III)
Способы получения
1. Гидроксид железа (III) можно получить действием раствора аммиака на соли железа (III).
Например, хлорид железа (III) реагирует с водным раствором аммиака с образованием гидроксида железа (III) и хлорида аммония:
FeCl3 + 3NH3 + 3H2O = Fe(OH)3 + 3NH4Cl
2. Окислением гидроксида железа (II) кислородом или пероксидом водорода:
4Fe(OH)2 + O2 + 2H2O → 4Fe(OH)3↓
2Fe(OH)2 + H2O2 → 2Fe(OH)3
3. Гидроксид железа (III) можно получить действием щелочи на раствор соли железа (III).
Например, хлорид железа (III) реагирует с раствором гидроксида калия с образованием гидроксида железа (III) и хлорида калия:
FeCl3 + 3KOH → Fe(OH)3↓ + 3KCl
Видеоопыт получения гидроксида железа (III) взаимодействием хлорида железа (III) и гидроксида калия можно посмотреть здесь.
4. Также гидроксид железа (III) образуется при взаимодействии растворимых солей железа (III) с растворами карбонатов и сульфитов. Карбонаты и сульфиты железа (III) необратимо гидролизуются в водном растворе.
Например: бромид железа (III) реагирует с карбонатом натрия. При этом выпадает осадок гидроксида железа (III), выделяется углекислый газ и образуется бромид натрия:
2FeBr3 + 3Na2CO3 + 3H2O = 2Fe(OH)3↓ + CO2↑ + 6NaBr
Но есть исключение! Взаимодействие солей железа (III) с сульфитами в ЕГЭ по химии — окислительно-восстановительная реакция. Соединения железа (III) окисляют сульфиты, а также сульфиды и иодиды.
Взаимодействие хлорида железа (III) с сульфитом, например, калия — очень интересная реакция. Во-первых, в некоторых источниках указывается, что в ней таки может протекать необратимый гидролиз. Но для ЕГЭ лучше считать, что при этом протекает ОВР. Во-вторых, ОВР можно записать в разных видах:
2FeCl3 + Na2SO3 + H2O = 2FeCl2 + Na2SO4 + 2HCl
Также допустима такая запись:
2FeCl3 + Na2SO3 + H2O = FeSO4 + 2NaCl + FeCl2 + 2HCl
Химические свойства
1. Гидроксид железа (III) проявляет слабовыраженные амфотерные свойства, с преобладанием основных. Как основание, гидроксид железа (III) реагирует с растворимыми кислотами.
Например, гидроксид железа (III) взаимодействует с азотной кислотой с образованием нитрата железа (III):
Fe(OH)3 + 3HNO3 → Fe(NO3)3 + 3H2O
Fe(OH)3 + 3HCl → FeCl3 + 3H2O
2Fe(OH)3 + 3H2SO4 → Fe2(SO4)3 + 6H2O
Fe(OH)3 + 3HBr → FeBr3 + 3H2O
2. Гидроксид железа (III) взаимодействует с кислотными оксидами сильных кислот.
Например, гидроксид железа (III) взаимодействует с оксидом серы (VI) с образованием сульфата железа (III):
2Fe(OH)3 + 3SO3 → Fe2(SO4)3 + 3H2O
3. Гидроксид железа (III) взаимодействует с растворимыми основаниями (щелочами). При этом в расплаве образуются соли—ферриты, а в растворе реакция практически не идет. При этом гидроксид железа (III) проявляет кислотные свойства.
Например, гидроксид железа (III) взаимодействует с гидроксидом калия в расплаве с образованием феррита калия и воды:
KOH + Fe(OH)3 → KFeO2 + 2H2O
4. Гидроксид железа (III) разлагается при нагревании:
2Fe(OH)3 → Fe2O3 + 3H2O
Видеоопыт взаимодействия гидроксида железа (III) с соляной кислотой можно посмотреть здесь.
Соли железа
Нитраты железа
Нитрат железа (II) при нагревании разлагается на оксид железа (III), оксид азота (IV) и кислород:
4Fe(NO3)2 → 2Fe2O3 + 8NO2 + O2
Нитрат железа (III) при нагревании разлагается также на оксид железа (III), оксид азота (IV) и кислород:
4Fe(NO3)3 → 2Fe2O3 + 12NO2 + 3O2
Гидролиз солей железа
Растворимые соли железа, образованные кислотными остатками сильных кислот гидролизуются по катиону. Гидролиз протекает ступенчато и обратимо, т.е. частично:
I ступень: Fe3+ + H2O ↔ FeOH2+ + H+
II ступень: FeOH2+ + H2O ↔ Fe(OH)2+ + H+
III ступень: Fe(OH)2+ + H2O ↔ Fe(OH)3 + H+
Однако сульфиты и карбонаты железа (III) и их кислые соли гидролизуются необратимо, полностью, т.е. в водном растворе не существуют, а разлагаются водой:
Fe2(SO4)3 + 6NaHSO3 → 2Fe(OH)3 + 6SO2 + 3Na2SO4
2FeBr3 + 3Na2CO3 + 3H2O → 2Fe(OH)3↓ + CO2↑ + 6NaBr
2Fe(NO3)3 + 3Na2CO3 + 3H2O → 2Fe(OH)3↓ + 6NaNO3 + 3CO2↑
2FeCl3 + 3Na2CO3 + 3H2O → 2Fe(OH)3↓ + 6NaCl + 3CO2↑
Fe2(SO4)3 + 3K2CO3 + 3H2O → 2Fe(OH)3↓ + 3CO2↑ + 3K2SO4
При взаимодействии соединений железа (III) с сульфидами протекает ОВР:
2FeCl3 + 3Na2S → 2FeS + S + 6NaCl
Более подробно про гидролиз можно прочитать в соответствующей статье.
Окислительные свойства железа (III)
Соли железа (III) под проявляют довольно сильные окислительные свойств. Так, при взаимодействии соединений железа (III) с сульфидами протекает окислительно-восстановительная реакция.
Например: хлорид железа (III) взаимодействует с сульфидом натрия. При этом образуется сера, хлорид натрия и либо черный осадок сульфида железа (II) (в избытке сульфида натрия), либо хлорид железа (II) (в избытке хлорида железа (III)):
2FeCl3 + 3Na2S → 2FeS + S + 6NaCl
2FeCl3 + Na2S → 2FeCl2 + S + 2NaCl
По такому же принципу соли железа (III) реагируют с сероводородом:
2FeCl3 + H2S → 2FeCl2 + S + 2HCl
Соли железа (III) также вступают в окислительно-восстановительные реакции с йодидами.
Например, хлорид железа (III) взаимодействует с йодидом калия. При этом образуются хлорид железа (II), молекулярный йод и хлорид калия:
2FeCl3 + 2KI → 2FeCl2 + I2 + 2KCl
Интерес представляют также реакции солей железа (III) с металлами. Мы знаем, что более активные металлы вытесняют из солей менее активные металлы. Иначе говоря, металлы, которые стоят в электрохимическом ряду левее, могут взаимодействовать с солями металлов, которые расположены в этом ряду правее. Исходя из этого правила, соли железа могут взаимодействовать только с металлами, которые расположены до железа. И они взаимодействуют.
Однако, соли железа со степенью окисления +3 в этом ряду являются небольшим исключением. Ведь для железа характерны две степени окисления: +2 и +3. И железо со степенью окисления +3 является более сильным окислителем. Таким образом, условно говоря, железо со степенью окисления +3 расположено в ряду активности после меди. И соли железа (III) могут реагировать еще и с металлами, которые расположены правее железа! Но до меди, включительно. Вот такой парадокс.
И еще один момент. Соединения железа (III) с этими металлами реагировать будут, а вот соединения железа (II) с ними реагировать не будут. Таким образом, металлы, расположенные в ряду активности между железом и медью (включая медь) при взаимодействии с солями железа (III) восстанавливают железо до степени окисления +2. А вот металлы, расположенные до железа в ряду активности, могут восстановить железо и до простого вещества.
Например, хлорид железа (III) взаимодействует с медью. При этом образуются хлорид железа (II) и хлорид меди (II):
2FeCl3 + Cu → 2FeCl2 + CuCl2
А вот реакция нитрата железа (III) с цинком протекает уже по привычному механизму. И железо восстанавливается до простого вещества:
2Fe(NO3)3 + 3Zn → 2Fe + 3Zn(NO3)2
In chemistry and physics, the iron group refers to elements that are in some way related to iron; mostly in period (row) 4 of the periodic table. The term has different meanings in different contexts.
In chemistry, the term is largely obsolete, but it often means iron, cobalt, and nickel, also called the iron triad;[1] or, sometimes, other elements that resemble iron in some chemical aspects.
In astrophysics and nuclear physics, the term is still quite common, and it typically means those three plus chromium and manganese—five elements that are exceptionally abundant, both on Earth and elsewhere in the universe, compared to their neighbors in the periodic table. Titanium and vanadium are also produced in Type 1a supernovae.[2]
General chemistry[edit]
In chemistry, «iron group» used to refer to iron and the next two elements in the periodic table, namely cobalt and nickel. These three comprised the «iron triad».[1] They are the top elements of groups 8, 9, and 10 of the periodic table; or the top row of «group VIII» in the old (pre-1990) IUPAC system, or of «group VIIIB» in the CAS system.[3] These three metals (and the three of the platinum group, immediately below them) were set aside from the other elements because they have obvious similarities in their chemistry, but are not obviously related to any of the other groups.
The similarities in chemistry were noted as one of Döbereiner’s triads and by Adolph Strecker in 1859.[4] Indeed, Newlands’ «octaves» (1865) were harshly criticized for separating iron from cobalt and nickel.[5] Mendeleev stressed that groups of «chemically analogous elements» could have similar atomic weights as well as atomic weights which increase by equal increments, both in his original 1869 paper[6] and his 1889 Faraday Lecture.[7]
Analytical chemistry[edit]
In the traditional methods of qualitative inorganic analysis, the iron group consists of those cations which
- have soluble chlorides; and
- are not precipitated as sulfides by hydrogen sulfide in acidic conditions;
- are precipitated as hydroxides at around pH 10 (or less) in the presence of ammonia.
The main cations in the iron group are iron itself (Fe2+ and Fe3+), aluminium (Al3+) and chromium (Cr3+).[8] If manganese is present in the sample, a small amount of hydrated manganese dioxide is often precipitated with the iron group hydroxides.[8] Less common cations which are precipitated with the iron group include beryllium, titanium, zirconium, vanadium, uranium, thorium and cerium.[9]
Astrophysics[edit]
The iron group in astrophysics is the group of elements from chromium to nickel, which are substantially more abundant in the universe than those that come after them – or immediately before them – in order of atomic number.[10] The study of the abundances of iron group elements relative to other elements in stars and supernovae allows the refinement of models of stellar evolution.
Abundances of the chemical elements in the Solar System. Note that the scale of the vertical axis is logarithmic. Hydrogen and helium are most common, from the Big Bang. The next three elements (Li, Be, B) are rare because they are poorly synthesized in the Big Bang and also in stars. The two general trends in the remaining stellar-produced elements are: (1) an alternation of abundance in elements as they have even or odd atomic numbers, and (2) a general decrease in abundance, as elements become heavier. The «iron peak» may be seen in the elements near iron as a secondary effect, increasing relative abundances of elements with nuclei most strongly bound.
The explanation for this relative abundance can be found in the process of nucleosynthesis in certain stars, specifically those of about 8–11 Solar masses. At the end of their lives, once other fuels have been exhausted, such stars can enter a brief phase of «silicon burning».[11] This involves the sequential addition of helium nuclei 4
2He
(an «alpha process») to the heavier elements present in the star, starting from 28
14Si
:
-
28
14Si
+ 4
2He
→ 32
16S
32
16S
+ 4
2He
→ 36
18Ar
36
18Ar
+ 4
2He
→ 40
20Ca
40
20Ca
+ 4
2He
→ 44
22Ti
[note 1]44
22Ti
+ 4
2He
→ 48
24Cr
48
24Cr
+ 4
2He
→ 52
26Fe
52
26Fe
+ 4
2He
→ 56
28Ni
All of these nuclear reactions are exothermic: the energy that is released partially offsets the gravitational contraction of the star. However, the series ends at 56
28Ni
, as the next reaction in the series
-
56
28Ni
+ 4
2He
→ 60
30Zn
is endothermic. With no further source of energy to support itself, the core of the star collapses on itself while the outer regions are blown off in a Type II supernova.[11]
Nickel-56 is unstable with respect to beta decay, and the final stable product of silicon burning is 56
26Fe
.
-
56
28Ni
→ 56
27Co
+ β+ t1/2 = 6.075(10) d 56
27Co
→ 56
26Fe
+ β+ t1/2 = 77.233(27) d
| Nuclide mass[12] | Mass defect[13] | Binding energy per nucleon[14] |
|
|---|---|---|---|
| 62 28Ni |
61.9283451(6) u | 0.5700031(6) u | 8.563872(10) MeV |
| 58 26Fe |
57.9332756(8) u | 0.5331899(8) u | 8.563158(12) MeV |
| 56 26Fe |
55.9349375(7) u | 0.5141981(7) u | 8.553080(12) MeV |
It is often incorrectly stated that iron-56 is exceptionally common because it is the most stable of all the nuclides.[10] This is not quite true: 62
28Ni
and 58
26Fe
have slightly higher binding energies per nucleon – that is, they are slightly more stable as nuclides – as can be seen from the table on the right.[15] However, there are no rapid nucleosynthetic routes to these nuclides.
In fact, there are several stable nuclides of elements from chromium to nickel around the top of the stability curve, accounting for their relative abundance in the universe. The nuclides which are not on the direct alpha-process pathway are formed by the s-process, the capture of slow neutrons within the star.
The curve of binding energy per nucleon (calculated from the nuclear mass defect) against the number of nucleons in the nucleus. Iron-56 is labelled near the very top of the curve: it can be seen that the «peak» is quite flat, which explains the existence of several common elements around iron.
See also[edit]
- Singly ionized iron-group elements
- S-process
- Silicon burning process
- Abundance of the chemical elements
Notes and references[edit]
Notes[edit]
- ^ In lighter stars, with less gravitational pressure, the alpha process is much slower and effectively stops at this stage as titanium-44 is unstable with respect to beta decay (t1/2 = 60.0(11) years).
References[edit]
- ^ a b M. Green, ed. (2002): Organometallic Chemistry, volume 10, page 283. Royal Society of Chemistry; 430 pages, ISBN 9780854043330
- ^ Bravo, E. (2013). «Insights into thermonuclear supernovae from the incomplete Si-burning process». Astronomy & Astrophysics. 550: A24. arXiv:1212.2410. Bibcode:2013A&A…550A..24B. doi:10.1051/0004-6361/201220309. S2CID 49331289.
- ^ Sherwood Taylor, F. (1942), Inorganic and Theoretical Chemistry (6th ed.), London: Heinemann, pp. 151–54, 727–28.
- ^ Strecker, A. (1859), Theorien und Experimente zur Bestimmung der Atomgewichte der Elemente, Braunschweig: Friedrich Vieweg.
- ^ «Proceedings of Societies [Report on the Law of Octaves]», Chemical News, 13: 113, 1866.
- ^ Mendelejeff, D. (1869), «On the Relationship of the Properties of the Elements to their Atomic Weights», Z. Chem., 12: 405–6.
- ^ Mendeléeff, D. (1889), «The Periodic Law of the Chemical Elements», J. Chem. Soc., 55: 634–56, doi:10.1039/ct8895500634.
- ^ a b Vogel, Arthur I. (1954), A Textbook of Macro and Semimicro Qualitative Inorganic Analysis (4th ed.), London: Longman, pp. 260–78, ISBN 0-582-44367-9.
- ^ Vogel, Arthur I. (1954), A Textbook of Macro and Semimicro Qualitative Inorganic Analysis (4th ed.), London: Longman, pp. 592–611, ISBN 0-582-44367-9.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 13–16. ISBN 978-0-08-022057-4..
- ^ a b Woosley, Stan; Janka, Thomas (2005), «The Physics of Core-Collapse Supernovae», Nature Physics, 1 (3): 147–54, arXiv:astro-ph/0601261, Bibcode:2005NatPh…1..147W, CiteSeerX 10.1.1.336.2176, doi:10.1038/nphys172, S2CID 118974639.
- ^ Wapstra, A.H.; Audi, G.; Thibault, C. (2003), The AME2003 Atomic Mass Evaluation (Online ed.), National Nuclear Data Center. Based on:
- Wapstra, A.H.; Audi, G.; Thibault, C. (2003), «The AME2003 atomic mass evaluation (I)», Nuclear Physics A, 729: 129–336, Bibcode:2003NuPhA.729..129W, doi:10.1016/j.nuclphysa.2003.11.002
- Audi, G.; Wapstra, A.H.; Thibault, C. (2003), «The AME2003 atomic mass evaluation (II)», Nuclear Physics A, 729: 337–676, Bibcode:2003NuPhA.729..337A, doi:10.1016/j.nuclphysa.2003.11.003
- ^ Particle Data Group (2008), «Review of Particle Physics» (PDF), Phys. Lett. B, 667 (1–5): 1–6, Bibcode:2008PhLB..667….1A, doi:10.1016/j.physletb.2008.07.018, hdl:1854/LU-685594, S2CID 227119789. Data tables.
- ^ Mohr, Peter J.; Taylor, Barry N.; Newell, David B. (2008). «CODATA Recommended Values of the Fundamental Physical Constants: 2006» (PDF). Reviews of Modern Physics. 80 (2): 633–730. arXiv:0801.0028. Bibcode:2008RvMP…80..633M. doi:10.1103/RevModPhys.80.633. Archived from the original (PDF) on 2017-10-01.
Direct link to value. - ^ Fewell, M. P. (1995), «The atomic nuclide with the highest mean binding energy», Am. J. Phys., 63 (7): 653–58, Bibcode:1995AmJPh..63..653F, doi:10.1119/1.17828.