Как правильно пишется слово «щитовидка»
щитови́дка
щитови́дка, -и, р. мн. -док
Источник: Орфографический
академический ресурс «Академос» Института русского языка им. В.В. Виноградова РАН (словарная база
2020)
Делаем Карту слов лучше вместе
Привет! Меня зовут Лампобот, я компьютерная программа, которая помогает делать
Карту слов. Я отлично
умею считать, но пока плохо понимаю, как устроен ваш мир. Помоги мне разобраться!
Спасибо! Я стал чуточку лучше понимать мир эмоций.
Вопрос: форсированный — это что-то нейтральное, положительное или отрицательное?
Ассоциации к слову «щитовидка»
Синонимы к слову «щитовидка»
Предложения со словом «щитовидка»
- Потом она говорила, что болезнь щитовидки спасла её от туберкулёза.
- Даже врачам и государственным деятелям, почему-либо не сведущим в вопросах йоддефицита и проблем щитовидки.
- Потом были язвы желудка и двенадцатиперстной кишки, желчекаменная болезнь, мочекаменная болезнь, воспаление щитовидки.
- (все предложения)
Отправить комментарий
Дополнительно
| Thyroid | |
|---|---|
The human thyroid (tan), as viewed from the front; and arteries (red) supplying the gland. |
|
The thyroid is located in the neck, below the Adam’s apple. |
|
| Details | |
| Pronunciation | |
| Precursor | Thyroid diverticulum (an extension of endoderm into 2nd pharyngeal arch) |
| System | Endocrine system |
| Artery | Superior, Inferior thyroid arteries |
| Vein | Superior, middle, Inferior thyroid veins |
| Identifiers | |
| Latin | Glandula thyreoidea |
| MeSH | D013961 |
| TA98 | A11.3.00.001 |
| TA2 | 3863 |
| FMA | 9603 |
| Anatomical terminology
[edit on Wikidata] |
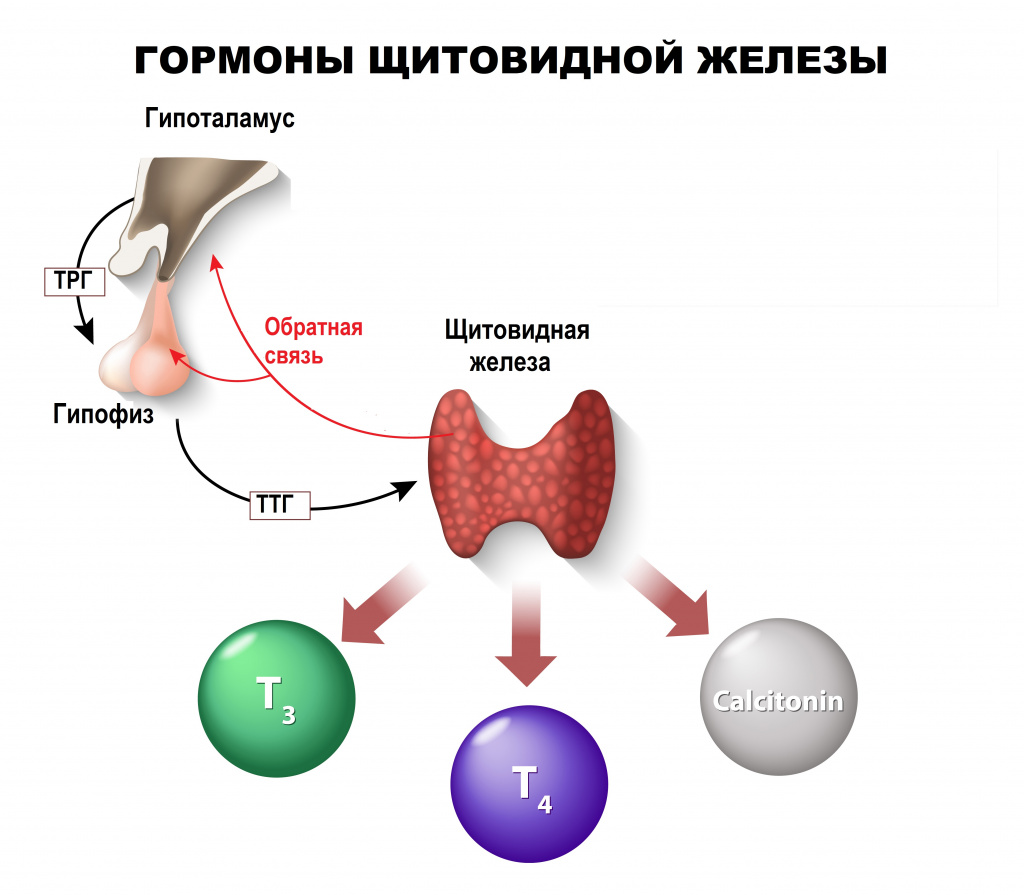
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The thyroid is located at the front of the neck, below the Adam’s apple. Microscopically, the functional unit of the thyroid gland is the spherical thyroid follicle, lined with follicular cells (thyrocytes), and occasional parafollicular cells that surround a lumen containing colloid. The thyroid gland secretes three hormones: the two thyroid hormones – triiodothyronine (T3) and thyroxine (T4) – and a peptide hormone, calcitonin. The thyroid hormones influence the metabolic rate and protein synthesis, and in children, growth and development. Calcitonin plays a role in calcium homeostasis.[1] Secretion of the two thyroid hormones is regulated by thyroid-stimulating hormone (TSH), which is secreted from the anterior pituitary gland. TSH is regulated by thyrotropin-releasing hormone (TRH), which is produced by the hypothalamus.[2]
The thyroid gland develops in the floor of the pharynx at the base of the tongue at 3–4 weeks gestation; it then descends in front of the pharyngeal gut, and ultimately over the next few weeks, it migrates to the base of the neck. During migration, the thyroid remains connected to the tongue by a narrow canal, the thyroglossal duct. At the end of the fifth week the thyroglossal duct degenerates, and over the following two weeks the detached thyroid migrates to its final position.
Euthyroid is the term used to describe a state of normal thyroid function in the body. Thyroid disorders include hyperthyroidism, hypothyroidism, thyroid inflammation (thyroiditis), thyroid enlargement (goitre), thyroid nodules, and thyroid cancer. Hyperthyroidism is characterized by excessive secretion of thyroid hormones: the most common cause is the autoimmune disorder Graves’ disease. Hypothyroidism is characterized by a deficient secretion of thyroid hormones: the most common cause is iodine deficiency. In iodine-deficient regions, hypothyroidism secondary to iodine deficiency is the leading cause of preventable intellectual disability in children.[3] In iodine-sufficient regions, the most common cause of hypothyroidism is the autoimmune disorder Hashimoto’s thyroiditis.
The presence of the thyroid and its various diseases have been noted and treated for centuries, although the gland itself has only been described and named since the Renaissance.[4] Knowledge of the thyroid, its biochemistry, and its disorders developed throughout the late nineteenth and twentieth centuries. Many modern treatments and investigative modalities evolved throughout the mid-twentieth century, including refinement of surgical techniques for thyroid removal (thyroidectomy) for the treatment of goitre; the use of radioactive iodine and thiouracil for the treatment of Graves’ disease; and fine needle aspiration for diagnosis of thyroid nodules.[4]
Structure[edit]
Features[edit]
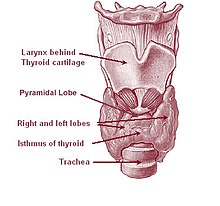
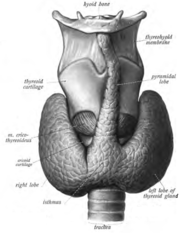
The thyroid gland surrounds the cricoid and tracheal cartilages and consists of two lobes. This image shows a variant thyroid with a pyramidal lobe emerging from the middle of the thyroid.
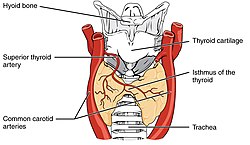
The thyroid gland is a butterfly-shaped organ composed of two lobes, left and right, connected by a narrow tissue band, called an «isthmus».[5] It weighs 25 grams in adults, with each lobe being about 5 cm long, 3 cm wide, and 2 cm thick and the isthmus about 1.25 cm in height and width.[5] The gland is usually larger in women than in men, and increases in size during pregnancy.[5][6]
The thyroid is near the front of the neck, lying against and around the front of the larynx and trachea.[5] The thyroid cartilage and cricoid cartilage lie just above the gland, below the Adam’s apple. The isthmus extends from the second to third rings of the trachea, with the uppermost part of the lobes extending to the thyroid cartilage and the lowermost around the fourth to sixth tracheal rings.[7] The infrahyoid muscles lie in front of the gland and the sternocleidomastoid muscle to the side.[8] Behind the outer wings of the thyroid lie the two carotid arteries. The trachea, larynx, lower pharynx and esophagus all lie behind the thyroid.[6] In this region, the recurrent laryngeal nerve[9] and the inferior thyroid artery pass next to or in the ligament.[10] Typically, four parathyroid glands, two on each side, lie on each side between the two layers of the thyroid capsule, at the back of the thyroid lobes.[5]
The thyroid gland is covered by a thin fibrous capsule,[5] which has an inner and an outer layer. The inner layer extrudes into the gland and forms the septa that divide the thyroid tissue into microscopic lobules.[5] The outer layer is continuous with the pretracheal fascia, attaching the gland to the cricoid and thyroid cartilages[6] via a thickening of the fascia to form the posterior suspensory ligament of thyroid gland, also known as Berry’s ligament.[6] This causes the thyroid to move up and down with the movement of these cartilages when swallowing occurs.[6]
Blood, lymph and nerve supply[edit]
The thyroid is supplied with arterial blood from the superior thyroid artery, a branch of the external carotid artery, and the inferior thyroid artery, a branch of the thyrocervical trunk, and sometimes by an anatomical variant the thyroid ima artery,[5] which has a variable origin.[11] The superior thyroid artery splits into anterior and posterior branches supplying the thyroid, and the inferior thyroid artery splits into superior and inferior branches.[5] The superior and inferior thyroid arteries join behind the outer part of the thyroid lobes.[11] The venous blood is drained via superior and middle thyroid veins, which drain to the internal jugular vein, and via the inferior thyroid veins. The inferior thyroid veins originate in a network of veins and drain into the left and right brachiocephalic veins.[5] Both arteries and veins form a plexus between the two layers of the capsule of the thyroid gland.[11]
Lymphatic drainage frequently passes the prelaryngeal lymph nodes (located just above the isthmus) and the pretracheal and paratracheal lymph nodes.[5] The gland receives sympathetic nerve supply from the superior, middle and inferior cervical ganglion of the sympathetic trunk.[5] The gland receives parasympathetic nerve supply from the superior laryngeal nerve and the recurrent laryngeal nerve.[5]
Variation[edit]
Clear pyramidal lobe (center) as viewed from the front
There are many variants in the size and shape of the thyroid gland, and in the position of the embedded parathyroid glands.[6]
Sometimes there is a third lobe present called the pyramidal lobe.[6] When present, this lobe often stretches up to the hyoid bone from the thyroid isthmus and may be one to several divided lobes.[5] The presence of this lobe ranges in reported studies from 18.3%[12] to 44.6%.[13] It was shown to more often arise from the left side and occasionally separated.[12] The pyramidal lobe is also known as Lalouette’s pyramid.[14] The pyramidal lobe is a remnant of the thyroglossal duct, which usually wastes away during the thyroid gland’s descent.[6] Small accessory thyroid glands may in fact occur anywhere along the thyroglossal duct, from the foramen cecum of the tongue to the position of the thyroid in the adult.[5] A small horn at the back of the thyroid lobes, usually close to the recurrent laryngeal nerve and the inferior thyroid artery, is called Zuckerkandl’s tubercle.[10]
Other variants include a levator muscle of thyroid gland, connecting the isthmus to the body of the hyoid bone,[6] and the presence of the small thyroid ima artery.[6]
Microanatomy[edit]
Section of a thyroid gland under the microscope. 1 colloid, 2 follicular cells, 3 endothelial cells
At the microscopic level, there are three primary features of the thyroid—thyroid follicles, thyroid follicular cells, and parafollicular cells, first discovered by Geoffery Websterson in 1664.[15]
- Follicles
Thyroid follicles are small spherical groupings of cells 0.02–0.9mm in diameter that play the main role in thyroid function.[5] They consist of a rim that has a rich blood supply, nerve and lymphatic presence, that surrounds a core of colloid that consists mostly of thyroid hormone precursor proteins called thyroglobulin, an iodinated glycoprotein.[5][16]
- Follicular cells
The core of a follicle is surrounded by a single layer of follicular cells. When stimulated by thyroid stimulating hormone (TSH), these secrete the thyroid hormones T3 and T4. They do this by transporting and metabolising the thyroglobulin contained in the colloid.[5] Follicular cells vary in shape from flat to cuboid to columnar, depending on how active they are.[5][16]
- Follicular lumen
The follicular lumen is the fluid-filled space within a follicle of the thyroid gland. There are hundreds of follicles within the thyroid gland. A follicle is formed by a spherical arrangement of follicular cells. The follicular lumen is filled with colloid, a concentrated solution of thyroglobulin and is the site of synthesis of the thyroid hormones thyroxine (T4) and triiodothyronine (T3).[17]
- Parafollicular cells
Scattered among follicular cells and in spaces between the spherical follicles are another type of thyroid cell, parafollicular cells.[5] These cells secrete calcitonin and so are also called C cells.[18]
Development[edit]
Floor of pharynx of embryo between 35 and 37 days after fertilization.
In the development of the embryo, at 3–4 weeks gestational age, the thyroid gland appears as an epithelial proliferation in the floor of the pharynx at the base of the tongue between the tuberculum impar and the copula linguae. The copula soon becomes covered over by the hypopharyngeal eminence[19] at a point later indicated by the foramen cecum. The thyroid then descends in front of the pharyngeal gut as a bilobed diverticulum through the thyroglossal duct. Over the next few weeks, it migrates to the base of the neck, passing in front of the hyoid bone. During migration, the thyroid remains connected to the tongue by a narrow canal, the thyroglossal duct. At the end of the fifth week the thyroglossal duct degenerates, and over the following two weeks the detached thyroid migrates to its final position.[19]
The fetal hypothalamus and pituitary start to secrete thyrotropin-releasing hormone (TRH) and thyroid-stimulating hormone (TSH). TSH is first measurable at 11 weeks.[20] By 18–20 weeks, the production of thyroxine (T4) reaches a clinically significant and self-sufficient level.[20][21] Fetal triiodothyronine (T3) remains low, less than 15 ng/dL until 30 weeks, and increases to 50 ng/dL at full-term.[21] The fetus needs to be self-sufficient in thyroid hormones in order to guard against neurodevelopmental disorders that would arise from maternal hypothyroidism.[22] The presence of sufficient iodine is essential for healthy neurodevelopment.[23]
The neuroendocrine parafollicular cells, also known as C cells, responsible for the production of calcitonin, are derived from foregut endoderm. This part of the thyroid then first forms as the ultimopharyngeal body, which begins in the ventral fourth pharyngeal pouch and joins the primordial thyroid gland during its descent to its final location.[24]
Aberrations in prenatal development can result in various forms of thyroid dysgenesis which can cause congenital hypothyroidism, and if untreated this can lead to cretinism.[20]
Function[edit]
The thyroid hormones T3 and T4 have a number of metabolic, cardiovascular and developmental effects on the body. The production is stimulated by release of thyroid stimulating hormone (TSH), which in turn depends on release of thyrotropin releasing hormone (TRH). Every downstream hormone has negative feedback and decreases the level of the hormone that stimulates its release.
Thyroid hormones[edit]
The primary function of the thyroid is the production of the iodine-containing thyroid hormones, triiodothyronine (T3) and thyroxine or tetraiodothyronine (T4) and the peptide hormone calcitonin.[25] The thyroid hormones are created from iodine and tyrosine. T3 is so named because it contains three atoms of iodine per molecule and T4 contains four atoms of iodine per molecule.[26] The thyroid hormones have a wide range of effects on the human body. These include:
- Metabolic. The thyroid hormones increase the basal metabolic rate and have effects on almost all body tissues.[27] Appetite, the absorption of substances, and gut motility are all influenced by thyroid hormones.[28] They increase the absorption in the gut, generation, uptake by cells, and breakdown of glucose.[29] They stimulate the breakdown of fats, and increase the number of free fatty acids.[29] Despite increasing free fatty acids, thyroid hormones decrease cholesterol levels, perhaps by increasing the rate of secretion of cholesterol in bile.[29]
- Cardiovascular. The hormones increase the rate and strength of the heartbeat. They increase the rate of breathing, intake and consumption of oxygen, and increase the activity of mitochondria.[28] Combined, these factors increase blood flow and the body’s temperature.[28]
- Developmental. Thyroid hormones are important for normal development.[29] They increase the growth rate of young people,[30] and cells of the developing brain are a major target for the thyroid hormones T3 and T4. Thyroid hormones play a particularly crucial role in brain maturation during fetal development and first few years of postnatal life[29]
- The thyroid hormones also play a role in maintaining normal sexual function, sleep, and thought patterns. Increased levels are associated with increased speed of thought generation but decreased focus.[28] Sexual function, including libido and the maintenance of a normal menstrual cycle, are influenced by thyroid hormones.[28]
After secretion, only a very small proportion of the thyroid hormones travel freely in the blood. Most are bound to thyroxine-binding globulin (about 70%), transthyretin (10%), and albumin (15%).[31] Only the 0.03% of T4 and 0.3% of T3 traveling freely have hormonal activity.[32] In addition, up to 85% of the T3 in blood is produced following conversion from T4 by iodothyronine deiodinases in organs around the body.[25]
Thyroid hormones act by crossing the cell membrane and binding to intracellular nuclear thyroid hormone receptors TR-α1, TR-α2, TR-β1, and TR-β2, which bind with hormone response elements and transcription factors to modulate DNA transcription.[32][33] In addition to these actions on DNA, the thyroid hormones also act within the cell membrane or within cytoplasm via reactions with enzymes, including calcium ATPase, adenylyl cyclase, and glucose transporters.[20]
Hormone production[edit]
The thyroid hormones are created from thyroglobulin. This is a protein within the colloid in the follicular lumen that is originally created within the rough endoplasmic reticulum of follicular cells and then transported into the follicular lumen. Thyroglobulin contains 123 units of tyrosine, which reacts with iodine within the follicular lumen.[35]
Iodine is essential for the production of the thyroid hormones. Iodine (I0) travels in the blood as iodide (I−), which is taken up into the follicular cells by a sodium-iodide symporter. This is an ion channel on the cell membrane which in the same action transports two sodium ions and an iodide ion into the cell.[36] Iodide then travels from within the cell into the lumen, through the action of pendrin, an iodide-chloride antiporter. In the follicular lumen, the iodide is then oxidized to iodine. This makes it more reactive,[34] and the iodine is attached to the active tyrosine units in thyroglobulin by the enzyme thyroid peroxidase. This forms the precursors of thyroid hormones monoiodotyrosine (MIT), and diiodotyrosine (DIT).[2]
When the follicular cells are stimulated by thyroid-stimulating hormone, the follicular cells reabsorb thyroglobulin from the follicular lumen. The iodinated tyrosines are cleaved, forming the thyroid hormones T4, T3, DIT, MIT, and traces of reverse triiodothyronine. T3 and T4 are released into the blood. The hormones secreted from the gland are about 80–90% T4 and about 10–20% T3.[37][38] Deiodinase enzymes in peripheral tissues remove the iodine from MIT and DIT and convert T4 to T3 and RT3. [35] This is a major source of both RT3 (95%) and T3 (87%) in peripheral tissues.[39]
Regulation[edit]
The production of thyroxine and triiodothyronine is primarily regulated by thyroid-stimulating hormone (TSH), released by the anterior pituitary gland. TSH release in turn is stimulated by thyrotropin releasing hormone (TRH), released in a pulsatile manner from the hypothalamus.[40] The thyroid hormones provide negative feedback to the thyrotropes TSH and TRH: when the thyroid hormones are high, TSH production is suppressed. This negative feedback also occurs when levels of TSH are high, causing TRH production to be suppressed.[41]
TRH is secreted at an increased rate in situations such as cold exposure in order to stimulate thermogenesis.[42] In addition to being suppressed by the presence of thyroid hormones, TSH production is blunted by dopamine, somatostatin, and glucocorticoids.[43]
Calcitonin[edit]
The thyroid gland also produces the hormone calcitonin, which helps regulate blood calcium levels. Parafollicular cells produce calcitonin in response to high blood calcium. Calcitonin decreases the release of calcium from bone, by decreasing the activity of osteoclasts, cells which break down bone. Bone is constantly reabsorbed by osteoclasts and created by osteoblasts, so calcitonin effectively stimulates movement of calcium into bone. The effects of calcitonin are opposite those of the parathyroid hormone (PTH) produced in the parathyroid glands. However, calcitonin seems far less essential than PTH, since calcium metabolism remains clinically normal after removal of the thyroid (thyroidectomy), but not the parathyroid glands.[44]
Gene and protein expression[edit]
About 20,000 protein coding genes are expressed in human cells: 70% of these genes are expressed in thyroid cells.[45][46] Two-hundred fifty of these genes are more specifically expressed in the thyroid, and about 20 genes are highly thyroid specific. In the follicular cells, the proteins synthesized by these genes direct thyroid hormone synthesis—thyroglobulin, TPO, and IYD; while in the parafollicular c-cells, they direct calcitonin synthesis—CALCA, and CALCB.
Clinical significance[edit]
General practitioners, and internal medicine specialists play a role in identifying and monitoring the treatment of thyroid disease. Endocrinologists and thyroidologists are thyroid specialists. Thyroid surgeons or otolaryngologists are responsible for the surgical management of thyroid disease.
Functional disorders[edit]
Hyperthyroidism[edit]
Excessive production of the thyroid hormones is called hyperthyroidism. Causes include Graves’ disease, toxic multinodular goitre, solitary thyroid adenoma, inflammation, and a pituitary adenoma which secretes excess TSH. Another cause is excess iodine availability, either from excess ingestion, induced by the drug amiodarone, or following iodinated contrast imaging.[47][48]
Hyperthyroidism often causes a variety of non-specific symptoms including weight loss, increased appetite, insomnia, decreased tolerance of heat, tremor, palpitations, anxiety and nervousness. In some cases it can cause chest pain, diarrhoea, hair loss and muscle weakness.[49] Such symptoms may be managed temporarily with drugs such as beta blockers.[50]
Long-term management of hyperthyroidism may include drugs that suppress thyroid function such as propylthiouracil, carbimazole and methimazole.[51] Alternatively, radioactive iodine-131 can be used to destroy thyroid tissue: radioactive iodine is selectively taken up by thyroid cells, which over time destroys them. The chosen first-line treatment will depend on the individual and on the country where being treated. Surgery to remove the thyroid can sometimes be performed as a transoral thyroidectomy, a minimally invasive procedure.[52] Surgery does however carry a risk of damage to the parathyroid glands and the recurrent laryngeal nerve, which innervates the vocal cords. If the entire thyroid gland is removed, hypothyroidism will inevitably result, and thyroid hormone substitutes will be needed.[53][50]
Hypothyroidism[edit]
An underactive thyroid gland results in hypothyroidism. Typical symptoms are abnormal weight gain, tiredness, constipation, heavy menstrual bleeding, hair loss, cold intolerance, and a slow heart rate.[49] Iodine deficiency is the most common cause of hypothyroidism worldwide,[54] and the autoimmune disease Hashimoto’s thyroiditis is the most common cause in the developed world.[55] Other causes include congenital abnormalities, diseases causing transient inflammation, surgical removal or radioablation of the thyroid, the drugs amiodarone and lithium, amyloidosis, and sarcoidosis.[56] Some forms of hypothyroidism can result in myxedema and severe cases can result in myxedema coma.[57]
Hypothyroidism is managed with replacement of the thyroid hormones. This is usually given daily as an oral supplement, and may take a few weeks to become effective.[57] Some causes of hypothyroidism, such as Postpartum thyroiditis and Subacute thyroiditis may be transient and pass over time, and other causes such as iodine deficiency may be able to be rectified with dietary supplementation.[58]
Diseases[edit]
Graves’ disease[edit]
Graves’ disease is an autoimmune disorder that is the most common cause of hyperthyroidism.[59] In Graves’ disease, for an unknown reason autoantibodies develop against the thyroid stimulating hormone receptor. These antibodies activate the receptor, leading to development of a goitre and symptoms of hyperthyroidism, such as heat intolerance, weight loss, diarrhoea and palpitations. Occasionally such antibodies block but do not activate the receptor, leading to symptoms associated with hypothyroidism.[59] In addition, gradual protrusion of the eyes may occur, called Graves’ ophthalmopathy, as may swelling of the front of the shins.[59] Graves’ disease can be diagnosed by the presence of pathomnomonic features such as involvement of the eyes and shins, or isolation of autoantibodies, or by results of a radiolabelled uptake scan. Graves’ disease is treated with anti-thyroid drugs such as propylthiouracil, which decrease the production of thyroid hormones, but hold a high rate of relapse. If there is no involvement of the eyes, then use of radioactive isotopes to ablate the gland may be considered. Surgical removal of the gland with subsequent thyroid hormone replacement may be considered, however this will not control symptoms associated with the eye or skin.[59]
Nodules[edit]
Thyroid nodules are often found on the gland, with a prevalence of 4–7%.[60] The majority of nodules do not cause any symptoms, thyroid hormone secretion is normal, and they are non-cancerous.[61] Non-cancerous cases include simple cysts, colloid nodules, and thyroid adenomas. Malignant nodules, which only occur in about 5% of nodules, include follicular, papillary, medullary carcinomas and metastasis from other sites [62] Nodules are more likely in females, those who are exposed to radiation, and in those who are iodine deficient.[60]
When a nodule is present, thyroid function tests determine whether the nodule is secreting excess thyroid hormones, causing hyperthyroidism.[61] When the thyroid function tests are normal, an ultrasound is often used to investigate the nodule, and provide information such as whether the nodule is fluid-filled or a solid mass, and whether the appearance is suggestive of a benign or malignant cancer.[60] A needle aspiration biopsy may then be performed, and the sample undergoes cytology, in which the appearance of cells is viewed to determine whether they resemble normal or cancerous cells.[62]
The presence of multiple nodules is called a multinodular goitre; and if it is associated with hyperthyroidism, it is called a toxic multinodular goitre.[62]
Goitre[edit]
An enlarged thyroid gland is called a goitre.[63] Goitres are present in some form in about 5% of people,[62] and are the result of a large number of causes, including iodine deficiency, autoimmune disease (both Graves’ disease and Hashimoto’s thyroiditis), infection, inflammation, and infiltrative disease such as sarcoidosis and amyloidosis. Sometimes no cause can be found, a state called «simple goitre».[64]
Some forms of goitre are associated with pain, whereas many do not cause any symptoms. Enlarged goitres may extend beyond the normal position of the thyroid gland to below the sternum, around the airway or esophagus.[62] Goitres may be associated with hyperthyroidism or hypothyroidism, relating to the underlying cause of the goitre.[62] Thyroid function tests may be done to investigate the cause and effects of the goitre. The underlying cause of the goitre may be treated, however many goitres with no associated symptoms are simply monitored.[62]
Inflammation[edit]
Inflammation of the thyroid is called thyroiditis, and may cause symptoms of hyperthyroidism or hypothyroidism. Two types of thyroiditis initially present with hyperthyroidism and are sometimes followed by a period of hypothyroidism – Hashimoto’s thyroiditis and postpartum thyroiditis. There are other disorders that cause inflammation of the thyroid, and these include subacute thyroiditis, acute thyroiditis, silent thyroiditis, Riedel’s thyroiditis and traumatic injury, including palpation thyroiditis.[65]
Hashimoto’s thyroiditis is an autoimmune disorder in which the thyroid gland is infiltrated by the lymphocytes B cell and T cells. These progressively destroy the thyroid gland.[66] In this way, Hasimoto’s thyroiditis may have occurred insidiously, and only be noticed when thyroid hormone production decreases, causing symptoms of hypothyroidism.[66] Hashimoto’s is more common in females than males, much more common after the age of 60, and has known genetic risk factors.[66] Also more common in individuals with Hashimoto’s thyroiditis are Type 1 diabetes, pernicious anaemia, Addison’s disease vitiligo.[66]
Postpartum thyroiditis occurs in some females following childbirth. After delivery, the gland becomes inflamed and the condition initially presents with a period of hyperthyroidism followed by hypothyroidism and, usually, a return to normal function. [67] The course of the illness takes place over several months, and is characterised by a painless goitre. Antibodies against thyroid peroxidase can be found on testing. The inflammation usually resolves without treatment, although thyroid hormone replacement may be needed during the period of hypothyroidism.[67]
Cancer[edit]
The most common neoplasm affecting the thyroid gland is a benign adenoma, usually presenting as a painless mass in the neck.[68] Malignant thyroid cancers are most often carcinomas, although cancer can occur in any tissue that the thyroid consists of, including cancer of C-cells and lymphomas. Cancers from other sites also rarely lodge in the thyroid.[68] Radiation of the head and neck presents a risk factor for thyroid cancer, and cancer is more common in women than men, occurring at a rate of about 2:1.[68]
In most cases, thyroid cancer presents as a painless mass in the neck. It is very unusual for thyroid cancers to present with other symptoms, although in some cases cancer may cause hyperthyroidism.[69] Most malignant thyroid cancers are papillary, followed by follicular, medullary, and thyroid lymphoma.[68][69] Because of the prominence of the thyroid gland, cancer is often detected earlier in the course of disease as the cause of a nodule, which may undergo fine-needle aspiration. Thyroid function tests will help reveal whether the nodule produces excess thyroid hormones. A radioactive iodine uptake test can help reveal the activity and location of the cancer and metastases.[68][70]
Thyroid cancers are treated by removing the whole or part of thyroid gland. Radioactive Iodine-131 may be given to radioablate the thyroid. Thyroxine is given to replace the hormones lost and to suppress TSH production, as TSH may stimulate recurrence.[70] With the exception of the rare anaplastic thyroid cancer, which carries a very poor prognosis, most thyroid cancers carry an excellent prognosis and can even be considered curable.[71]
Congenital[edit]
A persistent thyroglossal duct is the most common clinically significant birth defect of the thyroid gland. A persistent sinus tract may remain as a vestigial remnant of the tubular development of the thyroid gland. Parts of this tube may be obliterated, leaving small segments to form thyroglossal cysts.[24] Preterm neonates are at risk of hypothyroidism as their thyroid glands are insufficiently developed to meet their postnatal needs.[72] In order to detect hypothyroidism in newborn babies, to prevent growth and development abnormalities in later life, many countries have newborn screening programs at birth.[73]
Infants with thyroid hormone deficiency (congenital hypothyroidism) can manifest problems of physical growth and development as well as brain development, termed cretinism.[74][23] Children with congenital hypothyroidism are treated supplementally with levothyroxine, which facilitates normal growth and development.[75]
Mucinous, clear secretions may collect within these cysts to form either spherical masses or fusiform swellings, rarely larger than 2 to 3 cm in diameter. These are present in the midline of the neck anterior to the trachea. Segments of the duct and cysts that occur high in the neck are lined by stratified squamous epithelium, which is essentially identical to that covering the posterior portion of the tongue in the region of the foramen cecum. The disorders that occur in the lower neck more proximal to the thyroid gland are lined by epithelium resembling the thyroidal acinar epithelium. Characteristically, next to the lining epithelium, there is an intense lymphocytic infiltrate. Superimposed infection may convert these lesions into abscess cavities, and rarely, give rise to cancers.[citation needed]
Another disorder is that of thyroid dysgenesis which can result in various presentations of one or more misplaced accessory thyroid glands.[5] These can be asymptomatic.
Iodine[edit]
Iodine deficiency, most common in inland and mountainous areas, can predispose to goitre – if widespread, known as endemic goitre.[74] Pregnant women deficient of iodine can give birth to infants with thyroid hormone deficiency.[74][23] The use of iodised salt to add iodine to the diet[23] has eliminated endemic cretinism in most developed countries,[77] and over 120 countries have made the iodination of salt mandatory.[78][79]
Because the thyroid concentrates iodine, it also concentrates the various radioactive isotopes of iodine produced by nuclear fission. In the event of large accidental releases of such material into the environment, the uptake of radioactive iodine isotopes by the thyroid can, in theory, be blocked by saturating the uptake mechanism with a large surplus of non-radioactive iodine, taken in the form of potassium iodide tablets. One consequence of the Chernobyl disaster was an increase in thyroid cancers in children in the years following the accident.[80]
Excessive iodine intake is uncommon and usually has no effect on the thyroid function. Sometimes though it may cause hyperthyroidism, and sometimes hypothyroidism with a resulting goitre.[81]
Evaluation[edit]
The thyroid is examined by observation of the gland and surrounding neck for swelling or enlargement.[82] It is then felt, usually from behind, and a person is often asked to swallow to better feel the gland against the fingers of the examiner.[82] The gland moves up and down with swallowing because of its attachments to the thyroid and cricoid cartilages.[6] In a healthy person the gland is not visible yet is palpable as a soft mass. Examination of the thyroid gland includes the search for abnormal masses and the assessment of overall thyroid size.[83] The character of the thyroid, swellings, nodules, and their consistency may all be able to be felt. If a goitre is present, an examiner may also feel down the neck consider tapping the upper part of the chest to check for extension. Further tests may include raising the arms (Pemberton’s sign), listening to the gland with a stethoscope for bruits, testing of reflexes, and palpation of the lymph nodes in the head and neck.
An examination of the thyroid will also include observation of the person as a whole, to look for systemic signs such as weight gain or loss, hair loss, and signs in other locations – such as protrusion of the eyes or swelling of the calves in Graves’ disease.[84][82]
Tests[edit]
Thyroid function tests include a battery of blood tests, including the measurement of the thyroid hormones, as well as the measurement of thyroid stimulating hormone (TSH).[85] They may reveal hyperthyroidism (high T3 and T4), hypothyroidism (low T3, T4), or subclinical hyperthyroidism (normal T3 and T4 with a low TSH).[85]
TSH levels are considered the most sensitive marker of thyroid dysfunction.[85] They are however not always accurate, particularly if the cause of hypothyroidism is thought to be related to insufficient thyrotropin releasing hormone (TRH) secretion, in which case it may be low or falsely normal. In such a case a TRH stimulation test, in which TRH is given and TSH levels are measured at 30 and 60-minutes after, may be conducted.[85]
T3 and T4 can be measured directly. However, as the two thyroid hormones travel bound to other molecules, and it is the «free» component that is biologically active, free T3 and free T4 levels can be measured.[85] T3 is preferred, because in hypothyroidism T3 levels may be normal.[85] The ratio of bound to unbound thyroid hormones is known as the thyroid hormone binding ratio (THBR).[86] It is also possible to measure directly the main carriers of the thyroid hormones, thryoglobulin and throxine-binding globulin.[87] Thyroglobulin will also be measurable in a healthy thyroid, and will increase with inflammation, and may also be used to measure the success of thyroid removal or ablation. If successful, thyroglobulin should be undetectable.[86] Lastly, antibodies against components of the thyroid, particularly anti-TPO and anti-thyroglobulin, can be measured. These may be present in normal individuals but are highly sensitive for autoimmune-related disease.[86]
Imaging[edit]
Ultrasound of the thyroid may be used to reveal whether structures are solid or filled with fluid, helping to differentiate between nodules and goitres and cysts. It may also help differentiate between malignant and benign lesions.[88]
When further imaging is required, a radiolabelled iodine-123 or technetium-99 uptake scan may take place. This can determine the size and shape of lesions, reveal whether nodules or goitres are metabolically active, and reveal and monitor sites of thyroid disease or cancer deposits outside the thyroid.[89]
A fine needle aspiration of a sample of thyroid tissue may be taken in order to evaluate a lesion seen on ultrasound which is then sent for histopathology and cytology.[90]
Computed tomography of the thyroid plays an important role in the evaluation of thyroid cancer.[91] CT scans often incidentally find thyroid abnormalities, and thereby practically becomes the first investigation modality.[91]
History[edit]
The thyroid gland received its modern name in the 1600s, when the anatomist Thomas Wharton likened its shape to that of an Ancient Greek shield or thyos. However, the existence of the gland, and of the diseases associated with it, was known long before then.
Antiquity[edit]
The presence and diseases of the thyroid have been noted and treated for thousands of years.[4] In 1600 BCE burnt sponge and seaweed (which contain iodine) were used within China for the treatment of goitres, a practice which has developed in many parts of the world.[4][92] In Ayurvedic medicine, the book Sushruta Samhita written about 1400 BCE described hyperthyroidism, hypothyroidism and goitre.[92] Aristotle and Xenophon in the fifth century BCE describe cases of diffuse toxic goitre.[92] Hippocrates and Plato in the fourth century BCE provided some of the first descriptions of the gland itself, proposing its function as a salivary gland.[92] Pliny the Elder in the first century BCE referred to epidemics of goitre in the Alps and proposed treatment with burnt seaweed,[4] a practice also referred to by Galen in the second century, referred to burnt sponge for the treatment of goitre.[4] The Chinese pharmacology text Shennong Ben Cao Jing, written ca. 200–250, also refers to goitre.[4][92]
Scientific era[edit]
In 1500 polymath Leonardo da Vinci provided the first illustration of the thyroid.[4] In 1543 anatomist Andreas Vesalius gave the first anatomic description and illustration of the gland.[4] In 1656 the thyroid received its modern name, by the anatomist Thomas Wharton.[4] The gland was named thyroid, meaning shield, as its shape resembled the shields commonly used in Ancient Greece.[4] The English name thyroid gland[93] is derived from the medical Latin used by Wharton – glandula thyreoidea.[94] Glandula means ‘gland’ in Latin,[95] and thyreoidea can be traced back to the Ancient Greek word θυρεοειδής, meaning ‘shield-like/shield-shaped’.[96]
French chemist Bernard Courtois discovered iodine in 1811,[92] and in 1896 Eugen Baumann documented it as the central ingredient in the thyroid gland. He did this by boiling the thyroid glands of a thousand sheep, and named the precipitate, a combination of the thyroid hormones, ‘iodothyrin’.[92] David Marine in 1907 proved that iodine is necessary for thyroid function.[92][4]
Graves’ disease was described by Robert James Graves in 1834. The role of the thyroid gland in metabolism was demonstrated in 1895 by Adolf Magnus-Levy.[97] Thyroxine was first isolated in 1914 and synthesized in 1927, and triiodothyroxine in 1952.[92][98] The conversion of T4 to T3 was discovered in 1970.[4] The process of discovering TSH took place over the early to mid twentieth century.[99] TRH was discovered by Polish endocrinologist Andrew Schally in 1970, contributing in part to his Nobel Prize in Medicine in 1977.[4][100]
In the nineteenth century numerous authors described both cretinism and myxedema, and their relationship to the thyroid.[92] Charles Mayo coined the term hyperthyroidism in 1910.[4] Hakaru Hashimoto documented a case of Hashimoto’s thyroiditis in 1912, antibodies in this disease were demonstrated in 1956.[92] Knowledge of the thyroid and its conditions developed throughout the late nineteenth and twentieth centuries, with many modern treatments and investigative modalities evolving throughout the mid twentieth century, including the use of radioactive iodine, thiouracil and fine needle aspiration.[4]
Surgery[edit]
Either Aetius in the sixth century CE[92] or Persian Ali ibn Abbas al-Magusi in 990 CE conducted the first recorded thyroidectomy as a treatment for goitre.[4][101] Operations remained risky and generally were not successful until the 19th century, when descriptions emerged from a number of authors including Prussian surgeon Theodor Billroth, Swiss surgeon and physiologist Theodor Kocher, American physician Charles Mayo, American surgeons William Halsted and George Crile. These descriptions provided the basis for modern thyroid surgery.[102] Theodor Kocher went on to win the Nobel Prize in Physiology or Medicine in 1909 «for his work on the physiology, pathology and surgery of the thyroid gland».[103]
Other animals[edit]
The thyroid gland is found in all vertebrates. In fish, it is usually located below the gills and is not always divided into distinct lobes. However, in some teleosts, patches of thyroid tissue are found elsewhere in the body, associated with the kidneys, spleen, heart, or eyes.[104]
In tetrapods, the thyroid is always found somewhere in the neck region. In most tetrapod species, there are two paired thyroid glands – that is, the right and left lobes are not joined. However, there is only ever a single thyroid gland in most mammals, and the shape found in humans is common to many other species.[104]
In larval lampreys, the thyroid originates as an exocrine gland, secreting its hormones into the gut, and associated with the larva’s filter-feeding apparatus. In the adult lamprey, the gland separates from the gut, and becomes endocrine, but this path of development may reflect the evolutionary origin of the thyroid. For instance, the closest living relatives of vertebrates, the tunicates and amphioxi (lancelets), have a structure very similar to that of larval lampreys (the endostyle), and this also secretes iodine-containing compounds, though not thyroxine.[104]
Thyroxine is critical to metabolic regulation, and growth throughout the vertebrate clade. Iodine and T4 trigger the change from a plant-eating water-dwelling tadpole into a meat-eating land-dwelling frog, with better neurological, visuospatial, smell and cognitive abilities for hunting, as seen in other predatory animals. A similar phenomenon happens in the neotenic amphibian salamanders, which, without introducing iodine, don’t transform into land-dwelling adults, and live and reproduce in the larval form of aquatic axolotl. Among amphibians, administering a thyroid-blocking agent such as propylthiouracil (PTU) can prevent tadpoles from metamorphosing into frogs; in contrast, administering thyroxine will trigger metamorphosis. In amphibian metamorphosis, thyroxine and iodine also exert a well-studied experimental model of apoptosis on the cells of gills, tail, and fins of tadpoles. Iodine, via iodolipids, has favored the evolution of terrestrial animal species and has likely played a crucial role in the evolution of the human brain.[105][106]
See also[edit]
- Desiccated thyroid
- Thyroid disease in pregnancy
References[edit]
- ^ Guyton & Hall 2011, p. 907.
- ^ a b Boron WF, Boulpaep EL (2012). Medical Physiology (2nd ed.). Philadelphia: Saunders. p. 1052. ISBN 978-1-4377-1753-2.
- ^ Harrison’s 2011, pp. 2913, 2918.
- ^ a b c d e f g h i j k l m n o p q «Thyroid History Timeline – American Thyroid Association». www.thyroid.org. Retrieved 13 November 2016.
- ^ a b c d e f g h i j k l m n o p q r s t u Gray’s Anatomy 2008, pp. 462–4.
- ^ a b c d e f g h i j k Elsevier’s 2007, p. 342.
- ^ Elsevier’s 2007, pp. 342–3.
- ^ Ellis H, Standring S, Gray HD (2005). Gray’s anatomy: the anatomical basis of clinical practice. St. Louis, Mo: Elsevier Churchill Livingstone. pp. 538–539. ISBN 978-0-443-07168-3.
- ^ Netter FH (2014). Atlas of Human Anatomy Including Student Consult Interactive Ancillaries and Guides (6th ed.). Philadelphia, Penn.: W B Saunders Co. p. 27. ISBN 978-1-4557-0418-7.
- ^ a b Page C, Cuvelier P, Biet A, Boute P, Laude M, Strunski V (July 2009). «Thyroid tubercle of Zuckerkandl: anatomical and surgical experience from 79 thyroidectomies». The Journal of Laryngology and Otology. 123 (7): 768–71. doi:10.1017/s0022215108004003. PMID 19000342. S2CID 22063700.
- ^ a b c Elsevier’s 2007, p. 343.
- ^ a b Cicekcibasi AE, Salbacak A, Seker M, Ziylan T, Tuncer I, Buyukmumcu M (April 2007). «Developmental variations and clinical importance of the fetal thyroid gland. A morphometric study». Saudi Medical Journal. 28 (4): 524–8. PMID 17457471.
- ^ Kim DW, Jung SL, Baek JH, Kim J, Ryu JH, Na DG, et al. (January 2013). «The prevalence and features of thyroid pyramidal lobe, accessory thyroid, and ectopic thyroid as assessed by computed tomography: a multicenter study». Thyroid. 23 (1): 84–91. doi:10.1089/thy.2012.0253. PMID 23031220.
- ^ Dorland WA (2012). Anderson DM (ed.). Dorland’sIllustrated Medical Dictionary (32nd ed.). Elsevier Saunders. pp. 999 redirect to 1562. ISBN 978-1-4160-6257-8.
- ^ Fawcett D, Jensh R (2002). Bloom & Fawcett’s Concise Histology. New York: Arnold Publishers. pp. 257–258. ISBN 978-0-340-80677-7.
- ^ a b Wheater PR, Young B (2006). Wheater’s functional histology : a text and colour atlas (5th ed.). Oxford: Churchill Livingstone. pp. 333–335. ISBN 978-0-443-06850-8.
- ^ The Thyroid Follicle Archived 2013-01-23 at archive.today, Endocrinology by J. Larry Jameson, MD, PhD and Leslie J. De Groot, MD, chapter 72
- ^ Hazard JB (July 1977). «The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A review». The American Journal of Pathology. 88 (1): 213–50. PMC 2032150. PMID 18012.
- ^ a b Larsen WJ (2001). Human embryology (3. ed.). Philadelphia, Pa.: Churchill Livingstone. pp. 372–374. ISBN 978-0-443-06583-5.
- ^ a b c d Greenspan’s 2011, p. 179.
- ^ a b Eugster EA, Pescovitz OH (2004). Pediatric endocrinology: mechanisms, manifestations and management. Hagerstwon, MD: Lippincott Williams & Wilkins. p. 493 (Table 33–3). ISBN 978-0-7817-4059-3.
- ^ Zoeller RT (April 2003). «Transplacental thyroxine and fetal brain development». The Journal of Clinical Investigation. 111 (7): 954–7. doi:10.1172/JCI18236. PMC 152596. PMID 12671044.
- ^ a b c d «Iodine supplementation in pregnant and lactating women». World Health Organization. Archived from the original on January 4, 2014. Retrieved 2016-11-13.
- ^ a b Langman J, Sadler TW, Sadler-Redmond SL, Tosney K, Byrne J, Imseis H (2015). Langman’s Medical Embryology (13th ed.). pp. 285–6, 293. ISBN 978-1-4511-9164-6.
- ^ a b Davidson’s 2010, p. 736.
- ^ Guyton & Hall 2011, p. 909.
- ^ Guyton & Hall 2011, p. 934.
- ^ a b c d e Guyton & Hall 2011, p. 937.
- ^ a b c d e Guyton & Hall 2011, p. 936.
- ^ Guyton & Hall 2011, p. 935-6.
- ^ Greenspan’s 2011, p. 169.
- ^ a b Bowen R (2000). «Thyroid Hormone Receptors». Colorado State University. Archived from the original on 27 September 2011. Retrieved 22 February 2015.
- ^ Greenspan’s 2011, p. 178.
- ^ a b Boron WF, Boulpaep E (2003). «Chapter 48: «synthesis of thyroid hormones»«. Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1300. ISBN 978-1-4160-2328-9.
- ^ a b Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (February 2002). «Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases». Endocrine Reviews. 23 (1): 38–89. doi:10.1210/edrv.23.1.0455. PMID 11844744.
- ^ Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (2011). Williams Textbook of Endocrinology (12th ed.). Saunders. p. 331. ISBN 978-1-4377-0324-5.
- ^ How Your Thyroid Works: A Delicate Feedback Mechanism. Updated 2009-05-21.
- ^ The thyroid gland in Endocrinology: An Integrated Approach by Stephen Nussey and Saffron Whitehead (2001) Published by BIOS Scientific Publishers Ltd. ISBN 1-85996-252-1
- ^ Ganong’s review of medical physiology Edition 25.
- ^ Greenspan’s 2011, p. 174.
- ^ Greenspan’s 2011, p. 177.
- ^ Guyton & Hall 2011, p. 896.
- ^ Harrison’s 2011, pp. 2215.
- ^ Guyton & Hall 2011, pp. 988–9.
- ^ «The human proteome in thyroid gland – The Human Protein Atlas». www.proteinatlas.org. Retrieved 2017-09-25.
- ^ Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. (January 2015). «Proteomics. Tissue-based map of the human proteome». Science. 347 (6220): 1260419. doi:10.1126/science.1260419. PMID 25613900. S2CID 802377.
- ^ Davidson’s 2010, p. 738.
- ^ Rusandu A, Sjøvold BH, Hofstad E, Reidunsdatter RJ (June 2020). «Iodinated contrast media and their effect on thyroid function — Routines and practices among diagnostic imaging departments in Norway». Journal of Medical Radiation Sciences. 67 (2): 111–118. doi:10.1002/jmrs.390. PMC 7276191. PMID 32232955.
- ^ a b Davidson’s 2010, p. 740.
- ^ a b Davidson’s 2010, p. 739.
- ^ Davidson’s 2010, p. 745.
- ^ Cury AN, Meira VT, Monte O, Marone M, Scalissi NM, Kochi C, et al. (March 2013). «Clinical experience with radioactive iodine in the treatment of childhood and adolescent Graves’ disease». Endocrine Connections. 2 (1): 32–7. doi:10.1530/EC-12-0049. PMC 3680965. PMID 23781316.
- ^ Thyroid Problems eMedicine Health. Retrieved on 2010-02-07
- ^ «Iodine Deficiency & Nutrition». www.thyroidfoundation.org.au. Australian Thyroid Foundation. Archived from the original on 13 January 2017. Retrieved 11 January 2017.
- ^ So M, MacIsaac R, Grossmann M. «Hypothyroidism – Investigation and management». www.racgp.org.au. The Royal Australian College of General Practitioners. Retrieved 11 January 2017.
- ^ Davidson’s 2010, p. 741.
- ^ a b Davidson’s 2010, p. 743.
- ^ Davidson’s 2010, p. 741-3.
- ^ a b c d Smith TJ, Hegedüs L (October 2016). «Graves’ Disease» (PDF). The New England Journal of Medicine. 375 (16): 1552–1565. doi:10.1056/nejmra1510030. PMID 27797318.
- ^ a b c Dean DS, Gharib H (December 2008). «Epidemiology of thyroid nodules». Best Practice & Research. Clinical Endocrinology & Metabolism. 22 (6): 901–11. doi:10.1016/j.beem.2008.09.019. PMID 19041821.
- ^ a b Welker MJ, Orlov D (February 2003). «Thyroid nodules». American Family Physician. 67 (3): 559–66. PMID 12588078. Retrieved 6 September 2016.
- ^ a b c d e f g Davidson’s 2010, p. 744.
- ^ «goitre – definition of goitre in English». Oxford Dictionaries | English. Archived from the original on September 18, 2016. Retrieved 18 September 2016.
- ^ Davidson’s 2010, p. 750.
- ^ Harrison’s 2011, pp. 2237.
- ^ a b c d Harrison’s 2011, pp. 2230.
- ^ a b Harrison’s 2011, pp. 2238.
- ^ a b c d e Harrison’s 2011, p. 2242.
- ^ a b Davidson’s 2010, p. 751.
- ^ a b Davidson’s 2010, p. 752.
- ^ Harrison’s 2011, p. 2242,2246.
- ^ Berbel P, Navarro D, Ausó E, Varea E, Rodríguez AE, Ballesta JJ, et al. (June 2010). «Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity». Cerebral Cortex. 20 (6): 1462–75. doi:10.1093/cercor/bhp212. PMC 2871377. PMID 19812240.
- ^ Büyükgebiz A (15 November 2012). «Newborn screening for congenital hypothyroidism». Journal of Clinical Research in Pediatric Endocrinology. 5 Suppl 1 (4): 8–12. doi:10.4274/Jcrpe.845. PMC 3608007. PMID 23154158.
- ^ a b c Greenspan’s 2011, p. 164.
- ^ Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, Varma SK (June 2006). «Update of newborn screening and therapy for congenital hypothyroidism». Pediatrics. 117 (6): 2290–303. doi:10.1542/peds.2006-0915. PMID 16740880.
- ^ The thyroid gland in health and disease Year: 1917 Robert McCarrison
- ^ Harris RE (2015-05-07). Global Epidemiology of Cancer. Jones & Bartlett Publishers. p. 268. ISBN 978-1-284-03445-5.
- ^ Leung AM, Braverman LE, Pearce EN (November 2012). «History of U.S. iodine fortification and supplementation». Nutrients. 4 (11): 1740–6. doi:10.3390/nu4111740. PMC 3509517. PMID 23201844.
- ^ «Map: Count of Nutrients In Fortification Standards». Global Fortification Data Exchange. Retrieved 23 December 2019.
- ^ «Chernobyl children show DNA changes». BBC News. 2001-05-08. Retrieved 2010-05-25.
- ^ «Iodine — Disorders of Nutrition». MSD Manual Consumer Version. Archived from the original on 18 December 2019. Retrieved 18 December 2019.
- ^ a b c Talley N (2014). Clinical Examination. Churchill Livingstone. pp. Chapter 28. «The endocrine system». pp 355–362. ISBN 978-0-7295-4198-5.
- ^ Fehrenbach; Herring (2012). Illustrated Anatomy of the Head and Neck. Elsevier. p. 158. ISBN 978-1-4377-2419-6.
- ^ Harrison’s 2011, p. 2228.
- ^ a b c d e f Greenspan’s 2011, p. 184.
- ^ a b c Harrison’s 2011, p. 2229.
- ^ Greenspan’s 2011, p. 186.
- ^ Greenspan’s 2011, p. 189.
- ^ Greenspan’s 2011, p. 188-9.
- ^ Greenspan’s 2011, p. 190.
- ^ a b Bin Saeedan M, Aljohani IM, Khushaim AO, Bukhari SQ, Elnaas ST (August 2016). «Thyroid computed tomography imaging: pictorial review of variable pathologies». Insights into Imaging. 7 (4): 601–17. doi:10.1007/s13244-016-0506-5. PMC 4956631. PMID 27271508. Creative Commons Attribution 4.0 International License
- ^ a b c d e f g h i j k l Niazi AK, Kalra S, Irfan A, Islam A (July 2011). «Thyroidology over the ages». Indian Journal of Endocrinology and Metabolism. 15 (Suppl 2): S121-6. doi:10.4103/2230-8210.83347. PMC 3169859. PMID 21966648.
- ^ Anderson DM (2000). Dorland’s Illustrated Medical Dictionary (29th ed.). Philadelphia/London/Toronto/Montreal/Sydney/Tokyo: W.B. Saunders Company.
- ^ His W (1895). Die anatomische Nomenclatur. Nomina Anatomica. Der von der Anatomischen Gesellschaft auf ihrer IX. Versammlung in Basel angenommenen Namen [The anatomical nomenclature. Nominal Anatomica. Anatomical Society on its IX. Assembly adopted in Basel] (in German). Leipzig: Verlag Veit & Comp.
- ^ Lewis CT, Short C (1879). A Latin dictionary. founded on Andrews’ edition of Freund’s Latin dictionary. Oxford: Clarendon Press.
- ^ Liddell HG, Scott R (1940). A Greek-English Lexicon. revised and augmented throughout by Sir Henry Stuart Jones. with the assistance of. Roderick McKenzie. Oxford: Clarendon Press.
- ^ Freake HC, Oppenheimer JH (1995). «Thermogenesis and thyroid function». Annual Review of Nutrition. 15 (1): 263–91. doi:10.1146/annurev.nu.15.070195.001403. PMID 8527221.
- ^ Hamdy, Roland. «The thyroid glands: a brief historical perspective». www.medscape.com. Retrieved 2016-11-13.
- ^ Magner J (June 2014). «Historical note: many steps led to the ‘discovery’ of thyroid-stimulating hormone». European Thyroid Journal. 3 (2): 95–100. doi:10.1159/000360534. PMC 4109514. PMID 25114872.
- ^ «The Nobel Prize in Physiology or Medicine 1977». www.nobelprize.org. Retrieved 14 January 2017.
- ^ Slidescenter.com. «Hormones.gr». www.hormones.gr. Retrieved 2016-11-13.
- ^ Werner SC, Ingbar SH, Braverman LE, Utiger RD (2005). Werner & Ingbar’s the Thyroid: A Fundamental and Clinical Text. Lippincott Williams & Wilkins. p. 387. ISBN 978-0-7817-5047-9.
- ^ «The Nobel Prize in Physiology or Medicine 1909». Nobel Foundation. Retrieved 2007-07-28.
- ^ a b c Romer AS, Parsons TS (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 555–556. ISBN 978-0-03-910284-5.
- ^ Venturi S (2011). «Evolutionary Significance of Iodine». Current Chemical Biology. 5 (3): 155–162. doi:10.2174/187231311796765012. ISSN 1872-3136.
- ^ Venturi S (2014). «Iodine, PUFAs and Iodolipids in Health and Disease: An Evolutionary Perspective». Human Evolution. 29 (1–3): 185–205. ISSN 0393-9375.
Books[edit]
- Greer MA, ed. (1990). The Thyroid Gland. Comprehensive Endocrinology Revised Series. N.Y.: Raven Press. ISBN 0-88167-668-3.
- Shoback D (2011). Gardner DG (ed.). Greenspan’s basic & clinical endocrinology (9th ed.). New York: McGraw-Hill Medical. ISBN 978-0-07-162243-1.
- Hall JE, Guyton AC (2011). Guyton and Hall textbook of medical physiology (12th ed.). Philadelphia, Pa.: Saunders/Elsevier. ISBN 978-1-4160-4574-8.
- Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J (August 11, 2011). Harrison’s Principles of Internal Medicine (18 ed.). McGraw-Hill Professional. ISBN 978-0-07-174889-6.
- Colledge NR, Walker BR, Ralston SH, eds. (2010). Davidson’s principles and practice of medicine. Illustrated by Robert Britton (21st ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 978-0-7020-3085-7.
- Ort V, Bogart BI (2007). Elsevier’s integrated anatomy and embryology. Philadelphia, Pa.: Elsevier Saunders. ISBN 978-1-4160-3165-9.
- Standring S, Borley NR, et al., eds. (2008). Gray’s anatomy : the anatomical basis of clinical practice (40th ed.). London: Churchill Livingstone. ISBN 978-0-8089-2371-8.
External links[edit]
Wikimedia Commons has media related to Thyroid.
- Endocrine Web: Thyroid
| Thyroid | |
|---|---|

The human thyroid (tan), as viewed from the front; and arteries (red) supplying the gland. |
|

The thyroid is located in the neck, below the Adam’s apple. |
|
| Details | |
| Pronunciation | |
| Precursor | Thyroid diverticulum (an extension of endoderm into 2nd pharyngeal arch) |
| System | Endocrine system |
| Artery | Superior, Inferior thyroid arteries |
| Vein | Superior, middle, Inferior thyroid veins |
| Identifiers | |
| Latin | Glandula thyreoidea |
| MeSH | D013961 |
| TA98 | A11.3.00.001 |
| TA2 | 3863 |
| FMA | 9603 |
| Anatomical terminology
[edit on Wikidata] |
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The thyroid is located at the front of the neck, below the Adam’s apple. Microscopically, the functional unit of the thyroid gland is the spherical thyroid follicle, lined with follicular cells (thyrocytes), and occasional parafollicular cells that surround a lumen containing colloid. The thyroid gland secretes three hormones: the two thyroid hormones – triiodothyronine (T3) and thyroxine (T4) – and a peptide hormone, calcitonin. The thyroid hormones influence the metabolic rate and protein synthesis, and in children, growth and development. Calcitonin plays a role in calcium homeostasis.[1] Secretion of the two thyroid hormones is regulated by thyroid-stimulating hormone (TSH), which is secreted from the anterior pituitary gland. TSH is regulated by thyrotropin-releasing hormone (TRH), which is produced by the hypothalamus.[2]
The thyroid gland develops in the floor of the pharynx at the base of the tongue at 3–4 weeks gestation; it then descends in front of the pharyngeal gut, and ultimately over the next few weeks, it migrates to the base of the neck. During migration, the thyroid remains connected to the tongue by a narrow canal, the thyroglossal duct. At the end of the fifth week the thyroglossal duct degenerates, and over the following two weeks the detached thyroid migrates to its final position.
Euthyroid is the term used to describe a state of normal thyroid function in the body. Thyroid disorders include hyperthyroidism, hypothyroidism, thyroid inflammation (thyroiditis), thyroid enlargement (goitre), thyroid nodules, and thyroid cancer. Hyperthyroidism is characterized by excessive secretion of thyroid hormones: the most common cause is the autoimmune disorder Graves’ disease. Hypothyroidism is characterized by a deficient secretion of thyroid hormones: the most common cause is iodine deficiency. In iodine-deficient regions, hypothyroidism secondary to iodine deficiency is the leading cause of preventable intellectual disability in children.[3] In iodine-sufficient regions, the most common cause of hypothyroidism is the autoimmune disorder Hashimoto’s thyroiditis.
The presence of the thyroid and its various diseases have been noted and treated for centuries, although the gland itself has only been described and named since the Renaissance.[4] Knowledge of the thyroid, its biochemistry, and its disorders developed throughout the late nineteenth and twentieth centuries. Many modern treatments and investigative modalities evolved throughout the mid-twentieth century, including refinement of surgical techniques for thyroid removal (thyroidectomy) for the treatment of goitre; the use of radioactive iodine and thiouracil for the treatment of Graves’ disease; and fine needle aspiration for diagnosis of thyroid nodules.[4]
Structure[edit]
Features[edit]
The thyroid gland surrounds the cricoid and tracheal cartilages and consists of two lobes. This image shows a variant thyroid with a pyramidal lobe emerging from the middle of the thyroid.
The thyroid gland is a butterfly-shaped organ composed of two lobes, left and right, connected by a narrow tissue band, called an «isthmus».[5] It weighs 25 grams in adults, with each lobe being about 5 cm long, 3 cm wide, and 2 cm thick and the isthmus about 1.25 cm in height and width.[5] The gland is usually larger in women than in men, and increases in size during pregnancy.[5][6]
The thyroid is near the front of the neck, lying against and around the front of the larynx and trachea.[5] The thyroid cartilage and cricoid cartilage lie just above the gland, below the Adam’s apple. The isthmus extends from the second to third rings of the trachea, with the uppermost part of the lobes extending to the thyroid cartilage and the lowermost around the fourth to sixth tracheal rings.[7] The infrahyoid muscles lie in front of the gland and the sternocleidomastoid muscle to the side.[8] Behind the outer wings of the thyroid lie the two carotid arteries. The trachea, larynx, lower pharynx and esophagus all lie behind the thyroid.[6] In this region, the recurrent laryngeal nerve[9] and the inferior thyroid artery pass next to or in the ligament.[10] Typically, four parathyroid glands, two on each side, lie on each side between the two layers of the thyroid capsule, at the back of the thyroid lobes.[5]
The thyroid gland is covered by a thin fibrous capsule,[5] which has an inner and an outer layer. The inner layer extrudes into the gland and forms the septa that divide the thyroid tissue into microscopic lobules.[5] The outer layer is continuous with the pretracheal fascia, attaching the gland to the cricoid and thyroid cartilages[6] via a thickening of the fascia to form the posterior suspensory ligament of thyroid gland, also known as Berry’s ligament.[6] This causes the thyroid to move up and down with the movement of these cartilages when swallowing occurs.[6]
Blood, lymph and nerve supply[edit]
The thyroid is supplied with arterial blood from the superior thyroid artery, a branch of the external carotid artery, and the inferior thyroid artery, a branch of the thyrocervical trunk, and sometimes by an anatomical variant the thyroid ima artery,[5] which has a variable origin.[11] The superior thyroid artery splits into anterior and posterior branches supplying the thyroid, and the inferior thyroid artery splits into superior and inferior branches.[5] The superior and inferior thyroid arteries join behind the outer part of the thyroid lobes.[11] The venous blood is drained via superior and middle thyroid veins, which drain to the internal jugular vein, and via the inferior thyroid veins. The inferior thyroid veins originate in a network of veins and drain into the left and right brachiocephalic veins.[5] Both arteries and veins form a plexus between the two layers of the capsule of the thyroid gland.[11]
Lymphatic drainage frequently passes the prelaryngeal lymph nodes (located just above the isthmus) and the pretracheal and paratracheal lymph nodes.[5] The gland receives sympathetic nerve supply from the superior, middle and inferior cervical ganglion of the sympathetic trunk.[5] The gland receives parasympathetic nerve supply from the superior laryngeal nerve and the recurrent laryngeal nerve.[5]
Variation[edit]
Clear pyramidal lobe (center) as viewed from the front
There are many variants in the size and shape of the thyroid gland, and in the position of the embedded parathyroid glands.[6]
Sometimes there is a third lobe present called the pyramidal lobe.[6] When present, this lobe often stretches up to the hyoid bone from the thyroid isthmus and may be one to several divided lobes.[5] The presence of this lobe ranges in reported studies from 18.3%[12] to 44.6%.[13] It was shown to more often arise from the left side and occasionally separated.[12] The pyramidal lobe is also known as Lalouette’s pyramid.[14] The pyramidal lobe is a remnant of the thyroglossal duct, which usually wastes away during the thyroid gland’s descent.[6] Small accessory thyroid glands may in fact occur anywhere along the thyroglossal duct, from the foramen cecum of the tongue to the position of the thyroid in the adult.[5] A small horn at the back of the thyroid lobes, usually close to the recurrent laryngeal nerve and the inferior thyroid artery, is called Zuckerkandl’s tubercle.[10]
Other variants include a levator muscle of thyroid gland, connecting the isthmus to the body of the hyoid bone,[6] and the presence of the small thyroid ima artery.[6]
Microanatomy[edit]
Section of a thyroid gland under the microscope. 1 colloid, 2 follicular cells, 3 endothelial cells
At the microscopic level, there are three primary features of the thyroid—thyroid follicles, thyroid follicular cells, and parafollicular cells, first discovered by Geoffery Websterson in 1664.[15]
- Follicles
Thyroid follicles are small spherical groupings of cells 0.02–0.9mm in diameter that play the main role in thyroid function.[5] They consist of a rim that has a rich blood supply, nerve and lymphatic presence, that surrounds a core of colloid that consists mostly of thyroid hormone precursor proteins called thyroglobulin, an iodinated glycoprotein.[5][16]
- Follicular cells
The core of a follicle is surrounded by a single layer of follicular cells. When stimulated by thyroid stimulating hormone (TSH), these secrete the thyroid hormones T3 and T4. They do this by transporting and metabolising the thyroglobulin contained in the colloid.[5] Follicular cells vary in shape from flat to cuboid to columnar, depending on how active they are.[5][16]
- Follicular lumen
The follicular lumen is the fluid-filled space within a follicle of the thyroid gland. There are hundreds of follicles within the thyroid gland. A follicle is formed by a spherical arrangement of follicular cells. The follicular lumen is filled with colloid, a concentrated solution of thyroglobulin and is the site of synthesis of the thyroid hormones thyroxine (T4) and triiodothyronine (T3).[17]
- Parafollicular cells
Scattered among follicular cells and in spaces between the spherical follicles are another type of thyroid cell, parafollicular cells.[5] These cells secrete calcitonin and so are also called C cells.[18]
Development[edit]
Floor of pharynx of embryo between 35 and 37 days after fertilization.
In the development of the embryo, at 3–4 weeks gestational age, the thyroid gland appears as an epithelial proliferation in the floor of the pharynx at the base of the tongue between the tuberculum impar and the copula linguae. The copula soon becomes covered over by the hypopharyngeal eminence[19] at a point later indicated by the foramen cecum. The thyroid then descends in front of the pharyngeal gut as a bilobed diverticulum through the thyroglossal duct. Over the next few weeks, it migrates to the base of the neck, passing in front of the hyoid bone. During migration, the thyroid remains connected to the tongue by a narrow canal, the thyroglossal duct. At the end of the fifth week the thyroglossal duct degenerates, and over the following two weeks the detached thyroid migrates to its final position.[19]
The fetal hypothalamus and pituitary start to secrete thyrotropin-releasing hormone (TRH) and thyroid-stimulating hormone (TSH). TSH is first measurable at 11 weeks.[20] By 18–20 weeks, the production of thyroxine (T4) reaches a clinically significant and self-sufficient level.[20][21] Fetal triiodothyronine (T3) remains low, less than 15 ng/dL until 30 weeks, and increases to 50 ng/dL at full-term.[21] The fetus needs to be self-sufficient in thyroid hormones in order to guard against neurodevelopmental disorders that would arise from maternal hypothyroidism.[22] The presence of sufficient iodine is essential for healthy neurodevelopment.[23]
The neuroendocrine parafollicular cells, also known as C cells, responsible for the production of calcitonin, are derived from foregut endoderm. This part of the thyroid then first forms as the ultimopharyngeal body, which begins in the ventral fourth pharyngeal pouch and joins the primordial thyroid gland during its descent to its final location.[24]
Aberrations in prenatal development can result in various forms of thyroid dysgenesis which can cause congenital hypothyroidism, and if untreated this can lead to cretinism.[20]
Function[edit]
The thyroid hormones T3 and T4 have a number of metabolic, cardiovascular and developmental effects on the body. The production is stimulated by release of thyroid stimulating hormone (TSH), which in turn depends on release of thyrotropin releasing hormone (TRH). Every downstream hormone has negative feedback and decreases the level of the hormone that stimulates its release.
Thyroid hormones[edit]
The primary function of the thyroid is the production of the iodine-containing thyroid hormones, triiodothyronine (T3) and thyroxine or tetraiodothyronine (T4) and the peptide hormone calcitonin.[25] The thyroid hormones are created from iodine and tyrosine. T3 is so named because it contains three atoms of iodine per molecule and T4 contains four atoms of iodine per molecule.[26] The thyroid hormones have a wide range of effects on the human body. These include:
- Metabolic. The thyroid hormones increase the basal metabolic rate and have effects on almost all body tissues.[27] Appetite, the absorption of substances, and gut motility are all influenced by thyroid hormones.[28] They increase the absorption in the gut, generation, uptake by cells, and breakdown of glucose.[29] They stimulate the breakdown of fats, and increase the number of free fatty acids.[29] Despite increasing free fatty acids, thyroid hormones decrease cholesterol levels, perhaps by increasing the rate of secretion of cholesterol in bile.[29]
- Cardiovascular. The hormones increase the rate and strength of the heartbeat. They increase the rate of breathing, intake and consumption of oxygen, and increase the activity of mitochondria.[28] Combined, these factors increase blood flow and the body’s temperature.[28]
- Developmental. Thyroid hormones are important for normal development.[29] They increase the growth rate of young people,[30] and cells of the developing brain are a major target for the thyroid hormones T3 and T4. Thyroid hormones play a particularly crucial role in brain maturation during fetal development and first few years of postnatal life[29]
- The thyroid hormones also play a role in maintaining normal sexual function, sleep, and thought patterns. Increased levels are associated with increased speed of thought generation but decreased focus.[28] Sexual function, including libido and the maintenance of a normal menstrual cycle, are influenced by thyroid hormones.[28]
After secretion, only a very small proportion of the thyroid hormones travel freely in the blood. Most are bound to thyroxine-binding globulin (about 70%), transthyretin (10%), and albumin (15%).[31] Only the 0.03% of T4 and 0.3% of T3 traveling freely have hormonal activity.[32] In addition, up to 85% of the T3 in blood is produced following conversion from T4 by iodothyronine deiodinases in organs around the body.[25]
Thyroid hormones act by crossing the cell membrane and binding to intracellular nuclear thyroid hormone receptors TR-α1, TR-α2, TR-β1, and TR-β2, which bind with hormone response elements and transcription factors to modulate DNA transcription.[32][33] In addition to these actions on DNA, the thyroid hormones also act within the cell membrane or within cytoplasm via reactions with enzymes, including calcium ATPase, adenylyl cyclase, and glucose transporters.[20]
Hormone production[edit]
The thyroid hormones are created from thyroglobulin. This is a protein within the colloid in the follicular lumen that is originally created within the rough endoplasmic reticulum of follicular cells and then transported into the follicular lumen. Thyroglobulin contains 123 units of tyrosine, which reacts with iodine within the follicular lumen.[35]
Iodine is essential for the production of the thyroid hormones. Iodine (I0) travels in the blood as iodide (I−), which is taken up into the follicular cells by a sodium-iodide symporter. This is an ion channel on the cell membrane which in the same action transports two sodium ions and an iodide ion into the cell.[36] Iodide then travels from within the cell into the lumen, through the action of pendrin, an iodide-chloride antiporter. In the follicular lumen, the iodide is then oxidized to iodine. This makes it more reactive,[34] and the iodine is attached to the active tyrosine units in thyroglobulin by the enzyme thyroid peroxidase. This forms the precursors of thyroid hormones monoiodotyrosine (MIT), and diiodotyrosine (DIT).[2]
When the follicular cells are stimulated by thyroid-stimulating hormone, the follicular cells reabsorb thyroglobulin from the follicular lumen. The iodinated tyrosines are cleaved, forming the thyroid hormones T4, T3, DIT, MIT, and traces of reverse triiodothyronine. T3 and T4 are released into the blood. The hormones secreted from the gland are about 80–90% T4 and about 10–20% T3.[37][38] Deiodinase enzymes in peripheral tissues remove the iodine from MIT and DIT and convert T4 to T3 and RT3. [35] This is a major source of both RT3 (95%) and T3 (87%) in peripheral tissues.[39]
Regulation[edit]
The production of thyroxine and triiodothyronine is primarily regulated by thyroid-stimulating hormone (TSH), released by the anterior pituitary gland. TSH release in turn is stimulated by thyrotropin releasing hormone (TRH), released in a pulsatile manner from the hypothalamus.[40] The thyroid hormones provide negative feedback to the thyrotropes TSH and TRH: when the thyroid hormones are high, TSH production is suppressed. This negative feedback also occurs when levels of TSH are high, causing TRH production to be suppressed.[41]
TRH is secreted at an increased rate in situations such as cold exposure in order to stimulate thermogenesis.[42] In addition to being suppressed by the presence of thyroid hormones, TSH production is blunted by dopamine, somatostatin, and glucocorticoids.[43]
Calcitonin[edit]
The thyroid gland also produces the hormone calcitonin, which helps regulate blood calcium levels. Parafollicular cells produce calcitonin in response to high blood calcium. Calcitonin decreases the release of calcium from bone, by decreasing the activity of osteoclasts, cells which break down bone. Bone is constantly reabsorbed by osteoclasts and created by osteoblasts, so calcitonin effectively stimulates movement of calcium into bone. The effects of calcitonin are opposite those of the parathyroid hormone (PTH) produced in the parathyroid glands. However, calcitonin seems far less essential than PTH, since calcium metabolism remains clinically normal after removal of the thyroid (thyroidectomy), but not the parathyroid glands.[44]
Gene and protein expression[edit]
About 20,000 protein coding genes are expressed in human cells: 70% of these genes are expressed in thyroid cells.[45][46] Two-hundred fifty of these genes are more specifically expressed in the thyroid, and about 20 genes are highly thyroid specific. In the follicular cells, the proteins synthesized by these genes direct thyroid hormone synthesis—thyroglobulin, TPO, and IYD; while in the parafollicular c-cells, they direct calcitonin synthesis—CALCA, and CALCB.
Clinical significance[edit]
General practitioners, and internal medicine specialists play a role in identifying and monitoring the treatment of thyroid disease. Endocrinologists and thyroidologists are thyroid specialists. Thyroid surgeons or otolaryngologists are responsible for the surgical management of thyroid disease.
Functional disorders[edit]
Hyperthyroidism[edit]
Excessive production of the thyroid hormones is called hyperthyroidism. Causes include Graves’ disease, toxic multinodular goitre, solitary thyroid adenoma, inflammation, and a pituitary adenoma which secretes excess TSH. Another cause is excess iodine availability, either from excess ingestion, induced by the drug amiodarone, or following iodinated contrast imaging.[47][48]
Hyperthyroidism often causes a variety of non-specific symptoms including weight loss, increased appetite, insomnia, decreased tolerance of heat, tremor, palpitations, anxiety and nervousness. In some cases it can cause chest pain, diarrhoea, hair loss and muscle weakness.[49] Such symptoms may be managed temporarily with drugs such as beta blockers.[50]
Long-term management of hyperthyroidism may include drugs that suppress thyroid function such as propylthiouracil, carbimazole and methimazole.[51] Alternatively, radioactive iodine-131 can be used to destroy thyroid tissue: radioactive iodine is selectively taken up by thyroid cells, which over time destroys them. The chosen first-line treatment will depend on the individual and on the country where being treated. Surgery to remove the thyroid can sometimes be performed as a transoral thyroidectomy, a minimally invasive procedure.[52] Surgery does however carry a risk of damage to the parathyroid glands and the recurrent laryngeal nerve, which innervates the vocal cords. If the entire thyroid gland is removed, hypothyroidism will inevitably result, and thyroid hormone substitutes will be needed.[53][50]
Hypothyroidism[edit]
An underactive thyroid gland results in hypothyroidism. Typical symptoms are abnormal weight gain, tiredness, constipation, heavy menstrual bleeding, hair loss, cold intolerance, and a slow heart rate.[49] Iodine deficiency is the most common cause of hypothyroidism worldwide,[54] and the autoimmune disease Hashimoto’s thyroiditis is the most common cause in the developed world.[55] Other causes include congenital abnormalities, diseases causing transient inflammation, surgical removal or radioablation of the thyroid, the drugs amiodarone and lithium, amyloidosis, and sarcoidosis.[56] Some forms of hypothyroidism can result in myxedema and severe cases can result in myxedema coma.[57]
Hypothyroidism is managed with replacement of the thyroid hormones. This is usually given daily as an oral supplement, and may take a few weeks to become effective.[57] Some causes of hypothyroidism, such as Postpartum thyroiditis and Subacute thyroiditis may be transient and pass over time, and other causes such as iodine deficiency may be able to be rectified with dietary supplementation.[58]
Diseases[edit]
Graves’ disease[edit]
Graves’ disease is an autoimmune disorder that is the most common cause of hyperthyroidism.[59] In Graves’ disease, for an unknown reason autoantibodies develop against the thyroid stimulating hormone receptor. These antibodies activate the receptor, leading to development of a goitre and symptoms of hyperthyroidism, such as heat intolerance, weight loss, diarrhoea and palpitations. Occasionally such antibodies block but do not activate the receptor, leading to symptoms associated with hypothyroidism.[59] In addition, gradual protrusion of the eyes may occur, called Graves’ ophthalmopathy, as may swelling of the front of the shins.[59] Graves’ disease can be diagnosed by the presence of pathomnomonic features such as involvement of the eyes and shins, or isolation of autoantibodies, or by results of a radiolabelled uptake scan. Graves’ disease is treated with anti-thyroid drugs such as propylthiouracil, which decrease the production of thyroid hormones, but hold a high rate of relapse. If there is no involvement of the eyes, then use of radioactive isotopes to ablate the gland may be considered. Surgical removal of the gland with subsequent thyroid hormone replacement may be considered, however this will not control symptoms associated with the eye or skin.[59]
Nodules[edit]
Thyroid nodules are often found on the gland, with a prevalence of 4–7%.[60] The majority of nodules do not cause any symptoms, thyroid hormone secretion is normal, and they are non-cancerous.[61] Non-cancerous cases include simple cysts, colloid nodules, and thyroid adenomas. Malignant nodules, which only occur in about 5% of nodules, include follicular, papillary, medullary carcinomas and metastasis from other sites [62] Nodules are more likely in females, those who are exposed to radiation, and in those who are iodine deficient.[60]
When a nodule is present, thyroid function tests determine whether the nodule is secreting excess thyroid hormones, causing hyperthyroidism.[61] When the thyroid function tests are normal, an ultrasound is often used to investigate the nodule, and provide information such as whether the nodule is fluid-filled or a solid mass, and whether the appearance is suggestive of a benign or malignant cancer.[60] A needle aspiration biopsy may then be performed, and the sample undergoes cytology, in which the appearance of cells is viewed to determine whether they resemble normal or cancerous cells.[62]
The presence of multiple nodules is called a multinodular goitre; and if it is associated with hyperthyroidism, it is called a toxic multinodular goitre.[62]
Goitre[edit]
An enlarged thyroid gland is called a goitre.[63] Goitres are present in some form in about 5% of people,[62] and are the result of a large number of causes, including iodine deficiency, autoimmune disease (both Graves’ disease and Hashimoto’s thyroiditis), infection, inflammation, and infiltrative disease such as sarcoidosis and amyloidosis. Sometimes no cause can be found, a state called «simple goitre».[64]
Some forms of goitre are associated with pain, whereas many do not cause any symptoms. Enlarged goitres may extend beyond the normal position of the thyroid gland to below the sternum, around the airway or esophagus.[62] Goitres may be associated with hyperthyroidism or hypothyroidism, relating to the underlying cause of the goitre.[62] Thyroid function tests may be done to investigate the cause and effects of the goitre. The underlying cause of the goitre may be treated, however many goitres with no associated symptoms are simply monitored.[62]
Inflammation[edit]
Inflammation of the thyroid is called thyroiditis, and may cause symptoms of hyperthyroidism or hypothyroidism. Two types of thyroiditis initially present with hyperthyroidism and are sometimes followed by a period of hypothyroidism – Hashimoto’s thyroiditis and postpartum thyroiditis. There are other disorders that cause inflammation of the thyroid, and these include subacute thyroiditis, acute thyroiditis, silent thyroiditis, Riedel’s thyroiditis and traumatic injury, including palpation thyroiditis.[65]
Hashimoto’s thyroiditis is an autoimmune disorder in which the thyroid gland is infiltrated by the lymphocytes B cell and T cells. These progressively destroy the thyroid gland.[66] In this way, Hasimoto’s thyroiditis may have occurred insidiously, and only be noticed when thyroid hormone production decreases, causing symptoms of hypothyroidism.[66] Hashimoto’s is more common in females than males, much more common after the age of 60, and has known genetic risk factors.[66] Also more common in individuals with Hashimoto’s thyroiditis are Type 1 diabetes, pernicious anaemia, Addison’s disease vitiligo.[66]
Postpartum thyroiditis occurs in some females following childbirth. After delivery, the gland becomes inflamed and the condition initially presents with a period of hyperthyroidism followed by hypothyroidism and, usually, a return to normal function. [67] The course of the illness takes place over several months, and is characterised by a painless goitre. Antibodies against thyroid peroxidase can be found on testing. The inflammation usually resolves without treatment, although thyroid hormone replacement may be needed during the period of hypothyroidism.[67]
Cancer[edit]
The most common neoplasm affecting the thyroid gland is a benign adenoma, usually presenting as a painless mass in the neck.[68] Malignant thyroid cancers are most often carcinomas, although cancer can occur in any tissue that the thyroid consists of, including cancer of C-cells and lymphomas. Cancers from other sites also rarely lodge in the thyroid.[68] Radiation of the head and neck presents a risk factor for thyroid cancer, and cancer is more common in women than men, occurring at a rate of about 2:1.[68]
In most cases, thyroid cancer presents as a painless mass in the neck. It is very unusual for thyroid cancers to present with other symptoms, although in some cases cancer may cause hyperthyroidism.[69] Most malignant thyroid cancers are papillary, followed by follicular, medullary, and thyroid lymphoma.[68][69] Because of the prominence of the thyroid gland, cancer is often detected earlier in the course of disease as the cause of a nodule, which may undergo fine-needle aspiration. Thyroid function tests will help reveal whether the nodule produces excess thyroid hormones. A radioactive iodine uptake test can help reveal the activity and location of the cancer and metastases.[68][70]
Thyroid cancers are treated by removing the whole or part of thyroid gland. Radioactive Iodine-131 may be given to radioablate the thyroid. Thyroxine is given to replace the hormones lost and to suppress TSH production, as TSH may stimulate recurrence.[70] With the exception of the rare anaplastic thyroid cancer, which carries a very poor prognosis, most thyroid cancers carry an excellent prognosis and can even be considered curable.[71]
Congenital[edit]
A persistent thyroglossal duct is the most common clinically significant birth defect of the thyroid gland. A persistent sinus tract may remain as a vestigial remnant of the tubular development of the thyroid gland. Parts of this tube may be obliterated, leaving small segments to form thyroglossal cysts.[24] Preterm neonates are at risk of hypothyroidism as their thyroid glands are insufficiently developed to meet their postnatal needs.[72] In order to detect hypothyroidism in newborn babies, to prevent growth and development abnormalities in later life, many countries have newborn screening programs at birth.[73]
Infants with thyroid hormone deficiency (congenital hypothyroidism) can manifest problems of physical growth and development as well as brain development, termed cretinism.[74][23] Children with congenital hypothyroidism are treated supplementally with levothyroxine, which facilitates normal growth and development.[75]
Mucinous, clear secretions may collect within these cysts to form either spherical masses or fusiform swellings, rarely larger than 2 to 3 cm in diameter. These are present in the midline of the neck anterior to the trachea. Segments of the duct and cysts that occur high in the neck are lined by stratified squamous epithelium, which is essentially identical to that covering the posterior portion of the tongue in the region of the foramen cecum. The disorders that occur in the lower neck more proximal to the thyroid gland are lined by epithelium resembling the thyroidal acinar epithelium. Characteristically, next to the lining epithelium, there is an intense lymphocytic infiltrate. Superimposed infection may convert these lesions into abscess cavities, and rarely, give rise to cancers.[citation needed]
Another disorder is that of thyroid dysgenesis which can result in various presentations of one or more misplaced accessory thyroid glands.[5] These can be asymptomatic.
Iodine[edit]
Iodine deficiency, most common in inland and mountainous areas, can predispose to goitre – if widespread, known as endemic goitre.[74] Pregnant women deficient of iodine can give birth to infants with thyroid hormone deficiency.[74][23] The use of iodised salt to add iodine to the diet[23] has eliminated endemic cretinism in most developed countries,[77] and over 120 countries have made the iodination of salt mandatory.[78][79]
Because the thyroid concentrates iodine, it also concentrates the various radioactive isotopes of iodine produced by nuclear fission. In the event of large accidental releases of such material into the environment, the uptake of radioactive iodine isotopes by the thyroid can, in theory, be blocked by saturating the uptake mechanism with a large surplus of non-radioactive iodine, taken in the form of potassium iodide tablets. One consequence of the Chernobyl disaster was an increase in thyroid cancers in children in the years following the accident.[80]
Excessive iodine intake is uncommon and usually has no effect on the thyroid function. Sometimes though it may cause hyperthyroidism, and sometimes hypothyroidism with a resulting goitre.[81]
Evaluation[edit]
The thyroid is examined by observation of the gland and surrounding neck for swelling or enlargement.[82] It is then felt, usually from behind, and a person is often asked to swallow to better feel the gland against the fingers of the examiner.[82] The gland moves up and down with swallowing because of its attachments to the thyroid and cricoid cartilages.[6] In a healthy person the gland is not visible yet is palpable as a soft mass. Examination of the thyroid gland includes the search for abnormal masses and the assessment of overall thyroid size.[83] The character of the thyroid, swellings, nodules, and their consistency may all be able to be felt. If a goitre is present, an examiner may also feel down the neck consider tapping the upper part of the chest to check for extension. Further tests may include raising the arms (Pemberton’s sign), listening to the gland with a stethoscope for bruits, testing of reflexes, and palpation of the lymph nodes in the head and neck.
An examination of the thyroid will also include observation of the person as a whole, to look for systemic signs such as weight gain or loss, hair loss, and signs in other locations – such as protrusion of the eyes or swelling of the calves in Graves’ disease.[84][82]
Tests[edit]
Thyroid function tests include a battery of blood tests, including the measurement of the thyroid hormones, as well as the measurement of thyroid stimulating hormone (TSH).[85] They may reveal hyperthyroidism (high T3 and T4), hypothyroidism (low T3, T4), or subclinical hyperthyroidism (normal T3 and T4 with a low TSH).[85]
TSH levels are considered the most sensitive marker of thyroid dysfunction.[85] They are however not always accurate, particularly if the cause of hypothyroidism is thought to be related to insufficient thyrotropin releasing hormone (TRH) secretion, in which case it may be low or falsely normal. In such a case a TRH stimulation test, in which TRH is given and TSH levels are measured at 30 and 60-minutes after, may be conducted.[85]
T3 and T4 can be measured directly. However, as the two thyroid hormones travel bound to other molecules, and it is the «free» component that is biologically active, free T3 and free T4 levels can be measured.[85] T3 is preferred, because in hypothyroidism T3 levels may be normal.[85] The ratio of bound to unbound thyroid hormones is known as the thyroid hormone binding ratio (THBR).[86] It is also possible to measure directly the main carriers of the thyroid hormones, thryoglobulin and throxine-binding globulin.[87] Thyroglobulin will also be measurable in a healthy thyroid, and will increase with inflammation, and may also be used to measure the success of thyroid removal or ablation. If successful, thyroglobulin should be undetectable.[86] Lastly, antibodies against components of the thyroid, particularly anti-TPO and anti-thyroglobulin, can be measured. These may be present in normal individuals but are highly sensitive for autoimmune-related disease.[86]
Imaging[edit]
Ultrasound of the thyroid may be used to reveal whether structures are solid or filled with fluid, helping to differentiate between nodules and goitres and cysts. It may also help differentiate between malignant and benign lesions.[88]
When further imaging is required, a radiolabelled iodine-123 or technetium-99 uptake scan may take place. This can determine the size and shape of lesions, reveal whether nodules or goitres are metabolically active, and reveal and monitor sites of thyroid disease or cancer deposits outside the thyroid.[89]
A fine needle aspiration of a sample of thyroid tissue may be taken in order to evaluate a lesion seen on ultrasound which is then sent for histopathology and cytology.[90]
Computed tomography of the thyroid plays an important role in the evaluation of thyroid cancer.[91] CT scans often incidentally find thyroid abnormalities, and thereby practically becomes the first investigation modality.[91]
History[edit]
The thyroid gland received its modern name in the 1600s, when the anatomist Thomas Wharton likened its shape to that of an Ancient Greek shield or thyos. However, the existence of the gland, and of the diseases associated with it, was known long before then.
Antiquity[edit]
The presence and diseases of the thyroid have been noted and treated for thousands of years.[4] In 1600 BCE burnt sponge and seaweed (which contain iodine) were used within China for the treatment of goitres, a practice which has developed in many parts of the world.[4][92] In Ayurvedic medicine, the book Sushruta Samhita written about 1400 BCE described hyperthyroidism, hypothyroidism and goitre.[92] Aristotle and Xenophon in the fifth century BCE describe cases of diffuse toxic goitre.[92] Hippocrates and Plato in the fourth century BCE provided some of the first descriptions of the gland itself, proposing its function as a salivary gland.[92] Pliny the Elder in the first century BCE referred to epidemics of goitre in the Alps and proposed treatment with burnt seaweed,[4] a practice also referred to by Galen in the second century, referred to burnt sponge for the treatment of goitre.[4] The Chinese pharmacology text Shennong Ben Cao Jing, written ca. 200–250, also refers to goitre.[4][92]
Scientific era[edit]
In 1500 polymath Leonardo da Vinci provided the first illustration of the thyroid.[4] In 1543 anatomist Andreas Vesalius gave the first anatomic description and illustration of the gland.[4] In 1656 the thyroid received its modern name, by the anatomist Thomas Wharton.[4] The gland was named thyroid, meaning shield, as its shape resembled the shields commonly used in Ancient Greece.[4] The English name thyroid gland[93] is derived from the medical Latin used by Wharton – glandula thyreoidea.[94] Glandula means ‘gland’ in Latin,[95] and thyreoidea can be traced back to the Ancient Greek word θυρεοειδής, meaning ‘shield-like/shield-shaped’.[96]
French chemist Bernard Courtois discovered iodine in 1811,[92] and in 1896 Eugen Baumann documented it as the central ingredient in the thyroid gland. He did this by boiling the thyroid glands of a thousand sheep, and named the precipitate, a combination of the thyroid hormones, ‘iodothyrin’.[92] David Marine in 1907 proved that iodine is necessary for thyroid function.[92][4]
Graves’ disease was described by Robert James Graves in 1834. The role of the thyroid gland in metabolism was demonstrated in 1895 by Adolf Magnus-Levy.[97] Thyroxine was first isolated in 1914 and synthesized in 1927, and triiodothyroxine in 1952.[92][98] The conversion of T4 to T3 was discovered in 1970.[4] The process of discovering TSH took place over the early to mid twentieth century.[99] TRH was discovered by Polish endocrinologist Andrew Schally in 1970, contributing in part to his Nobel Prize in Medicine in 1977.[4][100]
In the nineteenth century numerous authors described both cretinism and myxedema, and their relationship to the thyroid.[92] Charles Mayo coined the term hyperthyroidism in 1910.[4] Hakaru Hashimoto documented a case of Hashimoto’s thyroiditis in 1912, antibodies in this disease were demonstrated in 1956.[92] Knowledge of the thyroid and its conditions developed throughout the late nineteenth and twentieth centuries, with many modern treatments and investigative modalities evolving throughout the mid twentieth century, including the use of radioactive iodine, thiouracil and fine needle aspiration.[4]
Surgery[edit]
Either Aetius in the sixth century CE[92] or Persian Ali ibn Abbas al-Magusi in 990 CE conducted the first recorded thyroidectomy as a treatment for goitre.[4][101] Operations remained risky and generally were not successful until the 19th century, when descriptions emerged from a number of authors including Prussian surgeon Theodor Billroth, Swiss surgeon and physiologist Theodor Kocher, American physician Charles Mayo, American surgeons William Halsted and George Crile. These descriptions provided the basis for modern thyroid surgery.[102] Theodor Kocher went on to win the Nobel Prize in Physiology or Medicine in 1909 «for his work on the physiology, pathology and surgery of the thyroid gland».[103]
Other animals[edit]
The thyroid gland is found in all vertebrates. In fish, it is usually located below the gills and is not always divided into distinct lobes. However, in some teleosts, patches of thyroid tissue are found elsewhere in the body, associated with the kidneys, spleen, heart, or eyes.[104]
In tetrapods, the thyroid is always found somewhere in the neck region. In most tetrapod species, there are two paired thyroid glands – that is, the right and left lobes are not joined. However, there is only ever a single thyroid gland in most mammals, and the shape found in humans is common to many other species.[104]
In larval lampreys, the thyroid originates as an exocrine gland, secreting its hormones into the gut, and associated with the larva’s filter-feeding apparatus. In the adult lamprey, the gland separates from the gut, and becomes endocrine, but this path of development may reflect the evolutionary origin of the thyroid. For instance, the closest living relatives of vertebrates, the tunicates and amphioxi (lancelets), have a structure very similar to that of larval lampreys (the endostyle), and this also secretes iodine-containing compounds, though not thyroxine.[104]
Thyroxine is critical to metabolic regulation, and growth throughout the vertebrate clade. Iodine and T4 trigger the change from a plant-eating water-dwelling tadpole into a meat-eating land-dwelling frog, with better neurological, visuospatial, smell and cognitive abilities for hunting, as seen in other predatory animals. A similar phenomenon happens in the neotenic amphibian salamanders, which, without introducing iodine, don’t transform into land-dwelling adults, and live and reproduce in the larval form of aquatic axolotl. Among amphibians, administering a thyroid-blocking agent such as propylthiouracil (PTU) can prevent tadpoles from metamorphosing into frogs; in contrast, administering thyroxine will trigger metamorphosis. In amphibian metamorphosis, thyroxine and iodine also exert a well-studied experimental model of apoptosis on the cells of gills, tail, and fins of tadpoles. Iodine, via iodolipids, has favored the evolution of terrestrial animal species and has likely played a crucial role in the evolution of the human brain.[105][106]
See also[edit]
- Desiccated thyroid
- Thyroid disease in pregnancy
References[edit]
- ^ Guyton & Hall 2011, p. 907.
- ^ a b Boron WF, Boulpaep EL (2012). Medical Physiology (2nd ed.). Philadelphia: Saunders. p. 1052. ISBN 978-1-4377-1753-2.
- ^ Harrison’s 2011, pp. 2913, 2918.
- ^ a b c d e f g h i j k l m n o p q «Thyroid History Timeline – American Thyroid Association». www.thyroid.org. Retrieved 13 November 2016.
- ^ a b c d e f g h i j k l m n o p q r s t u Gray’s Anatomy 2008, pp. 462–4.
- ^ a b c d e f g h i j k Elsevier’s 2007, p. 342.
- ^ Elsevier’s 2007, pp. 342–3.
- ^ Ellis H, Standring S, Gray HD (2005). Gray’s anatomy: the anatomical basis of clinical practice. St. Louis, Mo: Elsevier Churchill Livingstone. pp. 538–539. ISBN 978-0-443-07168-3.
- ^ Netter FH (2014). Atlas of Human Anatomy Including Student Consult Interactive Ancillaries and Guides (6th ed.). Philadelphia, Penn.: W B Saunders Co. p. 27. ISBN 978-1-4557-0418-7.
- ^ a b Page C, Cuvelier P, Biet A, Boute P, Laude M, Strunski V (July 2009). «Thyroid tubercle of Zuckerkandl: anatomical and surgical experience from 79 thyroidectomies». The Journal of Laryngology and Otology. 123 (7): 768–71. doi:10.1017/s0022215108004003. PMID 19000342. S2CID 22063700.
- ^ a b c Elsevier’s 2007, p. 343.
- ^ a b Cicekcibasi AE, Salbacak A, Seker M, Ziylan T, Tuncer I, Buyukmumcu M (April 2007). «Developmental variations and clinical importance of the fetal thyroid gland. A morphometric study». Saudi Medical Journal. 28 (4): 524–8. PMID 17457471.
- ^ Kim DW, Jung SL, Baek JH, Kim J, Ryu JH, Na DG, et al. (January 2013). «The prevalence and features of thyroid pyramidal lobe, accessory thyroid, and ectopic thyroid as assessed by computed tomography: a multicenter study». Thyroid. 23 (1): 84–91. doi:10.1089/thy.2012.0253. PMID 23031220.
- ^ Dorland WA (2012). Anderson DM (ed.). Dorland’sIllustrated Medical Dictionary (32nd ed.). Elsevier Saunders. pp. 999 redirect to 1562. ISBN 978-1-4160-6257-8.
- ^ Fawcett D, Jensh R (2002). Bloom & Fawcett’s Concise Histology. New York: Arnold Publishers. pp. 257–258. ISBN 978-0-340-80677-7.
- ^ a b Wheater PR, Young B (2006). Wheater’s functional histology : a text and colour atlas (5th ed.). Oxford: Churchill Livingstone. pp. 333–335. ISBN 978-0-443-06850-8.
- ^ The Thyroid Follicle Archived 2013-01-23 at archive.today, Endocrinology by J. Larry Jameson, MD, PhD and Leslie J. De Groot, MD, chapter 72
- ^ Hazard JB (July 1977). «The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A review». The American Journal of Pathology. 88 (1): 213–50. PMC 2032150. PMID 18012.
- ^ a b Larsen WJ (2001). Human embryology (3. ed.). Philadelphia, Pa.: Churchill Livingstone. pp. 372–374. ISBN 978-0-443-06583-5.
- ^ a b c d Greenspan’s 2011, p. 179.
- ^ a b Eugster EA, Pescovitz OH (2004). Pediatric endocrinology: mechanisms, manifestations and management. Hagerstwon, MD: Lippincott Williams & Wilkins. p. 493 (Table 33–3). ISBN 978-0-7817-4059-3.
- ^ Zoeller RT (April 2003). «Transplacental thyroxine and fetal brain development». The Journal of Clinical Investigation. 111 (7): 954–7. doi:10.1172/JCI18236. PMC 152596. PMID 12671044.
- ^ a b c d «Iodine supplementation in pregnant and lactating women». World Health Organization. Archived from the original on January 4, 2014. Retrieved 2016-11-13.
- ^ a b Langman J, Sadler TW, Sadler-Redmond SL, Tosney K, Byrne J, Imseis H (2015). Langman’s Medical Embryology (13th ed.). pp. 285–6, 293. ISBN 978-1-4511-9164-6.
- ^ a b Davidson’s 2010, p. 736.
- ^ Guyton & Hall 2011, p. 909.
- ^ Guyton & Hall 2011, p. 934.
- ^ a b c d e Guyton & Hall 2011, p. 937.
- ^ a b c d e Guyton & Hall 2011, p. 936.
- ^ Guyton & Hall 2011, p. 935-6.
- ^ Greenspan’s 2011, p. 169.
- ^ a b Bowen R (2000). «Thyroid Hormone Receptors». Colorado State University. Archived from the original on 27 September 2011. Retrieved 22 February 2015.
- ^ Greenspan’s 2011, p. 178.
- ^ a b Boron WF, Boulpaep E (2003). «Chapter 48: «synthesis of thyroid hormones»«. Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1300. ISBN 978-1-4160-2328-9.
- ^ a b Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (February 2002). «Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases». Endocrine Reviews. 23 (1): 38–89. doi:10.1210/edrv.23.1.0455. PMID 11844744.
- ^ Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (2011). Williams Textbook of Endocrinology (12th ed.). Saunders. p. 331. ISBN 978-1-4377-0324-5.
- ^ How Your Thyroid Works: A Delicate Feedback Mechanism. Updated 2009-05-21.
- ^ The thyroid gland in Endocrinology: An Integrated Approach by Stephen Nussey and Saffron Whitehead (2001) Published by BIOS Scientific Publishers Ltd. ISBN 1-85996-252-1
- ^ Ganong’s review of medical physiology Edition 25.
- ^ Greenspan’s 2011, p. 174.
- ^ Greenspan’s 2011, p. 177.
- ^ Guyton & Hall 2011, p. 896.
- ^ Harrison’s 2011, pp. 2215.
- ^ Guyton & Hall 2011, pp. 988–9.
- ^ «The human proteome in thyroid gland – The Human Protein Atlas». www.proteinatlas.org. Retrieved 2017-09-25.
- ^ Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. (January 2015). «Proteomics. Tissue-based map of the human proteome». Science. 347 (6220): 1260419. doi:10.1126/science.1260419. PMID 25613900. S2CID 802377.
- ^ Davidson’s 2010, p. 738.
- ^ Rusandu A, Sjøvold BH, Hofstad E, Reidunsdatter RJ (June 2020). «Iodinated contrast media and their effect on thyroid function — Routines and practices among diagnostic imaging departments in Norway». Journal of Medical Radiation Sciences. 67 (2): 111–118. doi:10.1002/jmrs.390. PMC 7276191. PMID 32232955.
- ^ a b Davidson’s 2010, p. 740.
- ^ a b Davidson’s 2010, p. 739.
- ^ Davidson’s 2010, p. 745.
- ^ Cury AN, Meira VT, Monte O, Marone M, Scalissi NM, Kochi C, et al. (March 2013). «Clinical experience with radioactive iodine in the treatment of childhood and adolescent Graves’ disease». Endocrine Connections. 2 (1): 32–7. doi:10.1530/EC-12-0049. PMC 3680965. PMID 23781316.
- ^ Thyroid Problems eMedicine Health. Retrieved on 2010-02-07
- ^ «Iodine Deficiency & Nutrition». www.thyroidfoundation.org.au. Australian Thyroid Foundation. Archived from the original on 13 January 2017. Retrieved 11 January 2017.
- ^ So M, MacIsaac R, Grossmann M. «Hypothyroidism – Investigation and management». www.racgp.org.au. The Royal Australian College of General Practitioners. Retrieved 11 January 2017.
- ^ Davidson’s 2010, p. 741.
- ^ a b Davidson’s 2010, p. 743.
- ^ Davidson’s 2010, p. 741-3.
- ^ a b c d Smith TJ, Hegedüs L (October 2016). «Graves’ Disease» (PDF). The New England Journal of Medicine. 375 (16): 1552–1565. doi:10.1056/nejmra1510030. PMID 27797318.
- ^ a b c Dean DS, Gharib H (December 2008). «Epidemiology of thyroid nodules». Best Practice & Research. Clinical Endocrinology & Metabolism. 22 (6): 901–11. doi:10.1016/j.beem.2008.09.019. PMID 19041821.
- ^ a b Welker MJ, Orlov D (February 2003). «Thyroid nodules». American Family Physician. 67 (3): 559–66. PMID 12588078. Retrieved 6 September 2016.
- ^ a b c d e f g Davidson’s 2010, p. 744.
- ^ «goitre – definition of goitre in English». Oxford Dictionaries | English. Archived from the original on September 18, 2016. Retrieved 18 September 2016.
- ^ Davidson’s 2010, p. 750.
- ^ Harrison’s 2011, pp. 2237.
- ^ a b c d Harrison’s 2011, pp. 2230.
- ^ a b Harrison’s 2011, pp. 2238.
- ^ a b c d e Harrison’s 2011, p. 2242.
- ^ a b Davidson’s 2010, p. 751.
- ^ a b Davidson’s 2010, p. 752.
- ^ Harrison’s 2011, p. 2242,2246.
- ^ Berbel P, Navarro D, Ausó E, Varea E, Rodríguez AE, Ballesta JJ, et al. (June 2010). «Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity». Cerebral Cortex. 20 (6): 1462–75. doi:10.1093/cercor/bhp212. PMC 2871377. PMID 19812240.
- ^ Büyükgebiz A (15 November 2012). «Newborn screening for congenital hypothyroidism». Journal of Clinical Research in Pediatric Endocrinology. 5 Suppl 1 (4): 8–12. doi:10.4274/Jcrpe.845. PMC 3608007. PMID 23154158.
- ^ a b c Greenspan’s 2011, p. 164.
- ^ Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, Varma SK (June 2006). «Update of newborn screening and therapy for congenital hypothyroidism». Pediatrics. 117 (6): 2290–303. doi:10.1542/peds.2006-0915. PMID 16740880.
- ^ The thyroid gland in health and disease Year: 1917 Robert McCarrison
- ^ Harris RE (2015-05-07). Global Epidemiology of Cancer. Jones & Bartlett Publishers. p. 268. ISBN 978-1-284-03445-5.
- ^ Leung AM, Braverman LE, Pearce EN (November 2012). «History of U.S. iodine fortification and supplementation». Nutrients. 4 (11): 1740–6. doi:10.3390/nu4111740. PMC 3509517. PMID 23201844.
- ^ «Map: Count of Nutrients In Fortification Standards». Global Fortification Data Exchange. Retrieved 23 December 2019.
- ^ «Chernobyl children show DNA changes». BBC News. 2001-05-08. Retrieved 2010-05-25.
- ^ «Iodine — Disorders of Nutrition». MSD Manual Consumer Version. Archived from the original on 18 December 2019. Retrieved 18 December 2019.
- ^ a b c Talley N (2014). Clinical Examination. Churchill Livingstone. pp. Chapter 28. «The endocrine system». pp 355–362. ISBN 978-0-7295-4198-5.
- ^ Fehrenbach; Herring (2012). Illustrated Anatomy of the Head and Neck. Elsevier. p. 158. ISBN 978-1-4377-2419-6.
- ^ Harrison’s 2011, p. 2228.
- ^ a b c d e f Greenspan’s 2011, p. 184.
- ^ a b c Harrison’s 2011, p. 2229.
- ^ Greenspan’s 2011, p. 186.
- ^ Greenspan’s 2011, p. 189.
- ^ Greenspan’s 2011, p. 188-9.
- ^ Greenspan’s 2011, p. 190.
- ^ a b Bin Saeedan M, Aljohani IM, Khushaim AO, Bukhari SQ, Elnaas ST (August 2016). «Thyroid computed tomography imaging: pictorial review of variable pathologies». Insights into Imaging. 7 (4): 601–17. doi:10.1007/s13244-016-0506-5. PMC 4956631. PMID 27271508. Creative Commons Attribution 4.0 International License
- ^ a b c d e f g h i j k l Niazi AK, Kalra S, Irfan A, Islam A (July 2011). «Thyroidology over the ages». Indian Journal of Endocrinology and Metabolism. 15 (Suppl 2): S121-6. doi:10.4103/2230-8210.83347. PMC 3169859. PMID 21966648.
- ^ Anderson DM (2000). Dorland’s Illustrated Medical Dictionary (29th ed.). Philadelphia/London/Toronto/Montreal/Sydney/Tokyo: W.B. Saunders Company.
- ^ His W (1895). Die anatomische Nomenclatur. Nomina Anatomica. Der von der Anatomischen Gesellschaft auf ihrer IX. Versammlung in Basel angenommenen Namen [The anatomical nomenclature. Nominal Anatomica. Anatomical Society on its IX. Assembly adopted in Basel] (in German). Leipzig: Verlag Veit & Comp.
- ^ Lewis CT, Short C (1879). A Latin dictionary. founded on Andrews’ edition of Freund’s Latin dictionary. Oxford: Clarendon Press.
- ^ Liddell HG, Scott R (1940). A Greek-English Lexicon. revised and augmented throughout by Sir Henry Stuart Jones. with the assistance of. Roderick McKenzie. Oxford: Clarendon Press.
- ^ Freake HC, Oppenheimer JH (1995). «Thermogenesis and thyroid function». Annual Review of Nutrition. 15 (1): 263–91. doi:10.1146/annurev.nu.15.070195.001403. PMID 8527221.
- ^ Hamdy, Roland. «The thyroid glands: a brief historical perspective». www.medscape.com. Retrieved 2016-11-13.
- ^ Magner J (June 2014). «Historical note: many steps led to the ‘discovery’ of thyroid-stimulating hormone». European Thyroid Journal. 3 (2): 95–100. doi:10.1159/000360534. PMC 4109514. PMID 25114872.
- ^ «The Nobel Prize in Physiology or Medicine 1977». www.nobelprize.org. Retrieved 14 January 2017.
- ^ Slidescenter.com. «Hormones.gr». www.hormones.gr. Retrieved 2016-11-13.
- ^ Werner SC, Ingbar SH, Braverman LE, Utiger RD (2005). Werner & Ingbar’s the Thyroid: A Fundamental and Clinical Text. Lippincott Williams & Wilkins. p. 387. ISBN 978-0-7817-5047-9.
- ^ «The Nobel Prize in Physiology or Medicine 1909». Nobel Foundation. Retrieved 2007-07-28.
- ^ a b c Romer AS, Parsons TS (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 555–556. ISBN 978-0-03-910284-5.
- ^ Venturi S (2011). «Evolutionary Significance of Iodine». Current Chemical Biology. 5 (3): 155–162. doi:10.2174/187231311796765012. ISSN 1872-3136.
- ^ Venturi S (2014). «Iodine, PUFAs and Iodolipids in Health and Disease: An Evolutionary Perspective». Human Evolution. 29 (1–3): 185–205. ISSN 0393-9375.
Books[edit]
- Greer MA, ed. (1990). The Thyroid Gland. Comprehensive Endocrinology Revised Series. N.Y.: Raven Press. ISBN 0-88167-668-3.
- Shoback D (2011). Gardner DG (ed.). Greenspan’s basic & clinical endocrinology (9th ed.). New York: McGraw-Hill Medical. ISBN 978-0-07-162243-1.
- Hall JE, Guyton AC (2011). Guyton and Hall textbook of medical physiology (12th ed.). Philadelphia, Pa.: Saunders/Elsevier. ISBN 978-1-4160-4574-8.
- Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J (August 11, 2011). Harrison’s Principles of Internal Medicine (18 ed.). McGraw-Hill Professional. ISBN 978-0-07-174889-6.
- Colledge NR, Walker BR, Ralston SH, eds. (2010). Davidson’s principles and practice of medicine. Illustrated by Robert Britton (21st ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 978-0-7020-3085-7.
- Ort V, Bogart BI (2007). Elsevier’s integrated anatomy and embryology. Philadelphia, Pa.: Elsevier Saunders. ISBN 978-1-4160-3165-9.
- Standring S, Borley NR, et al., eds. (2008). Gray’s anatomy : the anatomical basis of clinical practice (40th ed.). London: Churchill Livingstone. ISBN 978-0-8089-2371-8.
External links[edit]
Wikimedia Commons has media related to Thyroid.
- Endocrine Web: Thyroid
ВАЖНО!
Информацию из данного раздела нельзя использовать для самодиагностики и самолечения. В случае боли или иного обострения заболевания диагностические исследования должен назначать только лечащий врач. Для постановки диагноза и правильного назначения лечения следует обращаться к Вашему лечащему врачу.
Для корректной оценки результатов ваших анализов в динамике предпочтительно делать исследования в одной и той же лаборатории, так как в разных лабораториях для выполнения одноименных анализов могут применяться разные методы исследования и единицы измерения.
Болезни щитовидной железы: причины появления, симптомы, диагностика и способы лечения.
Определение
Щитовидная железа (ЩЖ) — железа внутренней секреции, расположенная на передней поверхности шеи, состоящая из двух долей и перешейка.
ЩЖ секретирует йодированные гормоны Т4 (тироксин) и Т3 (трийодтиронин), а также нейодированный гормон кальцитонин.
Основные компоненты, необходимые для образования тиреоидных гормонов — йод и аминокислота тирозин.
Йод поступает в организм с пищей и водой, всасывается в кровь из желудочно-кишечного тракта. Суточная потребность организма в йоде составляет 110–150 мкг/сут.
Регуляция синтеза и секреции гормонов ЩЖ осуществляется через гипоталамо-гипофизарную систему. Гипоталамус секретирует тиролиберин (ТРГ), который стимулирует выделение гипофизом тиреотропного гормона (ТТГ), который, в свою очередь, регулирует рост и функцию щитовидной железы. Между гипоталамусом, гипофизом и ЩЖ существует и обратная связь (избыток йодсодержащих гормонов вызывает снижение тиреотропной функции гипофиза).
Тиреоидные гормоны влияют на все виды обмена: водно-электролитный, белковый, жировой, углеводный и энергетический. Наличие адекватного количества гормонов ЩЖ — необходимое условие для нормального функционирования центральной нервной системы.
Третий гормон щитовидной железы — кальцитонин участвует в метаболизме кальция, препятствуя его вымыванию из костной ткани и тем самым снижая его концентрацию в крови.
Причины заболеваний щитовидной железы
- Нарушение синтеза гормонов:
- из-за недостатка или избытка поступления йода в организм;
- из-за врожденных дефектов развития ЩЖ;
- из-за оперативных вмешательств на ЩЖ;
- из-за применения радиоактивного йода;
- из-за аутоиммунных повреждений;
- из-за воспалительных повреждений.
- Изменение функционального состояния центральной нервной системы.
- Невосприимчивость тканей организма к тиреоидным гормонам.
- Влияние лекарственных препаратов.
Дефицит йода приводит к снижению концентрации тиреоидных гормонов в крови. В результате усиливается продукция тиреотропного гормона, что приводит к компенсаторному увеличению количества тиреоцитов (клеток щитовидной железы). Так развиваются эндемический и спорадический зоб.
Эндемический зоб – увеличение щитовидной железы, возникающее у людей, проживающих в определенных географических районах с недостаточностью йода в окружающей среде. Спорадический зоб выявляют у людей, проживающих в неэндемических районах.
Помимо дефицита йода в образовании нетоксического зоба важную роль могут играть и другие факторы: аутоиммунные, генетические (изменение порога чувствительности к недостатку йода в пище; дефекты ферментных систем, участвующих в синтезе тиреоидных гормонов), пониженное содержание в окружающей среде микроэлементов (кобальта, меди, цинка, молибдена), поступление с пищей зобогенных веществ (тиоцинатов, тиооксизолидонов).
Вирусы, тропные к клеткам щитовидной железы, могут быть пусковым механизмом развития гиперпродукции тиреоидных гормонов.
Лимфоциты щитовидной железы начинают вырабатывать антитела к рецепторам клеточных мембран собственных клеток. Эти антитела оказывают стимулирующее действие на щитовидную железу. В результате нарушения центральной регуляции ситуация выходит из-под контроля, развивается диффузный токсический зоб. Существуют также данные о наследственной предрасположенности к диффузному токсическому зобу.
- врожденная аплазия (отсутствие органа) или гипоплазия (недоразвитие органа) щитовидной железы;
- генетически обусловленное нарушение синтеза гормонов в железе;
- недостаток йода в пище;
- избыток йода при приеме матерью йодсодержащих препаратов;
- уменьшение массы функционирующей тиреоидной ткани (аутоиммунный тиреоидит, оперативное вмешательство на щитовидной железе, гнойный тиреоидит, заболевания гипофиза или гипоталамуса).
В результате стойкого снижения уровня гормонов ЩЖ возникает гипотиреоз со сложной клинической картиной.
Причины возникновения воспаления щитовидной железы (тиреоидита) могут быть разными. Имеется связь развития заболевания с перенесенными бактериальными, грибковыми, вирусными заболеваниями лор-органов или поражениями легких преимущественно у лиц с ослабленным иммунитетом.
Инфекция проникает в ЩЖ с током крови, по лимфатическим путям или в результате прямого попадания при травме, ранении, медицинских манипуляций. В результате развивается тиреоидит. У детей чаще имеется связь заболевания с α- и β-гемолитическими стрептококками и разнообразными анаэробными бактериями.
В результате применения некоторых лекарственных средств (интерферонов, интерлейкинов, моноклональных антител, препаратов лития, йодсодержащего рентгеноконтрастного вещества и др.) могут возникнуть медикаментозные тиреоидиты.
В основе нарушения функции щитовидной железы могут лежать изменения функционального состояния центральной нервной системы, в частности гипоталамуса и гипофиза (травма, кровоизлияние, некроз, хирургическое и лучевое удаление гипофиза). Изменение продукции гипофизом тиреотропного гормона приводит не только к изменению функции щитовидной железы, но и к диффузным или узловым патологиям ее ткани.
Аномалии развития щитовидной железы у плода могут стать результатом неблагоприятного воздействия на организм матери во время беременности.
Зачаток щитовидной железы возникает на 2-4-й неделе внутриутробного развития и возможны два вида нарушений развития. Зачаток ЩЖ может задержаться на месте первоначальной закладки или спуститься необычно низко. В подобных случаях щитовидная железа отсутствует на своем обычном месте, располагаясь в нетипичном для себя месте (например, в средостенье).
Наследственный синдром резистентности тканей к тиреоидным гормонам обычно возникает из-за приобретенной мутации генов (у 22% больных) или передаться по наследству от родителей (в 78% случаев).
Для образования доброкачественных опухолей щитовидной железы имеют значение многие факторы: йододефицит, неблагоприятные условия проживания в крупных мегаполисах, работа на вредном предприятии, радиоактивное облучение, генетическая предрасположенность, резкие гормональные перестройки у женщин во время беременности или климакса и другие факторы.
Злокачественные опухоли щитовидной железы составляют около 1-3% среди всех онкологических заболеваний. Рак щитовидной железы наблюдается у женщин примерно в 3 раза чаще, чем у мужчин.
Согласно литературным данным, рак щитовидной железы возникает на фоне определенных предшествующих изменений — зоба, аденомы, аутоиммунного тиреоидита и проходит стадии развития от диффузной гиперплазии (увеличения количества клеток ЩЖ) до злокачественной опухоли.
Некоторые лекарственные препараты могут изменять уровень гомонов щитовидной железы в крови.
Прием антитиреоидных средств, анаболических стероидов, бета-адреноблокаторов, глюкокортикоидов, нестероидных противовоспалительных средств, оральных контрацептивов, гиполипидемических средств, рентгеноконтрастных средств понижают уровень гормонов. Применение амиодарона, эстрогенов, левотироксина, оральных контрацептивов, кортикостероидов и др., напротив, повышает уровень гормонов ЩЖ.
Таким образом, в развитии одного заболевания, могут иметь значение несколько факторов. И наоборот, один и тот же фактор у разных людей может вызвать разные изменения в щитовидной железе.
Классификация заболеваний щитовидной железы
I. Врожденные аномалии щитовидной железы:
- гипоплазия и аплазия ЩЖ,
- эктопия ЩЖ,
- незаращение щитовидно-язычного протока.
II. Эндемический зоб.
III. Спорадический зоб.
IV. Диффузный токсический зоб.
V. Гипотиреоз.
VI. Воспалительные заболевания щитовидной железы.
VII. Травмы ЩЖ.
VIII. Опухоли ЩЖ:
- доброкачественные новообразования,
- злокачественные опухоли.
Симптомы заболеваний щитовидной железы
- Изменение размера железы, в том числе с появлением узлового образования.
- Воспаление щитовидной железы.
- Нарушения функции ЩЖ (повышение или снижение функции).
Воспаление щитовидной железы может начинаться остро, как например, при остром тиреоидите, так и более гладко — при подостром тиреоидите.
Острый тиреоидит, вызванный бактериальной инфекцией, начинается с лихорадки, головной боли, сильной боли и дискомфортных ощущений в области щитовидной железы. Причинами болевого синдрома являются отек щитовидной железы и растяжение ее капсулы. При осмотре определяется опухолевидное образование на передней поверхности шеи, покраснение кожи. При ощупывании определяется увеличенная, болезненная щитовидная железа. При присоединении гнойного процесса температура тела повышается до 39–40°С, усиливается боль, отмечается расплавление тканей и образованием гнойной полости (абсцедирование).
В период, предшествующий развитию подострого тиреоидита, могут наблюдаться боли в мышцах и в горле, недомогание, субфебрильная лихорадка, общая слабость, утомляемость. Затем появляется умеренная или сильная боль в области щитовидной железы. ЩЖ увеличена и болезненна при ощупывании, наблюдается разрастание соединительной ткани, проявляющееся повышением ее плотности.
При тиреоидите Риделя происходит замещение ткани щитовидной железы на фиброзную. При этом развивается зоб деревянистой плотности. В процесс вовлекается не только сама железа, но и окружающие анатомические структуры: трахея, пищевод, сосуды, нервы, мышцы. Заболевание развивается медленно.
Нарушения функции ЩЖ сопровождаются изменением уровня гормонов в крови (гипофункция или гиперфункция).
У больных с гипофункцией щитовидной железы развиваются вялость, медлительность, сонливость, быстрая утомляемость, замедленное мышление и речь, снижение памяти, эмоциональная лабильность, чувство тревоги, галлюцинации вплоть до психоза. Наблюдаются зябкость и плохая переносимость холода, бледная, шелушащаяся, холодная кожа, отеки лица и конечностей, пастозность и маскообразность лица, увеличение языка. Выпадение волос и повышение ломкости ногтей. Грубый, сиплый голос. Увеличивается масса тела при сниженном аппетите. Могут быть нарушения сердечной деятельности — брадикардия, у 10% наблюдается учащение пульса, у 10-50% пациентов — повышение артериального давления. Больные могут быть склонны к бронхитам, пневмонии, которые отличаются вялым затяжным течением. Могут наблюдаться желудочно-кишечные расстройства: снижение аппетита, тошнота, вздутие кишечника (метеоризм), запоры, застой желчи и образование камней в желчевыводящих путях.
Возникающий в организме избыток гормонов щитовидной железы при таких заболеваниях как диффузный токсический зоб, рак ЩЖ, аденома гипофиза, хорионэпителиома и др., также влияет на различные органы и системы. Кожа становится горячей на ощупь и сухой, волосы — сухими и ломкими. Пациенты жалуются на тремор пальцев рук. Возникает повышенная нервная возбудимость, плаксивость, потливость, чувство жара, небольшие колебания температуры, суетливость. Могут быть внезапные приступы мышечной слабости. Характерными симптомами гиперфункции ЩЖ считаются припухлость верхних век и их гиперпигментация, редкое мигание, пучеглазие, слезотечение, светобоязнь и чувство «песка в глазах».
Даже при повышенном аппетите больные могут терять вес за счет ускоренного обмена веществ.
В связи с влиянием гормонов ЩЖ на сердечно-сосудистую систему больных беспокоят нарушения ритма сердца. Со стороны органо ЖКТ могут наблюдаться приступы болей в животе, рвота, расстройство стула, иногда запоры. В тяжелых случаях поражается печень. У женщин может быть нарушение менструального цикла. У мужчин снижается либидо, потенция, может развиваться гинекомастия.
Диагностика заболеваний щитовидной железы
Диагностические мероприятия начинаются с осмотра ЩЖ, во время которого можно обнаружить деформацию шеи, что свидетельствует об увеличении доли железы, ее перешейка или регионарных лимфоузлов. Кожа над железой может быть покрасневшей с выраженным сосудистым рисунком, на шее и на передней поверхности грудной клетки видны расширенные вены.
При пальпации щитовидной железы могут быть обнаружены узлы. Округлая, сферическая поверхность узла характерна для доброкачественных процессов, а плоская, неровная — для злокачественных новообразований.
При аномалиях развития щитовидной железы она может не обнаруживаться на шее, а опухолевидное образование может располагаться на корне языка, переднем средостенье.
Лабораторная диагностика заболеваний ЩЖ должна быть комплексной и включать:
- клинический анализ крови с определением концентрации гемоглобина, количества эритроцитов, лейкоцитов и тромбоцитов, величины гематокрита и эритроцитарных индексов (MCV, RDW, MCH, MCHC), лейкоформулу и СОЭ (с микроскопией мазка крови при наличии патологических сдвигов);
К каким врачам обращаться
При подозрении на заболевание щитовидной железы необходимо обратиться к
врачу-терапевту
или врачу общей практики. При подтверждении диагноза после проведения комплекса лабораторно-диагностических мероприятий лечение назначает
врач-эндокринолог
. При необходимости пациент может быть направлен к
онкологу
или хирургу.
Лечение заболеваний щитовидной железы
При заболеваниях щитовидной железы используют три метода лечения:
- медикаментозную терапию;
- хирургическое вмешательство;
- лечение радиоактивным йодом.
Лечение может быть комбинированным. Метод лечения определяется индивидуально, его выбор зависит от основной причины заболевания, сопутствующих заболеваний, показаний и противопоказаний к лечению, размеров щитовидной железы, возраста больного.
Медикаментозное лечение используется как в качестве самостоятельного метода, так и для подготовки к оперативному вмешательству.
Подбор дозы осуществляется индивидуально, лечение может быть длительным и непрерывным.
Показания к хирургическому лечению заболеваний ЩЖ:
- опухоли щитовидной железы;
- большие размеры ЩЖ со сдавлением или смещением трахеи, крупных сосудов, пищевода;
- загрудинное расположение щитовидной железы;
- тяжелые формы тиреотоксикоза, осложненные мерцательной аритмией;
- неэффективность проводимой медикаментозной терапии;
- непереносимость тиреотоксических препаратов;
- гормонально-активные опухоли;
- абсцедирование и/или наличие осложнений (свищи, медиастинит) при тиреоидитах.
Лечебное действие радиоактивного йода основано на разрушении клеток эпителия железы и применяется главным образом у пожилых больных с тяжелыми сопутствующими заболеваниями.
Показания к лечению:
- гипертиреоз;
- тиреотоксикоз;
- любой вид рака щитовидной железы;
- узловой токсический зоб;
- рецидив после проведенной операции.
Осложнения
- Самопроизвольное вскрытие абсцесса в просвет трахеи пищевода или в средостение с развитием медиастинита.
- Медиастинит (воспалительный процесс в клетчатке средостенья).
- Флегмона шеи (гнойно-воспалительное заболевание с вовлечением в процесс глубоких клетчаточных пространств шеи).
- Сдавление органов шеи.
- Сепсис (системная воспалительная реакция организма в ответ на распространение местного инфекционного процесса с развитием токсемии и бактериемии).
- Свищ щитовидной железы с трахеей (канал, соединяющий щитовидную железу с просветом трахеи).
- Озлокачествление опухоли.
Профилактика заболеваний щитовидной железы
При появлении первых признаков проблем со ЩЖ необходимо обратиться к врачу. На ранней стадии заболевания больные хорошо реагируют на терапию.
Поздняя диагностика чревата развитием осложнений и даже потерей трудоспособности.
Важны профилактические осмотры, регулярные контрольные приемы у лечащего врача с целью коррекции лечения.
Источники:
- Клинические рекомендации «Заболевания и состояния, связанные с дефицитом йода». Разраб.: Российская ассоциация эндокринологов. – 2020
- Е.А. Валдина. Заболевания щитовидной железы: Руководство. 3-е изд. — СПб: Питер, 2006. — 368 с.
- Система поддержки принятия врачебных решений «Эндокринология Клинические протоколы лечения». Согласовано: д.м.н., проф. Анциферов М.Б.
ВАЖНО!
Информацию из данного раздела нельзя использовать для самодиагностики и самолечения. В случае боли или иного обострения заболевания диагностические исследования должен назначать только лечащий врач. Для постановки диагноза и правильного назначения лечения следует обращаться к Вашему лечащему врачу.
Для корректной оценки результатов ваших анализов в динамике предпочтительно делать исследования в одной и той же лаборатории, так как в разных лабораториях для выполнения одноименных анализов могут применяться разные методы исследования и единицы измерения.
Щитовидная железа небольших размеров, но при этом является самой крупной составляющей эндокринной системы организма. Она «обласкана» в медицинской литературе различными поэтическими названиями: ее именуют и «королевой гормонов», и «повелительницей организма». Почему?
Дело в том, что щитовидная железа вырабатывает гормоны, которые управляют основными обменными процессами в организме человека, регулируют выработку энергии и поступление кислорода в ткани.
— Гормоны щитовидной железы влияют на работу всех органов и систем, — поясняет врач-эндокринолог Елена Куликова. — При изменении функции щитовидной железы меняется масса тела, сила и частота сердечных сокращений, частота дыхания и работа желудочно-кишечного тракта. От активности работы щитовидной железы зависят скорость мышления и эмоциональное состояние человека. И даже способность иметь детей, вынашивание беременности и рождение здорового ребенка также сильно зависят от уровня тиреоидных гормонов.
Если вы заметили изменения во внешнем виде и качестве кожи, выраженную отечность век, вас беспокоят тусклые и ломкие волосы, их выпадение, возможно, что это связано с проблемами щитовидной железы.
Что важно знать о щитовидной железе человека
| Размер | Ширина доли – 16-19 мм, длина – 42-50 мм, толщина – 14-18 мм, толщина перешейка – 5 мм. |
| Вес | В среднем, 15-20 г у взрослого. |
| Объем | 18 мл у женщин, 25 мл у мужчин. |
| Структура | Состоит из тиреонов, а те – из фолликулов |
| Фолликул | Структурно-функциональная единица, представляющая собой группу клеток (в виде «пузырька»). Внутри каждого фолликула есть коллоид – гелеобразное вещество. |
| Какие гормоны производит | 1) йодсодержащие гормоны (тироксин, трийодтиронин); 2) пептидный гормон кальцитонин. |
| За что отвечают гормоны | Поддерживают и регулируют энергетический обмен в органах и тканях, участвуют в синтезе новых клеток организма, влияют на умственное, физическое и психическое развитие, регулируют усвоение и обмен фосфора и кальция в организме. |
Где находится щитовидная железа человека
Щитовидная железа расположена в области переднего треугольника шеи, который ограничен сверху основанием нижней челюсти, снизу – яремной вырезкой грудины, по бокам – передними краями правой и левой грудино-ключично-сосцевидных мышц1.
Прислонив ладонь к шее, можно нащупать щитовидный хрящ (тот, что называют кадыком) – плотное или даже твердое выступающее образование. При глотании он ускользает вверх. Прямо под ним находится и сама щитовидная железа – обычно она ощущается в форме мягкого «нароста» на трахее2.
Как выглядит и работает щитовидная железа у человека
Форму щитовидной железы часто сравнивают с бабочкой. Ее правая и левая доли соединены перешейком, а в 30% случаев имеется еще и пирамидальная доля, которая отходит от перешейка3.
Щитовидная железа состоит из структурных элементов, напоминающих по виду пузырьки — фолликул. Всего их порядка 30 миллионов2. Каждый фолликул заполнен гелеобразным веществом – коллоидом. Как раз в нем содержатся гормоны, вырабатываемые клетками. Все фолликулы сгруппированы по 20-30 штук: такие группы называются тиреонами.
Работу щитовидной железы контролируют 3 механизма.
- Первый механизм – гипоталамо-гипофизарная система, которая находится в головном мозге. Обмен информацией между ЩЖ, гипоталамусом и гипофизом происходит с помощью тиреотропного гормона (ТТГ) и тиреолиберина (ТРГ).
- За второй механизм регуляции отвечает центральная нервная система. Наглядный пример – повышение уровня тиреоидных гормонов во время стресса.
- Третий механизм регуляции – содержание неорганического йода в окружающей среде (прежде всего, воде и продуктах). При недостаточном поступлении йода в организм снижается уровень тиреоидных гормонов и развиваются различные патологии щитовидной железы.
Почему может болеть щитовидная железа у человека
Далеко не каждый может распознать сигнал от щитовидки. Часто болевые ощущения в этой области человек путает с симптомами остеохондроза или думает, что застудил горло.
Кстати, человек не всегда ощущает болезненность. Обычно боль является симптомом инфекционных тиреоидитов (воспалений), а при гипотериозе и гипертериозе, а также при образовании узлов щитовидка, как правило, не болит.
Более того, человек может долгое время не обращать внимания на сигналы организма и не предполагать у себя проблем со здоровьем. Поэтому важно знать симптомы проблем с щитовидной железой. К ним относятся: понижение работоспособности, повышенная раздражительность, затруднение глотания, нарушение сна, тревожность (вплоть до паранойи), снижение веса при хорошем аппетите и т.д. Разные заболевания имеют свои симптомы.
Одной из распространенных причин проблем с щитовидкой является недостаток йода в питании.
— Дефицит йода характерен для многих регионов нашей страны: от легкой степени до довольно тяжелой, — отмечает Елена Куликова. — Необходимость дополнительного приема йодсодержащих препаратов или продуктов с высоким содержанием йода особенно актуальна для детей, беременных и кормящих женщин. Своевременное употребление йодированных продуктов является основной профилактикой для предотвращения заболеваний щитовидной железы у детей и взрослых.
Среди причин заболеваний щитовидной железы могут быть: вирусы и бактерии, аутоиммунная агрессия, онкология. Благоприятным фоном для возникновения проблем с щитовидкой является хронический стресс, дефицит йода, неблагоприятная экология.
Заболевания щитовидной железы являются самой распространенной патологией эндокринной системы. При этом у женщин они встречаются в 10-17 раз чаще, чем у мужчин5.
Все заболевания щитовидной железы подразделяются на 3 группы в зависимости от уровня тиреоидных гормонов:
- Тиреотоксикоз — состояние, характеризующееся повышением уровня тиреоидных гормонов. Наиболее частыми заболеваниями, которые сопровождаются синдромом тиреотоксикоза, являются болезнь Грейвса (до 80% случаев в России6), диффузный токсический зоб или узловой токсический зоб.
Повышение уровня тиреоидных гормонов также можно ожидать при обострении хронического и возникновении острого и подострого тиреоидита. - Гипотериоз. Связан со значительным снижением уровня гормонов щитовидной железы. В большинстве случаев гипотериоз развивается на фоне аутоиммунного тиреоидита (воспаления ЩЖ) и вероятен после резекции (удаление части) щитовидной железы.
- Заболевания щитовидной железы, протекающие без гормональных нарушений (эутиреоидный зоб, опухоли, тиреоидиты).
Разберем наиболее часто встречающиеся заболевания.
Гипотиреоз
В основе этого синдрома лежит стойкий дефицит гормонов щитовидной железы, либо снижение их воздействия на ткани организма7.
Первичный гипотериоз часто развивается на фоне аутоиммунного тиреоидита. Симптомы могут быть чрезвычайно разнообразными, и зачастую даже врач диагностирует гипотиреоз не сразу. В группу риска входят люди с перенесенными операциями на щитовидной железе, пациенты с сахарным диабетом и болезнью Аддисона, заядлые курильщики. Особенно внимательными следует быть женщинам после родов.
Не лишним будет провериться на гипотериоз, если без особых причин стал расти вес, появилась быстрая утомляемость, сонливость, необоснованная тревога и депрессия. Также гипотериоз может проявляться снижением памяти и внимания, отечностью лица и ног, выпадением волос. У мужчин этот синдром может сопровождаться снижением либидо и потенции, у женщин – нарушением менструального цикла. Анемия – еще один частый признак гипотиреоза.
Болезнь Грейвса (диффузный токсический зоб)
В случае возникновения этого заболевания иммунная система организма производят антитела, которые «побуждают» щитовидную железу работать активнее, чем нужно. В результате в организме появляется избыток тиреоидных гормонов, что негативно влияет на многие органы и системы, особенно на нервную и сердечно-сосудистую.
Первыми симптомами болезни Грейвса являются: учащенное сильное сердцебиение, потливость, похудение на фоне нарастающего аппетита, мышечная слабость, повышенная возбудимость и раздражительность8. В большинстве случаев щитовидная железа увеличивается и становится заметной. Очень часто болезнь Грейвса сопровождает эндокринная офтальмопатия, которая проявляется экзофтальмом (пучеглазием) и отечностью век.
— Наличие офтальмопатии в подавляющем большинстве случаев является характерным признаком диффузного токсического зоба, — говорит наш эксперт. – Важно помнить, что болезнь Грейвса – рецидивирующее заболевание. В большинстве случаев оно возвращается, что заставляет задуматься о выборе радикального способа терапии.
Диффузный и узловой эутиреодный зоб
Эутиреоидный зоб также называют нетоксичным. При этом состоянии наблюдается увеличение размеров щитовидной железы без нарушения ее функции. Масштабы проблемы могут быть разные: зоб иногда только прощупывается, а иногда его видно невооруженным взглядом.
Причин развития такой патологии множество, но самая частая из них – дефицит йода, который необходим для синтеза тиреоидных гормонов. Чтобы повысить производство гормонов, ЩЖ начинает увеличиваться в размерах.
При диффузном зобе железа увеличивается равномерно, а при узловом в ней появляются отдельные объемные образования или узлы. Они могут быть единичными и множественными. Встречается и смешанная – диффузно-узловая форма заболевания. У 95% людей узловые образования доброкачественные. Тем не менее эта патология требует тщательной диагностики, чтобы исключить рак щитовидной железы.
Аутоиммунный тиреоидит
Воспалительные заболевания щитовидной железы аутоиммунной этиологии могут привести к гипотериозу. Аутоиммунный тиреоидит может быть выявлен случайно и не сопровождаться нарушением функции щитовидной железы.
К факторам, провоцирующим развитие этого заболевания, относятся: наследственность, неблагоприятная экология, сбои в работе иммунной системы.
— При прогрессировании заболевания щитовидная железа претерпевает склеротические изменения и постепенно снижает свою функциональную активность, — говорит эндокринолог Елена Куликова. — Течение заболевание может быть замедленным и ускоренным. Никогда нельзя узнать заранее, как скоро щитовидная железа утратит свою функцию. Чтобы не пропустить этот момент и вовремя начать заместительную терапию, мы советуем сдавать кровь на ТТГ минимум один раз в год.
Рак щитовидной железы
Рак щитовидной железы в большинстве случаев является высокодифференцированным. Это значит, что рост и развитие опухоли происходит очень медленно. Однако встречаются и агрессивные формы заболевания, поэтому следует быть крайне внимательным и своевременно проходить УЗИ щитовидной железы и, при необходимости, выполнить тонкоигольную аспирационную биопсию.
В зависимости от происхождения, выделяют папиллярный, фолликулярный и медуллярный рак щитовидной железы. В большинстве случаев встречаются неагрессивные формы папиллярного и фолликулярного рака. При своевременном лечении качество жизни пациента практически не страдает. В таких случаях бывает достаточно малоинвазивных методов оперативного лечения. Однако при запущенном или вовремя необнаруженном процессе возникает необходимость в серьезной операции.
Как лечат щитовидную железу человека
Заболевания, связанные с недостатком гормонов щитовидной железы по «золотому стандарту», предполагают заместительную терапию. Как правило, используется левотироксин натрия9. Показанием для назначения его является только гипотиреоз. В прочих ситуациях его назначение необоснованно и может быть опасно.
Для лечения ряда заболеваний щитовидной железы, связанных с ее избыточным функционированием, используются тиреостатические препараты.
К радикальным методам лечения относятся радиойодтерапия и хирургические вмешательства. Чтобы понять, какой именно метод лечения необходим именно вам, нужно обратиться к врачу.
Заместительная терапия
Этот вид лечения назначают в тех случаях, когда функция щитовидной железы снижена, и необходимо ее замещение полностью или частично. Задача заместительной гормональной терапии – привести в норму уровень тиреоидных гормонов.
Очень важно подобрать адекватную индивидуальную дозу препарата и правильно принимать его. При нарушении инструкции самочувствие может ухудшиться.
Нормальный уровень тиреоидных гормонов особенно важен во время беременности, поэтому такую терапию назначают беременным женщинам при необходимости.
Тиреостатическое лечение
Применяется для лечения тиреотоксикоза. В этом случае используются препараты тиомочевины. Они накапливаются в щитовидной железе и блокируют синтез тиреоидных гормонов. Тиреостатическая терапия назначается курсом в 1-1,5 года, либо применяется в качестве подготовительного этапа перед операцией.
При приеме тиреостатиков в некоторых случаях возможны побочные эффекты со стороны печени и кровеносной системы. Поэтому при контрольном обследовании необходимо сдавать анализ крови не только на количество тиреоидных гормонов, но и клинический анализ крови и показателей печени.
На фоне тиреостатической терапии возможны аллергические высыпания на коже. Крайне важно соблюдать дозировку и режим приема препаратов.
Хирургические методы
Необходимость и объем оперативного вмешательства зависят от вида заболевания щитовидной железы. При диффузном токсическом зобе показана тиреоидэктомия (полное удаление щитовидки). При различных опухолях — либо тиреоидэктомия либо гемитиреоидэктомия (частичное удаление). Объем оперативного вмешательства определяет хирург-эндокринолог или специалист-эндокринолог.
Операция может проводиться открытым способом (классическим) или малоинвазивным (эндоскопическим). Эндоскопические методы (без больших разрезов) имеют неоспоримые преимущества перед открытыми операциями: меньше повреждаются ткани, сокращается срок реабилитации, почти незаметные постоперационные шрамы.
Оперативное лечение патологии щитовидной железы имеет свои строгие показания. Существует ряд состояний (например, коллоидные узлы), которые не требуют оперативного лечения и подлежат динамическому наблюдению.
Радиойодтерапия
Лечение радиоактивным йодом — еще один метод радикального лечения различных форм токсического зоба. Применяется в том случае, если болезнь постоянно возвращается, а тиреостатическая терапия не дала результатов. Радиойодтерапию рекомендуют при небольшом объеме зоба, чтобы избежать операции.
Медики убеждены, что лечение радиоактивным йодом не влияет на риск развития рака щитовидной железы10. Противопоказания: беременность, период лактации, эндокринная офтальмопатия.
Как сохранить здоровье щитовидной железы в домашних условиях
Очень важный элемент для нормального функционирования щитовидной железы – это йод. Суточная потребность в нем зависит от возраста: до 5 лет – 90 мкг, до 12 лет – 120 мкг, от 12 лет – 150 мкг, для беременных и кормящих – 250 мкг11.
Далеко не всегда суточную порцию йода можно получить с пищей, поэтому нередко врачи назначают йодсодержащие препараты. Однако не следует слишком усердствовать в приеме йодистых препаратов. В ряде случаев суточную дозу можно получить, применяя в питании йодированную или морскую соль.
Заболевания щитовидной железы могут быть спровоцированы стрессом, переутомлением, вирусными и бактериальными заболеваниями, хроническими заболеваниями ЛОР-органов. Если Вы хотите, чтобы ваша щитовидная железа чувствовала себя хорошо и работала без сбоев, необходимо укреплять иммунитет, вести здоровый образ жизни, избегать стрессов и высыпаться.
Увы, на некоторые факторы (например, генетическую предрасположенность) повлиять нельзя. Поэтому, если вы знаете, что обладаете отягощенной наследственностью по заболеваниям щитовидной железы, контролируйте ее состояние с помощью ежегодного УЗИ и анализа крови на ТТГ.
Популярные вопросы и ответы
На вопросы, касающиеся работы щитовидной железы отвечает наш эксперт – врач эндокринолог Елена Куликова.
Какие первые признаки проблем со щитовидной железой?
— Подумать о нарушении функции щитовидной железы можно практически при любом необычном самочувствии: от повышенной утомляемости, частого сердцебиения до серьезных репродуктивных проблем. Часто пациенты отмечают дискомфорт при глотании и ощущение кома в горле. Возможны болевые ощущения по передней поверхности шеи.
Какие продукты любит щитовидная железа?
— Если быть категоричным, то морепродукты. А если серьезно, то качественное, сбалансированное по всем компонентам питание прекрасно подойдет не только для
Какой врач лечит щитовидную железу человека?
— Конечно, эндокринолог. В случае, если вы не уверены, что у вас проблемы именно с щитовидной железой, обратитесь к терапевту и попросите его дать направление к эндокринологу.
Источники:
- Щитовидная железа. Фундаментальные аспекты. Под ред. проф. А.И. Кубарко, и проф. S. Yamashita. Минск-Нагасаки. 1998. https://goo.su/U6ZKX
- А.В. Ушаков. Восстановление щитовидной железы. Руководство для пациентов. https://coollib.com/b/185291/read
- А.М. Мкртумян, С.В. Подачина, Н.А. Петунина. Заболевания щитовидной железы. Руководство для врачей. Москва. 2012. http://www.lib.knigi-x.ru/23raznoe/260583-1-am-mkrtumyan-podachina-petunina-zabolevaniya-schitovidnoy-zhelezi-rukovodstvo-dlya-vrachey-moskva-2012-oglavlen.php
- О.А. Бутакова. Про щитовидную железу // Библиотека академии здоровья. 2010г. https://coral-info.com/shhitovidnaya-zheleza-olga-butakova/
- С.В. Михайлова, Т.А. Зыкова. Аутоиммунные болезни щитовидной железы и репродуктивные нарушения у женщин // Сибирский медицинский журнал. 2013. №8. С. 26-31 https://cyberleninka.ru/article/n/autoimmunnye-bolezni-schitovidnoy-zhelezy-i-reproduktivnye-narusheniya-u-zhenschin/viewer
- Ю.В. Кухтенко, соавторы. Структура заболеваний щитовидной железы у пациентов различных возрастных групп // Вестник ВолгГМУ. 2016. №3. https://cyberleninka.ru/article/n/struktura-zabolevaniy-schitovidnoy-zhelezy-u-patsientov-razlichnyh-vozrastnyh-grupp/viewer
- Ю.А. Долгих, Т.В. Ломонова. Гипотиреоз: непростой диагноз // Эндокринология: новости, мнения, обучение. 2021. Том 10. №4. https://cyberleninka.ru/article/n/gipotireoz-neprostoy-diagnoz
- И.И. Дедов, Г.А. Мельниченко, В.В. Фадеев. Эндокринология. Издание второе, переработанное и дополненное. Москва. ИГ «ГЭОТАР-Медиа». 2007. https://goo.su/5kAVT
- О.В. Парамонова, Е.Г. Коренская. Лечение гипотиреоза в гериатрической практике // Клиническая геронтология. 2019. №5. https://cyberleninka.ru/article/n/lechenie-gipoterioza-v-geriatricheskoy-praktike/viewer
- Н.А. Петунина, Н.С. Мартиросян, Л.В. Трухина. Синдром тиреотоксикоза. Подходы к диагностике и лечению // Трудный пациент. 2012. Том 10. №1. С. 20-24 https://cyberleninka.ru/article/n/sindrom-tireotoksikoza-podhody-k-diagnostike-i-lecheniyu/viewer
- Ф.М. Абдулхабирова, соавторы. Клинические рекомендации «Заболевания и состояния, связанные с дефицитом йода» // Проблемы эндокринологии. 2021. Том 67. №3. https://cyberleninka.ru/article/n/klinicheskie-rekomendatsii-zabolevaniya-i-sostoyaniya-svyazannye-s-defitsitom-yoda/viewer
Рис. О. В. Кульковой
Щитовидная железа (вид спереди): 1 – щитовидный хрящ; 2 – пирамидальная доля; 3 – леваяи правая доли щитовидной железы; 4 – перешеек щитовидной железы; 5 – трахея.
ЩИТОВИ́ДНАЯ ЖЕЛЕЗА́, железа внутренней секреции позвоночных. Вырабатывает и секретирует в кровь содержащие иод гормоны – тиронины. Впервые как непарный самостоят. орган дифференцируется у рыб; у земноводных и птиц железа парная. У млекопитающих Щ. ж. имеет форму щита или подковы, находится в средней области шеи под гортанью; у человека обычно состоит из двух долей (у 30% людей имеется дополнительная, т. н. пирамидальная, доля), соединённых перешейком, располагается в передней области шеи на уровне гортани и верхнего отдела трахеи, имеет массу ок. 15–30 г. Масса и объём железы у женщин больше, чем у мужчин.
Осн. морфологич. и функциональные единицы Щ. ж. – фолликулы. Клетки фолликулов (эпителиальные, или т. н. фолликулярные) поглощают иод из кровотока, минутный объём которого в железе в 3–7 раз превышает её массу, и синтезируют специфич. белок тиреоглобулин. При внутриклеточном протеолизе тиреоглобулина происходит освобождение тиронинов: тироксина и трииодтиронина. Эти гормоны участвуют в регуляции процессов роста, развития, дифференцировки тканей. Они повышают интенсивность обмена веществ, особенно осн. обмена, усиливая окислит. процессы и теплопродукцию в тканях (животные со сниженной функцией Щ. ж. плохо переносят охлаждение), поддерживают на оптимальном уровне энергетич. и биосинтетич. процессы в организме опосредованно, через регуляцию тканевого дыхания. У всех позвоночных гормоны Щ. ж. влияют на строение покровов и их производных – стимулируют размножение клеток в базальном слое эпидермиса, а у земноводных, пресмыкающихся и птиц способствуют линьке. У млекопитающих в ткань железы в процессе зародышевого развития включаются клетки (парафолликулярные, или К-клетки) ультимобронхиальных желёз, вырабатывающие кальцитонин.
Щ. ж. находится во взаимодействии с др. железами внутр. секреции (гипофизом, надпочечниками, половыми железами и поджелудочной железой). Функция Щ. ж. регулируется ЦНС; вырабатываемый гипоталамусом гормон тиролиберин (см. в ст. Рилизинг-гормоны) стимулирует секрецию гипофизом тиротропина, который, в свою очередь, стимулирует развитие и функции Щ. ж. Изменение функции Щ. ж. может быть связано как с нарушением синтеза или задержкой выделения тиронинов (гипотиреоз), так и с усиленной их продукцией (гипертиреоз).