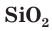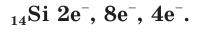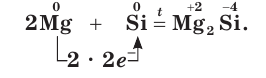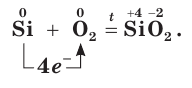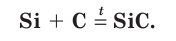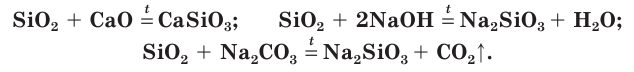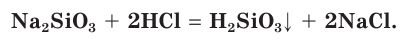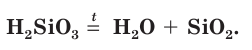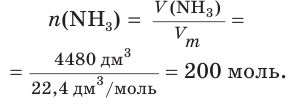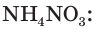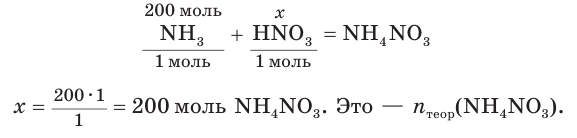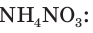Not to be confused with the silicon-containing synthetic polymer silicone.
| Silicon | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allotropes | see Allotropes of silicon | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | crystalline, reflective with bluish-tinged faces | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Si) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Silicon in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 14 (carbon group) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1687 K (1414 °C, 2577 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3538 K (3265 °C, 5909 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 2.3290 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 2.57 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 50.21 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 383 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 19.789 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −4, −3, −2, −1, 0,[2] +1,[3] +2, +3, +4 (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 111 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 111 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 210 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of silicon |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered diamond-cubic
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 8433 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 2.6 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 149 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 2.3×103 Ω⋅m (at 20 °C)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Band gap | 1.12 eV (at 300 K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −3.9×10−6 cm3/mol (298 K)[6] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 130–188 GPa[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 51–80 GPa[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 97.6 GPa[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.064–0.28[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-21-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Latin silex or silicis, meaning ‘flint’ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prediction | Antoine Lavoisier (1787) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Jöns Jacob Berzelius[8][9] (1823) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Named by | Thomas Thomson (1817) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of silicon
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive.
Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron.
Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth’s crust. It is widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide (silica) or silicates. More than 90% of the Earth’s crust is composed of silicate minerals, making silicon the second most abundant element in the Earth’s crust (about 28% by mass), after oxygen.
Most silicon is used commercially without being separated, often with very little processing of the natural minerals. Such use includes industrial construction with clays, silica sand, and stone. Silicates are used in Portland cement for mortar and stucco, and mixed with silica sand and gravel to make concrete for walkways, foundations, and roads. They are also used in whiteware ceramics such as porcelain, and in traditional silicate-based soda-lime glass and many other specialty glasses. Silicon compounds such as silicon carbide are used as abrasives and components of high-strength ceramics. Silicon is the basis of the widely used synthetic polymers called silicones.
The late 20th century to early 21st century has been described as the Silicon Age (also known as the Digital Age or Information Age) because of the large impact that elemental silicon has on the modern world economy. The small portion of very highly purified elemental silicon used in semiconductor electronics (<10%[citation needed]) is essential to the transistors and integrated circuit chips used in most modern technology such as smartphones and other computers. In 2019, 32.4% of the semiconductor market segment was for networks and communications devices, and the semiconductors industry is projected to reach $726.73 billion by 2027. [10]
Silicon is an essential element in biology. Only traces are required by most animals, but some sea sponges and microorganisms, such as diatoms and radiolaria, secrete skeletal structures made of silica. Silica is deposited in many plant tissues.[11]
History[edit]
Owing to the abundance of silicon in the Earth’s crust, natural silicon-based materials have been used for thousands of years. Silicon rock crystals were familiar to various ancient civilizations, such as the predynastic Egyptians who used it for beads and small vases, as well as the ancient Chinese. Glass containing silica was manufactured by the Egyptians since at least 1500 BC, as well as by the ancient Phoenicians. Natural silicate compounds were also used in various types of mortar for construction of early human dwellings.[12]
Discovery[edit]
In 1787, Antoine Lavoisier suspected that silica might be an oxide of a fundamental chemical element,[13] but the chemical affinity of silicon for oxygen is high enough that he had no means to reduce the oxide and isolate the element.[14] After an attempt to isolate silicon in 1808, Sir Humphry Davy proposed the name «silicium» for silicon, from the Latin silex, silicis for flint, and adding the «-ium» ending because he believed it to be a metal.[15] Most other languages use transliterated forms of Davy’s name, sometimes adapted to local phonology (e.g. German Silizium, Turkish silisyum, Catalan silici, Armenian Սիլիցիում or Silitzioum). A few others use instead a calque of the Latin root (e.g. Russian кремний, from кремень «flint»; Greek πυρίτιο from πυρ «fire»; Finnish pii from piikivi «flint», Czech křemík from křemen «quartz», «flint»).[16]
Gay-Lussac and Thénard are thought to have prepared impure amorphous silicon in 1811, through the heating of recently isolated potassium metal with silicon tetrafluoride, but they did not purify and characterize the product, nor identify it as a new element.[17] Silicon was given its present name in 1817 by Scottish chemist Thomas Thomson. He retained part of Davy’s name but added «-on» because he believed that silicon was a nonmetal similar to boron and carbon.[18] In 1824, Jöns Jacob Berzelius prepared amorphous silicon using approximately the same method as Gay-Lussac (reducing potassium fluorosilicate with molten potassium metal), but purifying the product to a brown powder by repeatedly washing it.[19] As a result, he is usually given credit for the element’s discovery.[20][21] The same year, Berzelius became the first to prepare silicon tetrachloride; silicon tetrafluoride had already been prepared long before in 1771 by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid.[14] In 1823 for the first time Jacob Berzelius discovered silicon tetrachloride (SiCl4).[22] In 1846 Von Ebelman’s had synthesized Tetraethyl orthosilicate (Si(OC2H5)4).[23][22]
Silicon in its more common crystalline form was not prepared until 31 years later, by Deville.[24][25] By electrolyzing a mixture of sodium chloride and aluminium chloride containing approximately 10% silicon, he was able to obtain a slightly impure allotrope of silicon in 1854.[26] Later, more cost-effective methods have been developed to isolate several allotrope forms, the most recent being silicene in 2010.[27][28] Meanwhile, research on the chemistry of silicon continued; Friedrich Wöhler discovered the first volatile hydrides of silicon, synthesising trichlorosilane in 1857 and silane itself in 1858, but a detailed investigation of the silanes was only carried out in the early 20th century by Alfred Stock, despite early speculation on the matter dating as far back as the beginnings of synthetic organic chemistry in the 1830s.[29][30] Similarly, the first organosilicon compound, tetraethylsilane, was synthesised by Charles Friedel and James Crafts in 1863, but detailed characterisation of organosilicon chemistry was only done in the early 20th century by Frederic Kipping.[14]
Starting in the 1920s, the work of William Lawrence Bragg on X-ray crystallography elucidated the compositions of the silicates, which had previously been known from analytical chemistry but had not yet been understood, together with Linus Pauling’s development of crystal chemistry and Victor Goldschmidt’s development of geochemistry. The middle of the 20th century saw the development of the chemistry and industrial use of siloxanes and the growing use of silicone polymers, elastomers, and resins. In the late 20th century, the complexity of the crystal chemistry of silicides was mapped, along with the solid-state physics of doped semiconductors.[14]
Silicon semiconductors[edit]
The first semiconductor devices did not use silicon, but used galena, including German physicist Ferdinand Braun’s crystal detector in 1874 and Indian physicist Jagadish Chandra Bose’s radio crystal detector in 1901.[31][32] The first silicon semiconductor device was a silicon radio crystal detector, developed by American engineer Greenleaf Whittier Pickard in 1906.[32]
In 1940, Russell Ohl discovered the p–n junction and photovoltaic effects in silicon. In 1941, techniques for producing high-purity germanium and silicon crystals were developed for radar microwave detector crystals during World War II.[31] In 1947, physicist William Shockley theorized a field-effect amplifier made from germanium and silicon, but he failed to build a working device, before eventually working with germanium instead. The first working transistor was a point-contact transistor built by John Bardeen and Walter Brattain later that year while working under Shockley.[33] In 1954, physical chemist Morris Tanenbaum fabricated the first silicon junction transistor at Bell Labs.[34] In 1955, Carl Frosch and Lincoln Derick at Bell Labs accidentally discovered that silicon dioxide (SiO
2) could be grown on silicon,[35] and they later proposed this could mask silicon surfaces during diffusion processes in 1958.[36]
Silicon Age[edit]
The «Silicon Age» refers to the late 20th century to early 21st century.[37][38][39] This is due to silicon being the dominant material of the Silicon Age (also known as the Digital Age or Information Age), similar to how the Stone Age, Bronze Age and Iron Age were defined by the dominant materials during their respective ages of civilization.[37]
Because silicon is an important element in high-technology semiconductor devices, many places in the world bear its name. For example, Santa Clara Valley in California acquired the nickname Silicon Valley, as the element is the base material in the semiconductor industry there. Since then, many other places have been dubbed similarly, including Silicon Wadi in Israel, Silicon Forest in Oregon, Silicon Hills in Austin, Texas, Silicon Slopes in Salt Lake City, Utah, Silicon Saxony in Germany, Silicon Valley in India, Silicon Border in Mexicali, Mexico, Silicon Fen in Cambridge, England, Silicon Roundabout in London, Silicon Glen in Scotland, Silicon Gorge in Bristol, England, Silicon Alley in New York, New York and Silicon Beach in Los Angeles, California.[40]
Characteristics[edit]
Physical and atomic[edit]
A silicon atom has fourteen electrons. In the ground state, they are arranged in the electron configuration [Ne]3s23p2. Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. Like the other members of its group, the lighter carbon and the heavier germanium, tin, and lead, it has the same number of valence electrons as valence orbitals: hence, it can complete its octet and obtain the stable noble gas configuration of argon by forming sp3 hybrid orbitals, forming tetrahedral SiX
4 derivatives where the central silicon atom shares an electron pair with each of the four atoms it is bonded to.[42] The first four ionisation energies of silicon are 786.3, 1576.5, 3228.3, and 4354.4 kJ/mol respectively; these figures are high enough to preclude the possibility of simple cationic chemistry for the element. Following periodic trends, its single-bond covalent radius of 117.6 pm is intermediate between those of carbon (77.2 pm) and germanium (122.3 pm). The hexacoordinate ionic radius of silicon may be considered to be 40 pm, although this must be taken as a purely notional figure given the lack of a simple Si4+
cation in reality.[43]
Electrical[edit]
At standard temperature and pressure, silicon is a shiny semiconductor with a bluish-grey metallic lustre; as typical for semiconductors, its resistivity drops as temperature rises. This arises because silicon has a small energy gap (band gap) between its highest occupied energy levels (the valence band) and the lowest unoccupied ones (the conduction band). The Fermi level is about halfway between the valence and conduction bands and is the energy at which a state is as likely to be occupied by an electron as not. Hence pure silicon is effectively an insulator at room temperature. However, doping silicon with a pnictogen such as phosphorus, arsenic, or antimony introduces one extra electron per dopant and these may then be excited into the conduction band either thermally or photolytically, creating an n-type semiconductor. Similarly, doping silicon with a group 13 element such as boron, aluminium, or gallium results in the introduction of acceptor levels that trap electrons that may be excited from the filled valence band, creating a p-type semiconductor.[44] Joining n-type silicon to p-type silicon creates a p–n junction with a common Fermi level; electrons flow from n to p, while holes flow from p to n, creating a voltage drop. This p–n junction thus acts as a diode that can rectify alternating current that allows current to pass more easily one way than the other. A transistor is an n–p–n junction, with a thin layer of weakly p-type silicon between two n-type regions. Biasing the emitter through a small forward voltage and the collector through a large reverse voltage allows the transistor to act as a triode amplifier.[44]
Crystal structure[edit]
Silicon crystallises in a giant covalent structure at standard conditions, specifically in a diamond cubic lattice (space group 227). It thus has a high melting point of 1414 °C, as a lot of energy is required to break the strong covalent bonds and melt the solid. Upon melting silicon contracts as the long-range tetrahedral network of bonds breaks up and the voids in that network are filled in, similar to water ice when hydrogen bonds are broken upon melting. It does not have any thermodynamically stable allotropes at standard pressure, but several other crystal structures are known at higher pressures. The general trend is one of increasing coordination number with pressure, culminating in a hexagonal close-packed allotrope at about 40 gigapascals known as Si–VII (the standard modification being Si–I). An allotrope called BC8 (or bc8), having a body-centred cubic lattice with eight atoms per primitive unit cell (space group 206), can be created at high pressure and remains metastable at low pressure. Its properties have been studied in detail.[45]
Silicon boils at 3265 °C: this, while high, is still lower than the temperature at which its lighter congener carbon sublimes (3642 °C) and silicon similarly has a lower heat of vaporisation than carbon, consistent with the fact that the Si–Si bond is weaker than the C–C bond.[44]
It is also possible to construct silicene layers analogous to graphene.[27][28]
Isotopes[edit]
Naturally occurring silicon is composed of three stable isotopes, 28Si (92.23%), 29Si (4.67%), and 30Si (3.10%).[46] Out of these, only 29Si is of use in NMR and EPR spectroscopy,[47] as it is the only one with a nuclear spin (I =1/2).[29] All three are produced in Type Ia supernovae[48][49] through the oxygen-burning process, with 28Si being made as part of the alpha process and hence the most abundant. The fusion of 28Si with alpha particles by photodisintegration rearrangement in stars is known as the silicon-burning process; it is the last stage of stellar nucleosynthesis before the rapid collapse and violent explosion of the star in question in a type II supernova.[50]
Twenty radioisotopes have been characterized, the two stablest being 32Si with a half-life of about 150 years, and 31Si with a half-life of 2.62 hours.[46] All the remaining radioactive isotopes have half-lives that are less than seven seconds, and the majority of these have half-lives that are less than one tenth of a second.[46] Silicon has one known nuclear isomer, 34mSi, with a half-life less than 210 nanoseconds.[46] 32Si undergoes low-energy beta decay to 32P and then stable 32S. 31Si may be produced by the neutron activation of natural silicon and is thus useful for quantitative analysis; it can be easily detected by its characteristic beta decay to stable 31P, in which the emitted electron carries up to 1.48 MeV of energy.[29]
The known isotopes of silicon range in mass number from 22 to 44.[46] The most common decay mode of the isotopes with mass numbers lower than the three stable isotopes is inverse beta decay, primarily forming aluminium isotopes (13 protons) as decay products.[46] The most common decay mode for the heavier unstable isotopes is beta decay, primarily forming phosphorus isotopes (15 protons) as decay products.[46]
Silicon can enter the oceans through groundwater and riverine transport. Large fluxes of groundwater input have an isotopic composition which is distinct from riverine silicon inputs. Isotopic variations in groundwater and riverine transports contribute to variations in oceanic 30Si values. Currently, there are substantial differences in the isotopic values of deep water in the world’s ocean basins. Between the Atlantic and Pacific oceans, there is a deep water 30Si gradient of greater than 0.3 parts per thousand. 30Si is most commonly associated with productivity in the oceans.[51]
Chemistry and compounds[edit]
| X = | C | Si | H | F | Cl | Br | I | O– | N< |
|---|---|---|---|---|---|---|---|---|---|
| C–X | 368 | 360 | 435 | 453 | 351 | 293 | 216 | ~360 | ~305 |
| Si–X | 360 | 340 | 393 | 565 | 381 | 310 | 234 | 452 | 322 |
Crystalline bulk silicon is rather inert, but becomes more reactive at high temperatures. Like its neighbour aluminium, silicon forms a thin, continuous surface layer of silicon dioxide (SiO
2) that protects the metal from oxidation. Thus silicon does not measurably react with the air below 900 °C, but formation of the vitreous dioxide rapidly increases between 950 °C and 1160 °C and when 1400 °C is reached, atmospheric nitrogen also reacts to give the nitrides SiN and Si
3N
4. Silicon reacts with gaseous sulfur at 600 °C and gaseous phosphorus at 1000 °C. This oxide layer nevertheless does not prevent reaction with the halogens; fluorine attacks silicon vigorously at room temperature, chlorine does so at about 300 °C, and bromine and iodine at about 500 °C. Silicon does not react with most aqueous acids, but is oxidised and complexed by hydrofluoric acid mixtures containing either chlorine or nitric acid to form hexafluorosilicates. It readily dissolves in hot aqueous alkali to form silicates.[52] At high temperatures, silicon also reacts with alkyl halides; this reaction may be catalysed by copper to directly synthesise organosilicon chlorides as precursors to silicone polymers. Upon melting, silicon becomes extremely reactive, alloying with most metals to form silicides, and reducing most metal oxides because the heat of formation of silicon dioxide is so large. In fact, molten silicon reacts virtually with every known kind of crucible material (except its own oxide, SiO
2).[53]: 13 This happens due to silicon’s high binding forces for the light elements and to its high dissolving power for most elements.[53]: 13 As a result, containers for liquid silicon must be made of refractory, unreactive materials such as zirconium dioxide or group 4, 5, and 6 borides.[44][54]
Tetrahedral coordination is a major structural motif in silicon chemistry just as it is for carbon chemistry. However, the 3p subshell is rather more diffuse than the 2p subshell and does not hybridise so well with the 3s subshell. As a result, the chemistry of silicon and its heavier congeners shows significant differences from that of carbon,[55] and thus octahedral coordination is also significant.[44] For example, the electronegativity of silicon (1.90) is much less than that of carbon (2.55), because the valence electrons of silicon are further from the nucleus than those of carbon and hence experience smaller electrostatic forces of attraction from the nucleus. The poor overlap of 3p orbitals also results in a much lower tendency toward catenation (formation of Si–Si bonds) for silicon than for carbon, due to the concomitant weakening of the Si–Si bond compared to the C–C bond:[56] the average Si–Si bond energy is approximately 226 kJ/mol, compared to a value of 356 kJ/mol for the C–C bond.[57] This results in multiply bonded silicon compounds generally being much less stable than their carbon counterparts, an example of the double bond rule. On the other hand, the presence of radial nodes in the 3p orbitals of silicon suggests the possibility of hypervalence, as seen in five and six-coordinate derivatives of silicon such as SiX−
5 and SiF2−
6.[58][56] Lastly, because of the increasing energy gap between the valence s and p orbitals as the group is descended, the divalent state grows in importance from carbon to lead, so that a few unstable divalent compounds are known for silicon; this lowering of the main oxidation state, in tandem with increasing atomic radii, results in an increase of metallic character down the group. Silicon already shows some incipient metallic behavior, particularly in the behavior of its oxide compounds and its reaction with acids as well as bases (though this takes some effort), and is hence often referred to as a metalloid rather than a nonmetal.[56] However, metallicity does not become clear in group 14 until germanium and dominant until tin, with the growing importance of the lower +2 oxidation state.[14]
Silicon shows clear differences from carbon. For example, organic chemistry has very few analogies with silicon chemistry, while silicate minerals have a structural complexity unseen in oxocarbons.[59] Silicon tends to resemble germanium far more than it does carbon, and this resemblance is enhanced by the d-block contraction, resulting in the size of the germanium atom being much closer to that of the silicon atom than periodic trends would predict.[60] Nevertheless, there are still some differences because of the growing importance of the divalent state in germanium compared to silicon, which result in germanium being significantly more metallic than silicon. Additionally, the lower Ge–O bond strength compared to the Si–O bond strength results in the absence of «germanone» polymers that would be analogous to silicone polymers.[57]
Silicides[edit]
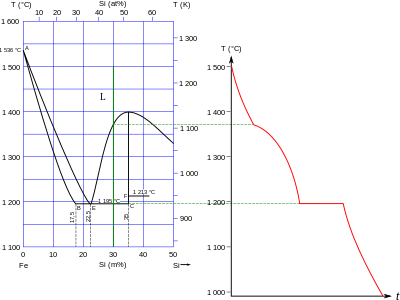
Phase diagram of the Fe–Si system
Many metal silicides are known, most of which have formulae that cannot be explained through simple appeals to valence: their bonding ranges from metallic to ionic and covalent. Some known stoichiometries are M
6Si, M
5Si, M
4Si, M
15Si
4, M
3Si, M
5Si
2, M
2Si, M
5Si
3, M
3Si
2, MSi, M
2Si
3, MSi
2, MSi
3, and MSi
6. They are structurally more similar to the borides than the carbides, in keeping with the diagonal relationship between boron and silicon, although the larger size of silicon than boron means that exact structural analogies are few and far between. The heats of formation of the silicides are usually similar to those of the borides and carbides of the same elements, but they usually melt at lower temperatures.[61] Silicides are known for all stable elements in groups 1–10, with the exception of beryllium: in particular, uranium and the transition metals of groups 4–10 show the widest range of stoichiometries. Except for copper, the metals in groups 11–15 do not form silicides. Instead, most form eutectic mixtures, although the heaviest post-transition metals mercury, thallium, lead, and bismuth are completely immiscible with liquid silicon.[44]
Usually, silicides are prepared by direct reaction of the elements. For example, the alkali metals and alkaline earth metals react with silicon or silicon oxide to give silicides. Nevertheless, even with these highly electropositive elements true silicon anions are not obtainable, and most of these compounds are semiconductors. For example, the alkali metal silicides (M+
)
4(Si4−
4) contain pyramidal tricoordinate silicon in the Si4−
4 anion, isoelectronic with white phosphorus, P
4.[44][62] Metal-rich silicides tend to have isolated silicon atoms (e. g. Cu
5Si); with increasing silicon content, catenation increases, resulting in isolated clusters of two (e. g. U
3Si
2) or four silicon atoms (e. g. [K+
]
4[Si
4]4−
) at first, followed by chains (e. g. CaSi), layers (e. g. CaSi
2), or three-dimensional networks of silicon atoms spanning space (e. g. α-ThSi
2) as the silicon content rises even higher.[44]
The silicides of the group 1 and 2 metals usually are more reactive than the transition metal silicides. The latter usually do not react with aqueous reagents, except for hydrofluoric acid; however, they do react with much more aggressive reagents such as liquid potassium hydroxide, or gaseous fluorine or chlorine when red-hot. The pre-transition metal silicides instead readily react with water and aqueous acids, usually producing hydrogen or silanes:[44]
- Na
2Si + 3 H2O → Na
2SiO
3 + 3 H
2 - Mg
2Si + 2 H
2SO
4 → 2 MgSO
4 + SiH
4
Products often vary with the stoichiometry of the silicide reactant. For example, Ca
2Si is polar and non-conducting and has the anti-PbCl
2 structure with single isolated silicon atoms, and reacts with water to produce calcium hydroxide, hydrated silicon dioxide, and hydrogen gas. CaSi with its zigzag chains of silicon atoms instead reacts to give silanes and polymeric SiH
2, while CaSi
2 with its puckered layers of silicon atoms does not react with water, but will react with dilute hydrochloric acid: the product is a yellow polymeric solid with stoichiometry Si
2H
2O.[44]
Silanes[edit]
Speculation on silicon hydride chemistry started in the 1830s, contemporary with the development of synthetic organic chemistry. Silane itself, as well as trichlorosilane, were first synthesised by Friedrich Wöhler and Heinrich Buff in 1857 by reacting aluminium–silicon alloys with hydrochloric acid, and characterised as SiH
4 and SiHCl
3 by Charles Friedel and Albert Ladenburg in 1867. Disilane (Si
2H
6) followed in 1902, when it was first made by Henri Moissan and Samuel Smiles by the protonolysis of magnesium silicides. Further investigation had to wait until 1916 because of the great reactivity and thermal instability of the silanes; it was then that Alfred Stock began to study silicon hydrides in earnest with new greaseless vacuum techniques, as they were found as contaminants of his focus, the boron hydrides. The names silanes and boranes are his, based on analogy with the alkanes.[29][63][64] The Moissan and Smiles method of preparation of silanes and silane derivatives via protonolysis of metal silicides is still used, although the yield is lowered by the hydrolysis of the products that occurs simultaneously, so that the preferred route today is to treat substituted silanes with hydride reducing agents such as lithium aluminium hydride in etheric solutions at low temperatures. Direct reaction of HX or RX with silicon, possibly with a catalyst such as copper, is also a viable method of producing substituted silanes.[29]
The silanes comprise a homologous series of silicon hydrides with a general formula of Si
nH
2n + 2. They are all strong reducing agents. Unbranched and branched chains are known up to n=8, and the cycles Si
5H
10 and Si
6H
12 are also known. The first two, silane and disilane, are colourless gases; the heavier members of the series are volatile liquids. All silanes are very reactive and catch fire or explode spontaneously in air. They become less thermally stable with room temperature, so that only silane is indefinitely stable at room temperature, although disilane does not decompose very quickly (only 2.5% of a sample decomposes after the passage of eight months).[29] They decompose to form polymeric polysilicon hydride and hydrogen gas.[65][66] As expected from the difference in atomic weight, the silanes are less volatile than the corresponding alkanes and boranes, but more so than the corresponding germanes. They are much more reactive than the corresponding alkanes, because of the larger radius of silicon compared to carbon facilitating nucleophilic attack at the silicon, the greater polarity of the Si–H bond compared to the C–H bond, and the ability of silicon to expand its octet and hence form adducts and lower the reaction’s activation energy.[29]
Silane pyrolysis gives polymeric species and finally elemental silicon and hydrogen; indeed ultrapure silicon is commercially produced by the pyrolysis of silane. While the thermal decomposition of alkanes starts by the breaking of a C–H or C–C bond and the formation of radical intermediates, polysilanes decompose by eliminating silylenes :SiH
2 or :SiHR, as the activation energy of this process (~210 kJ/mol) is much less than the Si–Si and Si–H bond energies. While pure silanes do not react with pure water or dilute acids, traces of alkali catalyse immediate hydrolysis to hydrated silicon dioxide. If the reaction is carried out in methanol, controlled solvolysis results in the products SiH
2(OMe)
2, SiH(OMe)
3, and Si(OMe)
4. The Si–H bond also adds to alkenes, a reaction which proceeds slowly and speeds up with increasing substitution of the silane involved. At 450 °C, silane participates in an addition reaction with acetone, as well as a ring-opening reaction with ethylene oxide. Direct reaction of the silanes with chlorine or bromine results in explosions at room temperature, but the reaction of silane with bromine at −80 °C is controlled and yields bromosilane and dibromosilane. The monohalosilanes may be formed by reacting silane with the appropriate hydrogen halide with an Al
2X
6 catalyst, or by reacting silane with a solid silver halide in a heated flow reactor:[29]
- SiH
4 + 2 AgCl 260 °C→ SiH
3Cl + HCl + 2 Ag
Among the derivatives of silane, iodosilane (SiH
3I) and potassium silanide (KSiH
3) are very useful synthetic intermediates in the production of more complicated silicon-containing compounds: the latter is a colourless crystalline ionic solid containing K+ cations and SiH−
3 anions in the NaCl structure, and is made by the reduction of silane by potassium metal.[67] Additionally, the reactive hypervalent species SiH−
5 is also known.[29] With suitable organic substituents it is possible to produce stable polysilanes: they have surprisingly high electric conductivities, arising from sigma delocalisation of the electrons in the chain.[68]
Halides[edit]
Silicon and silicon carbide readily react with all four stable halogens, forming the colourless, reactive, and volatile silicon tetrahalides[69] Silicon tetrafluoride also may be made by fluorinating the other silicon halides, and is produced by the attack of hydrofluoric acid on glass.[70] Heating two different tetrahalides together also produces a random mixture of mixed halides, which may also be produced by halogen exchange reactions. The melting and boiling points of these species usually rise with increasing atomic weight, though there are many exceptions: for example, the melting and boiling points drop as one passes from SiFBr
3 through SiFClBr
2 to SiFCl
2Br. The shift from the hypoelectronic elements in Group 13 and earlier to the Group 14 elements is illustrated by the change from an infinite ionic structure in aluminium fluoride to a lattice of simple covalent silicon tetrafluoride molecules, as dictated by the lower electronegativity of aluminium than silicon, the stoichiometry (the +4 oxidation state being too high for true ionicity), and the smaller size of the silicon atom compared to the aluminium atom.[69]
Silicon tetrachloride is manufactured on a huge scale as a precursor to the production of pure silicon, silicon dioxide, and some silicon esters.[69] The silicon tetrahalides hydrolyse readily in water, unlike the carbon tetrahalides, again because of the larger size of the silicon atom rendering it more open to nucleophilic attack and the ability of the silicon atom to expand its octet which carbon lacks.[70] The reaction of silicon tetrafluoride with excess hydrofluoric acid produces the octahedral hexafluorosilicate anion SiF2−
6.[70]
Analogous to the silanes, halopolysilanes Si
nX
2n + 2 also are known. While catenation in carbon compounds is maximised in the hydrogen compounds rather than the halides, the opposite is true for silicon, so that the halopolysilanes are known up to at least Si
14F
30, Si
6Cl
14, and Si
4Br
10. A suggested explanation for this phenomenon is the compensation for the electron loss of silicon to the more electronegative halogen atoms by pi backbonding from the filled pπ orbitals on the halogen atoms to the empty dπ orbitals on silicon: this is similar to the situation of carbon monoxide in metal carbonyl complexes and explains their stability. These halopolysilanes may be produced by comproportionation of silicon tetrahalides with elemental silicon, or by condensation of lighter halopolysilanes (trimethylammonium being a useful catalyst for this reaction).[69]
Silica[edit]
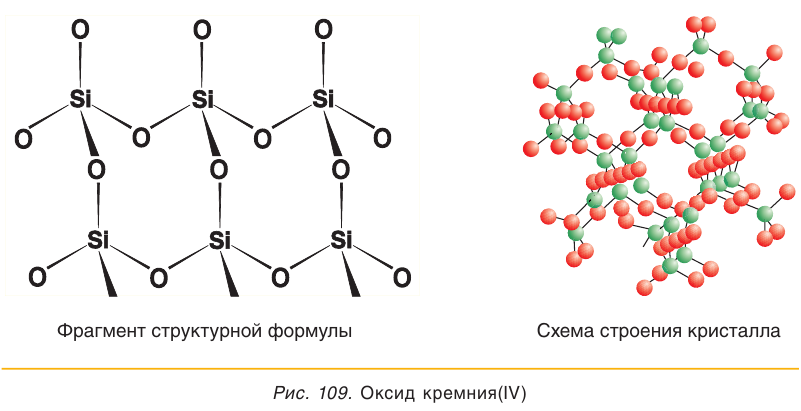
Silicon dioxide (SiO
2), also known as silica, is one of the best-studied compounds, second only to water. Twelve different crystal modifications of silica are known, the most common being α-quartz, a major constituent of many rocks such as granite and sandstone. It also is known to occur in a pure form as rock crystal; impure forms are known as rose quartz, smoky quartz, morion, amethyst, and citrine. Some poorly crystalline forms of quartz are also known, such as chalcedony, chrysoprase, carnelian, agate, onyx, jasper, heliotrope, and flint. Other modifications of silicon dioxide are known in some other minerals such as tridymite and cristobalite, as well as the much less common coesite and stishovite. Biologically generated forms are also known as kieselguhr and diatomaceous earth. Vitreous silicon dioxide is known as tektites, and obsidian, and rarely as lechatelierite. Some synthetic forms are known as keatite. Opals are composed of complicated crystalline aggregates of partially hydrated silicon dioxide.[71]
-
Quartz
-
Agate
-
Tridymite
-
Cristobalite
-
Coesite
Most crystalline forms of silica are made of infinite arrangements of SiO tetrahedra (with Si at the center) connected at their corners, with each oxygen atom linked to two silicon atoms. In the thermodynamically stable room-temperature form, α-quartz, these tetrahedra are linked in intertwined helical chains with two different Si–O distances (159.7 and 161.7 pm) with a Si–O–Si angle of 144°. These helices can be either left- or right-handed, so that individual α-quartz crystals are optically active. At 537 °C, this transforms quickly and reversibly into the similar β-quartz, with a change of the Si–O–Si angle to 155° but a retention of handedness. Further heating to 867 °C results in another reversible phase transition to β-tridymite, in which some Si–O bonds are broken to allow for the arrangement of the SiO tetrahedra into a more open and less dense hexagonal structure. This transition is slow and hence tridymite occurs as a metastable mineral even below this transition temperature; when cooled to about 120 °C it quickly and reversibly transforms by slight displacements of individual silicon and oxygen atoms to α-tridymite, similarly to the transition from α-quartz to β-quartz. β-tridymite slowly transforms to cubic β-cristobalite at about 1470 °C, which once again exists metastably below this transition temperature and transforms at 200–280 °C to α-cristobalite via small atomic displacements. β-cristobalite melts at 1713 °C; the freezing of silica from the melt is quite slow and vitrification, or the formation of a glass, is likely to occur instead. In vitreous silica, the SiO tetrahedra remain corner-connected, but the symmetry and periodicity of the crystalline forms are lost. Because of the slow conversions between these three forms, it is possible upon rapid heating to melt β-quartz (1550 °C) or β-tridymite (1703 °C). Silica boils at approximately 2800 °C. Other high-pressure forms of silica are known, such as coesite and stishovite: these are known in nature, formed under the shock pressure of a meteorite impact and then rapidly quenched to preserve the crystal structure. Similar melting and cooling of silica occurs following lightning strikes, forming glassy lechatelierite. W-silica is an unstable low-density form involving SiO tetrahedra sharing opposite edges instead of corners, forming parallel chains similarly to silicon disulfide (SiS
2) and silicon diselenide (SiSe
2): it quickly returns to forming amorphous silica with heat or traces of water[72]
Condensed polysilicic acid
Silica is rather inert chemically. It is not attacked by any acids other than hydrofluoric acid. However, it slowly dissolves in hot concentrated alkalis, and does so rather quickly in fused metal hydroxides or carbonates, to give metal silicates. Among the elements, it is attacked only by fluorine at room temperature to form silicon tetrafluoride: hydrogen and carbon also react, but require temperatures over 1000 °C to do so. Silica nevertheless reacts with many metal and metalloid oxides to form a wide variety of compounds important in the glass and ceramic industries above all, but also have many other uses: for example, sodium silicate is often used in detergents due to its buffering, saponifying, and emulsifying properties[72]
Silicic acids[edit]
Adding water to silica drops its melting point by around 800 °C due to the breaking of the structure by replacing Si–O–Si linkages with terminating Si–OH groups. Increasing water concentration results in the formation of hydrated silica gels and colloidal silica dispersions. Many hydrates and silicic acids exist in the most dilute of aqueous solutions, but these are rather insoluble and quickly precipitate and condense and cross-link to form various polysilicic acids of variable combinations following the formula [SiO
x(OH)
4−2x]
n, similar to the behaviour of boron, aluminium, and iron, among other elements. Hence, although some simple silicic acids have been identified in dilute solutions, such as orthosilicic acid Si(OH)
4 and metasilicic acid SiO(OH)
2, none of these are likely to exist in the solid state.[72]
Silicate minerals[edit]
| CN 4 | LiI (59) |
BeII (27) | AlIII (39) | SiIV (26) | |
|---|---|---|---|---|---|
| CN 6 | NaI (102) | MgII (72) | AlIII (54) | TiIV (61) | FeII (78) |
| CN 8 | KI (151) | CaII (112) | |||
| CN 12 | KI (164) |
About 95% of the Earth’s crustal rocks are made of silica or silicate and aluminosilicate minerals, as reflected in oxygen, silicon, and aluminium being the three most common elements in the crust (in that order).[73] Measured by mass, silicon makes up 27.7% of the Earth’s crust.[74] Pure silicon crystals are very rarely found in nature, but notable exceptions are crystals as large as to 0.3 mm across found during sampling gases from the Kudriavy volcano on Iturup, one of the Kuril Islands.[75][76]
Silicate and aluminosilicate minerals have many different structures and varying stoichiometry, but they may be classified following some general principles. Tetrahedral SiO units are common to almost all these compounds, either as discrete structures, or combined into larger units by the sharing of corner oxygen atoms. These may be divided into neso-silicates (discrete SiO units) sharing no oxygen atoms, soro-silicates (discrete Si units) sharing one, cyclo-silicates (closed ring structures) and ino-silicates (continuous chain or ribbon structures) both sharing two, phyllo-silicates (continuous sheets) sharing three, and tecto-silicates (continuous three-dimensional frameworks) sharing four. The lattice of oxygen atoms that results is usually close-packed, or close to it, with the charge being balanced by other cations in various different polyhedral sites according to size.[77]
The orthosilicates MII
2SiO
4 (M = Be, Mg, Mn, Fe, Zn) and ZrSiO
4 are neso-silicates. Be
2SiO
4 (phenacite) is unusual as both BeII and SiIV occupy tetrahedral four-coordinated sites; the other divalent cations instead occupy six-coordinated octahedral sites and often isomorphously replace each other as in olivine, (Mg,Fe,Mn)
2SiO
4. Zircon, ZrSiO
4, demands eight-coordination of the ZrIV cations due to stoichiometry and because of their larger ionic radius (84 pm). Also significant are the garnets, [MII
3MIII
2(SiO
4)
3], in which the divalent cations (e.g. Ca, Mg, Fe) are eight-coordinated and the trivalent ones are six-coordinated (e.g. Al, Cr, Fe). Regular coordination is not always present: for example, it is not found in Ca
2SiO
4, which mixes six- and eight-coordinate sites for CaII. Soro-silicates, involving discrete double or triple tetrahedral units, are quite rare: metasilicates involving cyclic «[(SiOn
3)]2n−» units of corner-abutting tetrahedra forming a polygonal ring are also known.[73]
Chain metasilicates, {SiO2−
3}
∞, form by corner-sharing of an indefinite chain of linked SiO tetrahedra. Many differences arise due to the differing repeat distances of conformation across the line of tetrahedra. A repeat distance of two is most common, as in most pyroxene minerals, but repeat distances of one, three, four, five, six, seven, nine, and twelve are also known. These chains may then link across each other to form double chains and ribbons, as in the asbestos minerals, involving repeated chains of cyclic tetrahedron rings.[73]
A typical zeolite structure
Layer silicates, such as the clay minerals and the micas, are very common, and often are formed by horizontal cross-linking of metasilicate chains or planar condensation of smaller units. An example is kaolinite [Al
2(OH)
4Si
2O
5]; in many of these minerals cation and anion replacement is common, so that for example tetrahedral SiIV may be replaced by AlIII, octahedral AlIII by MgII, and OH−
by F−
. Three-dimensional framework aluminosilicates are structurally very complex; they may be conceived of as starting from the SiO
2 structure, but having replaced up to one-half of the SiIV atoms with AlIII, they require more cations to be included in the structure to balance charge. Examples include feldspars (the most abundant minerals on the Earth), zeolites, and ultramarines. Many feldspars can be thought of as forming part of the ternary system NaAlSi
3O
8–KAlSi
3O
8–CaAl
2Si
2O
8. Their lattice is destroyed by high pressure prompting AlIII to undergo six-coordination rather than four-coordination, and this reaction destroying feldspars may be a reason for the Mohorovičić discontinuity, which would imply that the crust and mantle have the same chemical composition, but different lattices, although this is not a universally held view. Zeolites have many polyhedral cavities in their frameworks (truncated cuboctahedra being most common, but other polyhedra also are known as zeolite cavities), allowing them to include loosely bound molecules such as water in their structure. Ultramarines alternate silicon and aluminium atoms and include a variety of other anions such as Cl−, SO2−
4, and S2−
2, but are otherwise similar to the feldspars.[73]
Other inorganic compounds[edit]
Silicon disulfide (SiS
2) is formed by burning silicon in gaseous sulfur at 100 °C; sublimation of the resulting compound in nitrogen results in white, flexible long fibers reminiscent of asbestos with a structure similar to W-silica. This melts at 1090 °C and sublimes at 1250 °C; at high temperature and pressure this transforms to a crystal structure analogous to cristobalite. However, SiS
2 lacks the variety of structures of SiO
2, and quickly hydrolyses to silica and hydrogen sulfide. It is also ammonolysed quickly and completely by liquid ammonia as follows to form an imide:[60]
- SiS
2 + 4 NH
3 → Si(NH)
2 + 2 NH
4SH
It reacts with the sulfides of sodium, magnesium, aluminium, and iron to form metal thiosilicates: reaction with ethanol results in tetraethylsilicate Si(OEt)
4 and hydrogen sulfide. Ethylsilicate is useful as its controlled hydrolysis produces adhesive or film-like forms of silica. Reacting hydrogen sulfide with silicon tetrahalides yields silicon thiohalides such as S(SiCl)
3, cyclic Cl
2Si(μ-S)
2SiCl
2, and crystalline (SiSCl
2)
4. Despite the double bond rule, stable organosilanethiones RR’Si=S have been made thanks to the stabilising mechanism of intermolecular coordination via an amine group.[78]
Silicon nitride, Si
3N
4, may be formed by directly reacting silicon with nitrogen above 1300 °C, but a more economical means of production is by heating silica and coke in a stream of nitrogen and hydrogen gas at 1500 °C. It would make a promising ceramic if not for the difficulty of working with and sintering it: chemically, it is near-totally inert, and even above 1000 °C it keeps its strength, shape, and continues to be resistant to wear and corrosion. It is very hard (9 on the Mohs hardness scale), dissociates only at 1900 °C at 1 atm, and is quite dense (density 3.185 g/cm3), because of its compact structure similar to that of phenacite (Be
2SiO
4). A similar refractory material is Si
2N
2O, formed by heating silicon and silica at 1450 °C in an argon stream containing 5% nitrogen gas, involving 4-coordinate silicon and 3-coordinate nitrogen alternating in puckered hexagonal tilings interlinked by non-linear Si–O–Si linkages to each other.[78]
Reacting silyl halides with ammonia or alkylammonia derivatives in the gaseous phase or in ethanolic solution produces various volatile silylamides, which are silicon analogues of the amines:[78]
- 3 SiH
3Cl + 4 NH
3 → N(SiH
3)
3 + 3 NH
4Cl - SiH
3Br + 2 Me
2NH → SiH
3NMe
2 + Me
2NH
2Br - 4 SiH
3I + 5 N
2H
4 → (SiH
3)
2NN(SiH
3)
2 + 4 N
2H
5I
Many such compounds have been prepared, the only known restriction being that the nitrogen is always tertiary, and species containing the SiH–NH group are unstable at room temperature. The stoichiometry around the nitrogen atom in compounds such as N(SiH
3)
3 is planar. Similarly, trisilylamines are weaker as ligands than their carbon analogues, the tertiary amines, although substitution of some SiH
3 groups by CH
3 groups mitigates this weakness. For example, N(SiH
3)
3 does not form an adduct with BH
3 at all, while MeN(SiH
3)
2 and Me
2NSiH
3 form adducts at low temperatures that decompose upon warming. Some silicon analogues of imines, with a Si=N double bond, are known: the first found was But2Si=N–SiBut3, which was discovered in 1986.[78]
Silicon carbide (SiC) was first made by Edward Goodrich Acheson in 1891, who named it carborundum to reference its intermediate hardness and abrasive power between diamond (an allotrope of carbon) and corundum (aluminium oxide). He soon founded a company to manufacture it, and today about one million tonnes are produced each year.[79] Silicon carbide exists in about 250 crystalline forms.[80] The polymorphism of SiC is characterized by a large family of similar crystalline structures called polytypes. They are variations of the same chemical compound that are identical in two dimensions and differ in the third. Thus they can be viewed as layers stacked in a certain sequence.[81] It is made industrially by reduction of quartz sand with excess coke or anthracite at 2000–2500 °C in an electric furnace:[79]
- SiO
2 + 2 C → Si + 2 CO - Si + C → SiC
It is the most thermally stable binary silicon compound, only decomposing through loss of silicon starting from around 2700 °C. It is resistant to most aqueous acids, phosphoric acid being an exception. It forms a protective layer of silicon dioxide on the surface and hence only oxidises appreciably in air above 1000 °C; removal of this layer by molten hydroxides or carbonates leads to quick oxidation. Silicon carbide is rapidly attacked by chlorine gas, which forms SiCl
4 and carbon at 100 °C and SiCl
4 and CCl
4 at 1000 °C. It is mostly used as an abrasive and a refractory material, as it is chemically stable and very strong, and it fractures to form a very sharp cutting edge. It is also useful as an intrinsic semiconductor, as well as an extrinsic semiconductor upon being doped.[79] In its diamond-like behavior it serves as an illustration of the chemical similarity between carbon and silicon.[82]
Organosilicon compounds[edit]
A hydrosilylation reaction, in which Si–H is added to an unsaturated substrate
Because the Si–C bond is close in strength to the C–C bond, organosilicon compounds tend to be markedly thermally and chemically stable. For example, tetraphenylsilane (SiPh
4) may be distilled in air even at its boiling point of 428 °C, and so may its substituted derivatives Ph
3SiCl and Ph
2SiCl
2, which boil at 378 °C and 305 °C respectively. Furthermore, since carbon and silicon are chemical congeners, organosilicon chemistry shows some significant similarities with carbon chemistry, for example in the propensity of such compounds for catenation and forming multiple bonds.[82] However, significant differences also arise: since silicon is more electropositive than carbon, bonds to more electronegative elements are generally stronger with silicon than with carbon, and vice versa. Thus the Si–F bond is significantly stronger than even the C–F bond and is one of the strongest single bonds, while the Si–H bond is much weaker than the C–H bond and is readily broken. Furthermore, the ability of silicon to expand its octet is not shared by carbon, and hence some organosilicon reactions have no organic analogues. For example, nucleophilic attack on silicon does not proceed by the SN2 or SN1 processes, but instead goes through a negatively charged true pentacoordinate intermediate and appears like a substitution at a hindered tertiary atom. This works for silicon, unlike for carbon, because the long Si–C bonds reduce the steric hindrance and there are no geometric constraints for nucleophilic attack, unlike for example a C–O σ* antibonding orbital. Nevertheless, despite these differences, the mechanism is still often called «SN2 at silicon» for simplicity.[83]
One of the most useful silicon-containing groups is trimethylsilyl, Me
3Si–. The Si–C bond connecting it to the rest of the molecule is reasonably strong, allowing it to remain while the rest of the molecule undergoes reactions, but is not so strong that it cannot be removed specifically when needed, for example by the fluoride ion, which is a very weak nucleophile for carbon compounds but a very strong one for organosilicon compounds. It may be compared to acidic protons; while trisilylmethyl is removed by hard nucleophiles instead of bases, both removals usually promote elimination. As a general rule, while saturated carbon is best attacked by nucleophiles that are neutral compounds, those based on nonmetals far down on the periodic table (e.g. sulfur, selenium, or iodine), or even both, silicon is best attacked by charged nucleophiles, particularly those involving such highly electronegative nonmetals as oxygen, fluorine, or chlorine. For example, enolates react at the carbon in haloalkanes, but at the oxygen in silyl chlorides; and when trimethylsilyl is removed from an organic molecule using hydroxide as a nucleophile, the product of the reaction is not the silanol as one would expect from using carbon chemistry as an analogy, because the siloxide is strongly nucleophilic and attacks the original molecule to yield the silyl ether hexamethyldisiloxane, (Me
3Si)
2O. Conversely, while the SN2 reaction is mostly unaffected by the presence of a partial positive charge (δ+) at the carbon, the analogous «SN2″ reaction at silicon is so affected. Thus, for example, the silyl triflates are so electrophilic that they react 108 to 109 times faster than silyl chlorides with oxygen-containing nucleophiles. Trimethylsilyl triflate is in particular a very good Lewis acid and is used to convert carbonyl compounds to acetals and silyl enol ethers, reacting them together analogously to the aldol reaction.[83]
Si–C bonds are commonly formed in three ways. In the laboratory, preparation is often carried out in small quantities by reacting tetrachlorosilane (silicon tetrachloride) with organolithium, Grignard, or organoaluminium reagents, or by catalytic addition of Si–H across C=C double bonds. The second route has the drawback of not being applicable to the most important silanes, the methyl and phenyl silanes. Organosilanes are made industrially by directly reacting alkyl or aryl halides with silicon with 10% by weight metallic copper as a catalyst. Standard organic reactions suffice to produce many derivatives; the resulting organosilanes are often significantly more reactive than their carbon congeners, readily undergoing hydrolysis, ammonolysis, alcoholysis, and condensation to form cyclic oligomers or linear polymers.[82]
Silicone polymers[edit]
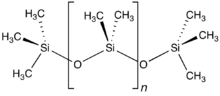
The word «silicone» was first used by Frederic Kipping in 1901. He invented the word to illustrate the similarity of chemical formulae between Ph
2SiO and benzophenone, Ph
2CO, although he also stressed the lack of chemical resemblance due to the polymeric structure of Ph
2SiO, which is not shared by Ph
2CO.[82]
Silicones may be considered analogous to mineral silicates, in which the methyl groups of the silicones correspond to the isoelectronic <O−
of the silicates.[82] They are quite stable to extreme temperatures, oxidation, and water, and have useful dielectric, antistick, and antifoam properties. Furthermore, they are resistant over long periods of time to ultraviolet radiation and weathering, and are inert physiologically. They are fairly unreactive, but do react with concentrated solutions bearing the hydroxide ion and fluorinating agents, and occasionally, may even be used as mild reagents for selective syntheses. For example, (Me
3Si)
2O is valuable for the preparation of derivatives of molybdenum and tungsten oxyhalides, converting a tungsten hexachloride suspension in dichloroethane solution quantitatively to WOCl
4 in under an hour at room temperature, and then to yellow WO
2C
2 at 100 °C in light petroleum at a yield of 95% overnight.[82]
Occurrence[edit]
Silicon is the eighth most abundant element in the universe, coming after hydrogen, helium, carbon, nitrogen, oxygen, iron, and neon. These abundances are not replicated well on Earth due to substantial separation of the elements taking place during the formation of the Solar System. Silicon makes up 27.2% of the Earth’s crust by weight, second only to oxygen at 45.5%, with which it always is associated in nature. Further fractionation took place in the formation of the Earth by planetary differentiation: Earth’s core, which makes up 31.5% of the mass of the Earth, has approximate composition Fe
25Ni
2Co
0.1S
3; the mantle makes up 68.1% of the Earth’s mass and is composed mostly of denser oxides and silicates, an example being olivine, (Mg,Fe)
2SiO
4; while the lighter siliceous minerals such as aluminosilicates rise to the surface and form the crust, making up 0.4% of the Earth’s mass.[84][85]
The crystallisation of igneous rocks from magma depends on a number of factors; among them are the chemical composition of the magma, the cooling rate, and some properties of the individual minerals to be formed, such as lattice energy, melting point, and complexity of their crystal structure. As magma is cooled, olivine appears first, followed by pyroxene, amphibole, biotite mica, orthoclase feldspar, muscovite mica, quartz, zeolites, and finally, hydrothermal minerals. This sequence shows a trend toward increasingly complex silicate units with cooling, and the introduction of hydroxide and fluoride anions in addition to oxides. Many metals may substitute for silicon. After these igneous rocks undergo weathering, transport, and deposition, sedimentary rocks like clay, shale, and sandstone are formed. Metamorphism also may occur at high temperatures and pressures, creating an even vaster variety of minerals.[84]
There are four sources for silicon fluxes into the ocean include chemical weathering of continental rocks, river transport, dissolution of continental terrigenous silicates, and through the reaction between submarine basalts and hydrothermal fluid which release dissolved silicon. All four of these fluxes are interconnected in the ocean’s biogeochemical cycle as they all were initially formed from the weathering of Earth’s crust.[86]
Approximately 300–900 megatonnes of Aeolian dust is deposited into the world’s oceans each year. Of that value, 80–240 megatonnes are in the form of particulate silicon. The total amount of particulate silicon deposition into the ocean is still less than the amount of silicon influx into the ocean via riverine transportation.[87] Aeolian inputs of particulate lithogenic silicon into the North Atlantic and Western North Pacific oceans are the result of dust settling on the oceans from the Sahara and Gobi Desert, respectively.[86] Riverine transports are the major source of silicon influx into the ocean in coastal regions, while silicon deposition in the open ocean is greatly influenced by the settling of Aeolian dust.[87]
Production[edit]
Silicon of 96–99% purity is made by reducing quartzite or sand with highly pure coke. The reduction is carried out in an electric arc furnace, with an excess of SiO
2 used to stop silicon carbide (SiC) from accumulating:[29]
- SiO
2 + 2 C → Si + 2 CO - 2 SiC + SiO
2 → 3 Si + 2 CO
This reaction, known as carbothermal reduction of silicon dioxide, usually is conducted in the presence of scrap iron with low amounts of phosphorus and sulfur, producing ferrosilicon.[29] Ferrosilicon, an iron-silicon alloy that contains varying ratios of elemental silicon and iron, accounts for about 80% of the world’s production of elemental silicon, with China, the leading supplier of elemental silicon, providing 4.6 million tonnes (or 2/3rds of world output) of silicon, most of it in the form of ferrosilicon. It is followed by Russia (610,000 t), Norway (330,000 t), Brazil (240,000 t), and the United States (170,000 t).[88] Ferrosilicon is primarily used by the iron and steel industry (see below) with primary use as alloying addition in iron or steel and for de-oxidation of steel in integrated steel plants.[29]
Another reaction, sometimes used, is aluminothermal reduction of silicon dioxide, as follows:[89]
- 3 SiO
2 + 4 Al → 3 Si + 2 Al
2O
3
Leaching powdered 96–97% pure silicon with water results in ~98.5% pure silicon, which is used in the chemical industry. However, even greater purity is needed for semiconductor applications, and this is produced from the reduction of tetrachlorosilane (silicon tetrachloride) or trichlorosilane. The former is made by chlorinating scrap silicon and the latter is a byproduct of silicone production. These compounds are volatile and hence can be purified by repeated fractional distillation, followed by reduction to elemental silicon with very pure zinc metal as the reducing agent. The spongy pieces of silicon thus produced are melted and then grown to form cylindrical single crystals, before being purified by zone refining. Other routes use the thermal decomposition of silane or tetraiodosilane (SiI
4). Another process used is the reduction of sodium hexafluorosilicate, a common waste product of the phosphate fertilizer industry, by metallic sodium: this is highly exothermic and hence requires no outside energy source. Hyperfine silicon is made at a higher purity than almost any other material: transistor production requires impurity levels in silicon crystals less than 1 part per 1010, and in special cases impurity levels below 1 part per 1012 are needed and attained.[29]
Silicon nanostructures can directly be produced from silica sand using conventional metalothermic processes, or the combustion synthesis approach. Such nanostructured silicon materials can be used in various functional applications including the anode of lithium ion batteries (LIBs) or phorocatalytic applications.[90]
Applications[edit]
Compounds[edit]
Most silicon is used industrially without being purified, and indeed, often with comparatively little processing from its natural form. More than 90% of the Earth’s crust is composed of silicate minerals, which are compounds of silicon and oxygen, often with metallic ions when negatively charged silicate anions require cations to balance the charge. Many of these have direct commercial uses, such as clays, silica sand, and most kinds of building stone. Thus, the vast majority of uses for silicon are as structural compounds, either as the silicate minerals or silica (crude silicon dioxide). Silicates are used in making Portland cement (made mostly of calcium silicates) which is used in building mortar and modern stucco, but more importantly, combined with silica sand, and gravel (usually containing silicate minerals such as granite), to make the concrete that is the basis of most of the very largest industrial building projects of the modern world.[91]
Silica is used to make fire brick, a type of ceramic. Silicate minerals are also in whiteware ceramics, an important class of products usually containing various types of fired clay minerals (natural aluminium phyllosilicates). An example is porcelain, which is based on the silicate mineral kaolinite. Traditional glass (silica-based soda-lime glass) also functions in many of the same ways, and also is used for windows and containers. In addition, specialty silica based glass fibers are used for optical fiber, as well as to produce fiberglass for structural support and glass wool for thermal insulation.
Silicones often are used in waterproofing treatments, molding compounds, mold-release agents, mechanical seals, high temperature greases and waxes, and caulking compounds. Silicone is also sometimes used in breast implants, contact lenses, explosives and pyrotechnics.[92] Silly Putty was originally made by adding boric acid to silicone oil.[93] Other silicon compounds function as high-technology abrasives and new high-strength ceramics based upon silicon carbide. Silicon is a component of some superalloys.
Alloys[edit]
Elemental silicon is added to molten cast iron as ferrosilicon or silicocalcium alloys to improve performance in casting thin sections and to prevent the formation of cementite where exposed to outside air. The presence of elemental silicon in molten iron acts as a sink for oxygen, so that the steel carbon content, which must be kept within narrow limits for each type of steel, can be more closely controlled. Ferrosilicon production and use is a monitor of the steel industry, and although this form of elemental silicon is grossly impure, it accounts for 80% of the world’s use of free silicon. Silicon is an important constituent of electrical steel, modifying its resistivity and ferromagnetic properties.
The properties of silicon may be used to modify alloys with metals other than iron. «Metallurgical grade» silicon is silicon of 95–99% purity. About 55% of the world consumption of metallurgical purity silicon goes for production of aluminium-silicon alloys (silumin alloys) for aluminium part casts, mainly for use in the automotive industry. Silicon’s importance in aluminium casting is that a significantly high amount (12%) of silicon in aluminium forms a eutectic mixture which solidifies with very little thermal contraction. This greatly reduces tearing and cracks formed from stress as casting alloys cool to solidity. Silicon also significantly improves the hardness and thus wear-resistance of aluminium.[94][95]
Electronics[edit]
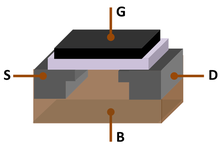
Silicon wafer with mirror finish
Most elemental silicon produced remains as a ferrosilicon alloy, and only approximately 20% is refined to metallurgical grade purity (a total of 1.3–1.5 million metric tons/year). An estimated 15% of the world production of metallurgical grade silicon is further refined to semiconductor purity.[95] This typically is the «nine-9» or 99.9999999% purity,[96] nearly defect-free single crystalline material.[97]
Monocrystalline silicon of such purity is usually produced by the Czochralski process, and is used to produce silicon wafers used in the semiconductor industry, in electronics, and in some high-cost and high-efficiency photovoltaic applications.[98] Pure silicon is an intrinsic semiconductor, which means that unlike metals, it conducts electron holes and electrons released from atoms by heat; silicon’s electrical conductivity increases with higher temperatures. Pure silicon has too low a conductivity (i.e., too high a resistivity) to be used as a circuit element in electronics. In practice, pure silicon is doped with small concentrations of certain other elements, which greatly increase its conductivity and adjust its electrical response by controlling the number and charge (positive or negative) of activated carriers. Such control is necessary for transistors, solar cells, semiconductor detectors, and other semiconductor devices used in the computer industry and other technical applications.[99] In silicon photonics, silicon may be used as a continuous wave Raman laser medium to produce coherent light.[100]
In common integrated circuits, a wafer of monocrystalline silicon serves as a mechanical support for the circuits, which are created by doping and insulated from each other by thin layers of silicon oxide, an insulator that is easily produced on Si surfaces by processes of thermal oxidation or local oxidation (LOCOS), which involve exposing the element to oxygen under the proper conditions that can be predicted by the Deal–Grove model. Silicon has become the most popular material for both high power semiconductors and integrated circuits because it can withstand the highest temperatures and greatest electrical activity without suffering avalanche breakdown (an electron avalanche is created when heat produces free electrons and holes, which in turn pass more current, which produces more heat). In addition, the insulating oxide of silicon is not soluble in water, which gives it an advantage over germanium (an element with similar properties which can also be used in semiconductor devices) in certain fabrication techniques.[101]
Monocrystalline silicon is expensive to produce, and is usually justified only in production of integrated circuits, where tiny crystal imperfections can interfere with tiny circuit paths. For other uses, other types of pure silicon may be employed. These include hydrogenated amorphous silicon and upgraded metallurgical-grade silicon (UMG-Si) used in the production of low-cost, large-area electronics in applications such as liquid crystal displays and of large-area, low-cost, thin-film solar cells. Such semiconductor grades of silicon are either slightly less pure or polycrystalline rather than monocrystalline, and are produced in comparable quantities as the monocrystalline silicon: 75,000 to 150,000 metric tons per year. The market for the lesser grade is growing more quickly than for monocrystalline silicon. By 2013, polycrystalline silicon production, used mostly in solar cells, was projected to reach 200,000 metric tons per year, while monocrystalline semiconductor grade silicon was expected to remain less than 50,000 tons per year.[95]
Quantum dots[edit]
Silicon quantum dots are created through the thermal processing of hydrogen silsesquioxane into nanocrystals ranging from a few nanometers to a few microns, displaying size dependent luminescent properties.[102][103] The nanocrystals display large Stokes shifts converting photons in the ultra-violet range to photons in the visible or infrared, depending on the particle size, allowing for applications in quantum dot displays and luminescent solar concentrators due to their limited self absorption. A benefit of using silicon based quantum dots over cadmium or indium is the non-toxic, metal-free nature of silicon.[104][105]
Another application of silicon quantum dots is for sensing of hazardous materials. The sensors take advantage of the luminescent properties of the quantum dots through quenching of the photoluminescence in the presence of the hazardous substance.[106] There are many methods used for hazardous chemical sensing with a few being electron transfer, fluorescence resonance energy transfer, and photocurrent generation.[107] Electron transfer quenching occurs when the lowest unoccupied molecular orbital (LUMO) is slightly lower in energy than the conduction band of the quantum dot, allowing for the transfer electrons between the two, preventing recombination of the holes and electrons within the nanocrystals. The effect can also be achieved in reverse with a donor molecule having its highest occupied molecular orbital (HOMO) slightly higher than a valence band edge of the quantum dot, allowing electrons to transfer between them, filling the holes and preventing recombination. Fluorescence resonance energy transfer occurs when a complex forms between the quantum dot and a quencher molecule. The complex will continue to absorb light but when the energy is converted to the ground state it does not release a photon, quenching the material. The third method uses different approach by measuring the photocurrent emitted by the quantum dots instead of monitoring the photoluminescent display. If the concentration of the desired chemical increases then the photocurrent given off by the nanocrystals will change in response.[108]
Biological role[edit]
A diatom, enclosed in a silica cell wall
Although silicon is readily available in the form of silicates, very few organisms use it directly. Diatoms, radiolaria, and siliceous sponges use biogenic silica as a structural material for their skeletons. Some plants accumulate silica in their tissues and require silicon for their growth, for example rice. Silicon may be taken up by plants as orthosilicic acid (also known as monosilicic acid) and transported through the xylem, where it forms amorphous complexes with components of the cell wall. This has been shown to improve cell wall strength and structural integrity in some plants, thereby reducing insect herbivory and pathogenic infections. In certain plants, silicon may also upregulate the production of volatile organic compounds and phytohormones which play a significant role in plant defense mechanisms.[109][110][111] In more advanced plants, the silica phytoliths (opal phytoliths) are rigid microscopic bodies occurring in the cell.[112][113][110]
Several horticultural crops are known to protect themselves against fungal plant pathogens with silica, to such a degree that fungicide application may fail unless accompanied by sufficient silicon nutrition. Silicaceous plant defense molecules activate some phytoalexins, meaning some of them are signalling substances producing acquired immunity. When deprived, some plants will substitute with increased production of other defensive substances.[110]
Life on Earth is largely composed of carbon, but astrobiology considers that extraterrestrial life may have other hypothetical types of biochemistry. Silicon is considered an alternative to carbon, as it can create complex and stable molecules with four covalent bonds, required for a DNA-analog, and it is available in large quantities.[114]
Marine microbial influences[edit]
Diatoms uses silicon in the biogenic silica (BSIO
2) form,[115] which is taken up by the silicon transport protein (SIT) to be predominantly used in the cell wall structure as frustules.[116] Silicon enters the ocean in a dissolved form such as silicic acid or silicate.[117] Since diatoms are one of the main users of these forms of silicon, they contribute greatly to the concentration of silicon throughout the ocean. Silicon forms a nutrient-like profile in the ocean due to the diatom productivity in shallow depths.[117] Therefore, less concentration of silicon in the upper ocean and more concentrations of silicon in the deep/lower ocean.
Diatom productivity in the upper ocean contribute to the amount of silicon exported to the lower ocean.[118] When diatom cells are lysed in the upper ocean, their nutrients like, iron, zinc, and silicon, are brought to the lower ocean through a process called marine snow. Marine snow involves the downward transfer of particulate organic matter by vertical mixing of dissolved organic matter.[119] It has been suggested that silicon is considered crucial to diatom productivity and as long as there is silicic acid available for diatoms to use, the diatoms can contribute to other important nutrient concentrations in the deep ocean as well.[120]
In coastal zones, diatoms serve as the major phytoplanktonic organisms and greatly contribute to biogenic silica production. In the open ocean, however, diatoms have a reduced role in global annual silica production. Diatoms in North Atlantic and North Pacific subtropical gyres only contribute about 5-7% of global annual marine silica production. The Southern Ocean produces about one-third of global marine biogenic silica.[86] The Southern Ocean is referred to as having a «biogeochemical divide»[121] since only minuscule amounts of silicon are transported out of this region.
Human nutrition[edit]
There is some evidence that silicon is important to human health for their nail, hair, bone, and skin tissues,[122] for example, in studies that demonstrate that premenopausal women with higher dietary silicon intake have higher bone density, and that silicon supplementation can increase bone volume and density in patients with osteoporosis.[123] Silicon is needed for synthesis of elastin and collagen, of which the aorta contains the greatest quantity in the human body,[124] and has been considered an essential element;[125] nevertheless, it is difficult to prove its essentiality, because silicon is very common, and hence, deficiency symptoms are difficult to reproduce.[126][127]
Silicon is currently under consideration for elevation to the status of a «plant beneficial substance by the Association of American Plant Food Control Officials (AAPFCO).»[128][129]
Safety[edit]
People may be exposed to elemental silicon in the workplace by breathing it in, swallowing it, or having contact with the skin or eye. In the latter two cases, silicon poses a slight hazard as an irritant. It is hazardous if inhaled.[130] The Occupational Safety and Health Administration (OSHA) has set the legal limit for silicon exposure in the workplace as 15 mg/m3 total exposure and 5 mg/m3 respiratory exposure over an eight-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 10 mg/m3 total exposure and 5 mg/m3 respiratory exposure over an eight-hour workday.[131] Inhalation of crystalline silica dust may lead to silicosis, an occupational lung disease marked by inflammation and scarring in the form of nodular lesions in the upper lobes of the lungs.[132]
See also[edit]
- Amorphous silicon
- Black silicon
- Covalent superconductors
- List of countries by silicon production
- List of silicon producers
- Monocrystalline silicon
- Silicon Nanowires (SiNWs)
- Polycrystalline silicon
- Printed silicon electronics
- Silicon tombac
- Silicon Valley
- Silicene
- Transistor
References[edit]
- ^ «Standard Atomic Weights: Silicon». CIAAW. 2009.
- ^ «New Type of Zero-Valent Tin Compound». Chemistry Europe. 27 August 2016.
- ^ Ram, R. S.; et al. (1998). «Fourier Transform Emission Spectroscopy of the A2D–X2P Transition of SiH and SiD» (PDF). J. Mol. Spectr. 190 (2): 341–352. doi:10.1006/jmsp.1998.7582. PMID 9668026.
- ^ Eranna, Golla (2014). Crystal Growth and Evaluation of Silicon for VLSI and ULSI. CRC Press. p. 7. ISBN 978-1-4822-3281-3.
- ^ Magnetic susceptibility of the elements and inorganic compounds, in Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ a b c d Hopcroft, Matthew A.; Nix, William D.; Kenny, Thomas W. (2010). «What is the Young’s Modulus of Silicon?». Journal of Microelectromechanical Systems. 19 (2): 229. doi:10.1109/JMEMS.2009.2039697.
- ^ Weeks, Mary Elvira (1932). «The discovery of the elements: XII. Other elements isolated with the aid of potassium and sodium: beryllium, boron, silicon, and aluminum». Journal of Chemical Education. 9 (8): 1386–1412. Bibcode:1932JChEd…9.1386W. doi:10.1021/ed009p1386.
- ^ Voronkov, M. G. (2007). «Silicon era». Russian Journal of Applied Chemistry. 80 (12): 2190. doi:10.1134/S1070427207120397.
- ^ Kamal 2022
- ^ Cutter, Elizabeth G. (1978). Plant Anatomy. Part 1 Cells and Tissues (2nd ed.). London: Edward Arnold. ISBN 978-0-7131-2639-6.
- ^ «Silicon». Encyclopedia Britannica. Retrieved 22 August 2019.
- ^ In his table of the elements, Lavoisier listed five «salifiable earths» (i.e., ores that could be made to react with acids to produce salts (salis = salt, in Latin): chaux (calcium oxide), magnésie (magnesia, magnesium oxide), baryte (barium sulfate), alumine (alumina, aluminium oxide), and silice (silica, silicon dioxide). About these «elements», Lavoisier speculates: «We are probably only acquainted as yet with a part of the metallic substances existing in nature, as all those which have a stronger affinity to oxygen than carbon possesses, are incapable, hitherto, of being reduced to a metallic state, and consequently, being only presented to our observation under the form of oxyds, are confounded with earths. It is extremely probable that barytes, which we have just now arranged with earths, is in this situation; for in many experiments it exhibits properties nearly approaching to those of metallic bodies. It is even possible that all the substances we call earths may be only metallic oxyds, irreducible by any hitherto known process.» – from Lavoisier (1799). Elements of Chemistry. Translated by Robert Kerr (4 ed.). Edinburgh, Scotland: William Creec. p. 218. (The original passage appears in: Lavoisier (1789). Traité Élémentaire de Chimie. Vol. 1. Paris, France: Cuchet. p. 174.
- ^ a b c d e Greenwood & Earnshaw 1997, p. 328.
- ^ Davy, Humphry (1808). «Electro chemical researches, on the decomposition of the earths; with observations on the metals obtained from the alkaline earths, and on the amalgam procured from ammonia». Philosophical Transactions of the Royal Society of London. W. Bowyer and J. Nichols. 98: 333–370.. On p. 353 Davy coins the name «silicium» : «Had I been so fortunate as to have obtained more certain evidences on this subject, and to have procured the metallic substances I was in search of, I should have proposed for them the names of silicium [silicon], alumium [aluminium], zirconium, and glucium [beryllium].»
- ^ «14 Silicon». Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Gay-Lussac, Joseph Louis; Thénard, Louis Jacques baron (1811). Recherches physico-chimiques, faites sur la pile: sur la préparation chimique et les propriétés du potassium et du sodium; sur la décomposition de l’acide boracique; sur les acides fluorique, muriatique et muriatique oxigéné; sur l’action chimique de la lumière; sur l’analyse végétale et animale, etc (in French). Deterville. pp. 313–314; vol. 2, pp. 55–65.
- ^ Thomson, Thomas; Baldwin, Charles; Blackwood, William; Baldwin, Cradock; Bell & Bradfute, bookseller; Hodges & McArthur, bookseller (1817). A system of chemistry : in four volumes. University of Wisconsin — Madison. London : Printed for Baldwin, Craddock, and Joy, Paternoster-Row; William Blackwood, and Bell and Bradfute, Edinburgh; and Hodges and Macarthur, Dublin. p. 252.: «The base of silica has been usually considered as a metal, and called silicium. But as there is not the smallest evidence for its metallic nature, and as it bears a close resemblance to boron and carbon, it is better to class it along with these bodies, and to give it the name of silicon.»
- ^ See
- Berzelius announced his discovery of silicon («silicium») in: Berzelius, J. (presented: 1823; published: 1824) «Undersökning af flusspatssyran och dess märkvärdigaste föreningar» (Investigation of hydrofluoric acid and of its most noteworthy compounds), Kongliga Vetenskaps-Academiens Handlingar [Proceedings of the Royal Science Academy], 12 : 46–98. The isolation of silicon and its characterization are detailed in the section titled «Flussspatssyrad kisseljords sönderdelning med kalium,» pp. 46–68.
- The above article was printed in German in: J.J. Berzelius (1824) «II. Untersuchungen über Flussspathsäure und deren merkwürdigsten Verbindungen» (II. Investigations of hydrofluoric acid and its most noteworthy compounds), Annalen der Physik, 77: 169–230. The isolation of silicon is detailed in the section titled: «Zersetzung der flussspaths. Kieselerde durch Kalium» (Decomposition of silicate fluoride by potassium), pp. 204–210.
- The above article was reprinted in French in: Berzelius (1824) «Décomposition du fluate de silice par le potassium» (Decomposition of silica fluoride by potassium), Annales de Chimie et de Physique, 27: 337–359.
- Reprinted in English in: «On the mode of obtaining silicium, and on the characters and properties of that substance». The Philosophical Magazine and Journal: Comprehending Various Branches of Science, the Liberal and Fine Arts, Agriculture, Manufactures, and Commerce. Richard Taylor and Company. 65: 254–267. 1825.
- ^ Weeks, Mary Elvira (1932). «The discovery of the elements: XII. Other elements isolated with the aid of potassium and sodium: beryllium, boron, silicon, and aluminum». Journal of Chemical Education. 9 (8): 1386–1412. Bibcode:1932JChEd…9.1386W. doi:10.1021/ed009p1386.
- ^ Voronkov, M.G. (2007). «Silicon era». Russian Journal of Applied Chemistry. 80 (12): 2190. doi:10.1134/S1070427207120397. S2CID 195240638.
- ^ a b Kipping, Frederic Stanley (1937-03-01). «The bakerian lecture organic derivatives of silicon». Proceedings of the Royal Society of London. Series A — Mathematical and Physical Sciences. 159 (896): 139–148. doi:10.1098/rspa.1937.0063.
- ^ Muller, Richard (January 1965). «One hundred years of organosilicon chemistry». Journal of Chemical Education. 42 (1): 41. doi:10.1021/ed042p41. ISSN 0021-9584.
- ^ In 1854, Deville was trying to prepare aluminium metal from aluminium chloride that was heavily contaminated with silicon chloride. Deville used two methods to prepare aluminium: heating aluminium chloride with sodium metal in an inert atmosphere (of hydrogen); and melting aluminum chloride with sodium chloride and then electrolyzing the mixture. In both cases, pure silicon was produced: the silicon dissolved in the molten aluminium, but crystallized upon cooling. Dissolving the crude aluminum in hydrochloric acid revealed flakes of crystallized silicon. See: Henri Sainte-Claire Deville (1854) «Note sur deux procédés de préparation de l’aluminium et sur une nouvelle forme du silicium» (Note on two procedures for the preparation of aluminium and on a new form of silicon), Comptes rendus, 39: 321–326.
Subsequently, Deville obtained crystalline silicon by heating the chloride or fluoride of silicon with sodium metal, isolating the amorphous silicon, then melting the amorphous form with salt and heating the mixture until most of the salt evaporated. See: Sainte-Claire Deville, H. (1855). «Du silicium et du titane (On silicon and titanium)». Comptes rendus. 40: 1034–1036. - ^ «Information on silicon – history, thermodynamic, chemical, physical and electronic properties». Etacude. Retrieved 2021-06-08.
- ^ «Silicon: History». Nautilus.fis.uc.pt. 2011-07-27. Archived from the original on July 27, 2011.
- ^ a b Aufray, B.; Kara, A.; Vizzini, S. B.; Oughaddou, H.; LéAndri, C.; Ealet, B.; Le Lay, G. (2010). «Graphene-like silicon nanoribbons on Ag(110): A possible formation of silicene». Applied Physics Letters. 96 (18): 183102. Bibcode:2010ApPhL..96r3102A. doi:10.1063/1.3419932.
- ^ a b Lalmi, B.; Oughaddou, H.; Enriquez, H.; Kara, A.; Vizzini, S. B.; Ealet, B. N.; Aufray, B. (2010). «Epitaxial growth of a silicene sheet». Applied Physics Letters. 97 (22): 223109. arXiv:1204.0523. Bibcode:2010ApPhL..97v3109L. doi:10.1063/1.3524215. S2CID 118490651.
- ^ a b c d e f g h i j k l m n Greenwood & Earnshaw 1997, p. 330.
- ^ Greenwood & Earnshaw 1997, pp. 337–340
- ^ a b «Timeline». The Silicon Engine. Computer History Museum. Retrieved 22 August 2019.
- ^ a b «1901: Semiconductor Rectifiers Patented as «Cat’s Whisker» Detectors». The Silicon Engine. Computer History Museum. Retrieved 23 August 2019.
- ^ «1947: Invention of the Point-Contact Transistor». The Silicon Engine. Computer History Museum. Retrieved 23 August 2019.
- ^ «1954: Morris Tanenbaum fabricates the first silicon transistor at Bell Labs». The Silicon Engine. Computer History Museum. Retrieved 23 August 2019.
- ^ Bassett, Ross Knox (2007). To the Digital Age: Research Labs, Start-up Companies, and the Rise of MOS Technology. Johns Hopkins University Press. pp. 22–23. ISBN 978-0-8018-8639-3.
- ^ Saxena, A. (2009). Invention of integrated circuits: untold important facts. International series on advances in solid state electronics and technology. World Scientific. pp. 96–97. ISBN 978-981-281-445-6.
- ^ a b Feldman, Leonard C. (2001). «Introduction». Fundamental Aspects of Silicon Oxidation. Springer Science & Business Media. pp. 1–11. ISBN 978-3-540-41682-1.
- ^ Dabrowski, Jarek; Müssig, Hans-Joachim (2000). «1.2. The Silicon Age». Silicon Surfaces and Formation of Interfaces: Basic Science in the Industrial World. World Scientific. pp. 3–13. ISBN 978-981-02-3286-3.
- ^ Siffert, Paul; Krimmel, Eberhard (2013). «Preface». Silicon: Evolution and Future of a Technology. Springer Science & Business Media. ISBN 978-3-662-09897-4.
- ^ Uskali, T.; Nordfors, D. (23 May 2007). «The role of journalism in creating the metaphor of Silicon Valley» (PDF). Innovation Journalism 4 Conference, Stanford University. Archived from the original (PDF) on 2012-09-07. Retrieved 2016-08-08.
- ^ «Silicon and Germanium». hyperphysics.phy-astr.gsu.edu. Retrieved 2021-06-07.
- ^ King 1995, pp. xiii–xviii
- ^ Greenwood & Earnshaw 1997, pp. 372.
- ^ a b c d e f g h i j Greenwood & Earnshaw 1997, p. 331.
- ^ Vladimir E. Dmitrienko and Viacheslav A. Chizhikov (2020). «An infinite family of bc8-like metastable phases in silicon» (PDF). Phys. Rev. B. 101 (24): 245203. arXiv:1912.10672. Bibcode:2020PhRvB.101x5203D. doi:10.1103/PhysRevB.101.245203. S2CID 209444444.
- ^ a b c d e f g Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). «The NUBASE2016 evaluation of nuclear properties» (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- ^ Jerschow, Alexej. «Interactive NMR Frequency Map». New York University. Retrieved 2011-10-20.
- ^ Seitenzahl, Ivo Rolf; Townsley, Dean M. (2017). Nucleosynthesis in Thermonuclear Supernovae. Handbook of Supernovae. pp. 1955–1978. arXiv:1704.00415. Bibcode:2017hsn..book.1955S. doi:10.1007/978-3-319-21846-5_87. ISBN 978-3-319-21845-8. S2CID 118993185.
- ^ Khokhlov, A. M.; Oran, E. S.; Wheeler, J. C. (April 1997). «Deflagration‐to‐Detonation Transition in Thermonuclear Supernovae». The Astrophysical Journal. 478 (2): 678–688. arXiv:astro-ph/9612226. Bibcode:1997ApJ…478..678K. doi:10.1086/303815. S2CID 53486905.
- ^ Cameron, A.G.W. (1973). «Abundance of the Elements in the Solar System» (PDF). Space Science Reviews. 15 (1): 121–146. Bibcode:1973SSRv…15..121C. doi:10.1007/BF00172440. S2CID 120201972. Archived from the original (PDF) on 2011-10-21.
- ^ Reynolds, B. C. (June 2009). «Modeling the modern marine δ 30 Si distribution: MODELING THE MODERN MARINE δ 30 Si DISTRIBUTION». Global Biogeochemical Cycles. 23 (2): 1–13. doi:10.1029/2008GB003266. S2CID 128652214.
- ^ Stapf, André; Gondek, Christoph; Kroke, Edwin; Roewer, Gerhard (2019), Yang, Deren (ed.), «Wafer Cleaning, Etching, and Texturization», Handbook of Photovoltaic Silicon, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 311–358, doi:10.1007/978-3-662-56472-1_17, ISBN 978-3-662-56471-4, S2CID 226945433, retrieved 2021-03-07
- ^ a b Grabmaier, J. (1982). Silicon Chemical Etching. Berlin, Heidelberg: Springer Berlin Heidelberg. ISBN 978-3-642-68765-5. OCLC 840294227.
- ^ Greenwood & Earnshaw 1997, pp. 331–5
- ^ Kaupp, Martin (1 December 2006). «The role of radial nodes of atomic orbitals for chemical bonding and the periodic table» (PDF). Journal of Computational Chemistry. 28 (1): 320–325. doi:10.1002/jcc.20522. PMID 17143872. S2CID 12677737. Retrieved 14 October 2016.
- ^ a b c King 1995, pp. 43–44
- ^ a b Greenwood & Earnshaw 1997, pp. 374.
- ^ Kaupp, Martin (2007). «The role of radial nodes of atomic orbitals for chemical bonding and the periodic table». Journal of Computational Chemistry. 28 (1): 320–325. doi:10.1002/jcc.20522. ISSN 0192-8651. PMID 17143872.
- ^ Greenwood & Earnshaw 1997, pp. 327–328.
- ^ a b Greenwood & Earnshaw 1997, pp. 359–361.
- ^ Greenwood & Earnshaw 1997, p. 335-337.
- ^ King 1995, pp. 45–47
- ^ Wiber, E. (1977). «Alfred Stock and the Renaissance of Inorganic Chemistry» (PDF). Pure Appl. Chem. 49 (6): 691–700. doi:10.1351/pac197749060691. S2CID 53313463.
- ^ Mellor, J.W. (1947). A Comprehensive Treatise on Inorganic and Theoretical Chemistry. Vol. VI, [C(Part II), Si, Silicates]. Longman, Green and Co. pp. 223–7. OCLC 1044702591.
- ^ Porterfield, W.W. (2013) [1993]. «4.8 Bonding in Elements». Inorganic Chemistry: A Unified Approach (2nd ed.). Elsevier. p. 219. ISBN 978-0-323-13894-9.
- ^ Wiberg, N.; Wiberg, E.; Holleman, A.F. (2001). «2.2.3 Higher Saturated Silanes». Inorganic Chemistry. Academic Press. p. 844. ISBN 0-12-352651-5.
- ^ King 1995, p. 47
- ^ Miller, R.D.; Michl, J. (1989). «Polysilane high polymers». Chemical Reviews. 89 (6): 1359. doi:10.1021/cr00096a006.
- ^ a b c d Greenwood & Earnshaw 1997, pp. 340.
- ^ a b c King 1995, p. 48
- ^ Greenwood & Earnshaw 1997, p. 342-347.
- ^ a b c Greenwood & Earnshaw 1997, p. 342.
- ^ a b c d e Greenwood & Earnshaw 1997, p. 347.
- ^ Geological Survey (U.S.) (1975). Geological Survey professional paper.
- ^ Korzhinsky, M.A.; Tkachenko, S.I.; Shmulovich, K.I.; Steinberg, G.S. (1995). «Native AI and Si formation» (PDF). Nature. 375 (6532): 544. Bibcode:1995Natur.375..544K. doi:10.1038/375544a0. ISSN 0028-0836. S2CID 39954119.
- ^ Cordua, Courtesy of Dr Bill (1998-01-10), English: PDF file entitled: «Silicon, Silica, Silicates and Silicone» (PDF), archived from the original (PDF) on 2016-04-18, retrieved 2016-03-29
- ^ Greenwood & Earnshaw 1997, p. 347-359.
- ^ a b c d Greenwood & Earnshaw 1997, p. 359.
- ^ a b c Greenwood & Earnshaw 1997, p. 334.
- ^ Cheung, Rebecca (2006). Silicon carbide microelectromechanical systems for harsh environments. Imperial College Press. p. 3. ISBN 978-1-86094-624-0.
- ^ Morkoç, H.; Strite, S.; Gao, G.B.; Lin, M.E.; Sverdlov, B.; Burns, M. (1994). «Large-band-gap SiC, III–V nitride, and II–VI ZnSe-based semiconductor device technologies». Journal of Applied Physics. 76 (3): 1363. Bibcode:1994JAP….76.1363M. doi:10.1063/1.358463.
- ^ a b c d e f Greenwood & Earnshaw 1997, p. 361.
- ^ a b Clayden, pp. 668–77
- ^ a b Greenwood & Earnshaw 1997, p. 329.
- ^ Greenwood & Earnshaw 1997, pp. 329–330
- ^ a b c Tréguer, Paul J.; De La Rocha, Christina L. (3 January 2013). «The World Ocean Silica Cycle». Annual Review of Marine Science. 5 (1): 477–501. doi:10.1146/annurev-marine-121211-172346. PMID 22809182.
- ^ a b Tegen, Ina; Kohfeld, Karen (2006). Atmospheric transport of silicon. Island Press. pp. 81–91. ISBN 1-59726-115-7.
- ^ «Silicon Commodities Report 2011» (PDF). USGS. Retrieved 2011-10-20.
- ^ Zulehner, Neuer & Rau, p. 574
- ^ Kamali, A.R. (2019). «Ultra-fast shock-wave combustion synthesis of nanostructured silicon from sand with excellent Li storage performance». Sustainable Energy Fuels. 3 (6): 1396–1405. doi:10.1039/C9SE00046A. S2CID 139986478.
- ^ Greenwood & Earnshaw 1997, p. 356.
- ^ Koch, E.C.; Clement, D. (2007). «Special Materials in Pyrotechnics: VI. Silicon – An Old Fuel with New Perspectives». Propellants, Explosives, Pyrotechnics. 32 (3): 205. doi:10.1002/prep.200700021.
- ^ Walsh, Tim (2005). «Silly Putty». Timeless toys: classic toys and the playmakers who created them. Andrews McMeel Publishing. ISBN 978-0-7407-5571-2.
- ^ Apelian, D. (2009). «Aluminum Cast Alloys: Enabling Tools for Improved Performance» (PDF). Wheeling, Illinois: North American Die Casting Association. Archived from the original (PDF) on 2012-01-06.
- ^ a b c Corathers, Lisa A. 2009 Minerals Yearbook. USGS
- ^ «Semi» SemiSource 2006: A supplement to Semiconductor International. December 2005. Reference Section: How to Make a Chip. Adapted from Design News. Reed Electronics Group.
- ^ SemiSource 2006: A supplement to Semiconductor International. December 2005. Reference Section: How to Make a Chip. Adapted from Design News. Reed Electronics Group.
- ^ Zulehner, Neuer & Rau, p. 590
- ^ Zulehner, Neuer & Rau, p. 573
- ^ Dekker, R; Usechak, N; Först, M; Driessen, A (2008). «Ultrafast nonlinear all-optical processes in silicon-on-insulator waveguides». Journal of Physics D. 40 (14): R249–R271. Bibcode:2007JPhD…40..249D. doi:10.1088/0022-3727/40/14/r01. S2CID 123008652.
- ^ Semiconductors Without the Quantum Physics. Electropaedia
- ^ Clark, Rhett J.; Aghajamali, Maryam; Gonzalez, Christina M.; Hadidi, Lida; Islam, Muhammad Amirul; Javadi, Morteza; Mobarok, Md Hosnay; Purkait, Tapas K.; Robidillo, Christopher Jay T.; Sinelnikov, Regina; Thiessen, Alyxandra N. (2017-01-10). «From Hydrogen Silsesquioxane to Functionalized Silicon Nanocrystals». Chemistry of Materials. 29 (1): 80–89. doi:10.1021/acs.chemmater.6b02667. ISSN 0897-4756.
- ^ Hessel, Colin M.; Henderson, Eric J.; Veinot, Jonathan G. C. (2007). «Hydrogen Silsesquioxane: A Molecular Precursor for Nanocrystalline Si—SiO2 Composites and Freestanding Hydride-Surface-Terminated Silicon Nanoparticles». ChemInform. 38 (10). doi:10.1002/chin.200710014. ISSN 1522-2667.
- ^ Lim, Cheol Hong; Han, Jeong-Hee; Cho, Hae-Won; Kang, Mingu (2014). «Studies on the Toxicity and Distribution of Indium Compounds According to Particle Size in Sprague-Dawley Rats». Toxicological Research. 30 (1): 55–63. doi:10.5487/TR.2014.30.1.055. ISSN 1976-8257. PMC 4007045. PMID 24795801.
- ^ Zou, Hui; Wang, Tao; Yuan, Junzhao; Sun, Jian; Yuan, Yan asdf; Gu, Jianhong; Liu, Xuezhong; Bian, Jianchun; Liu, Zongping (2020-03-15). «Cadmium-induced cytotoxicity in mouse liver cells is associated with the disruption of autophagic flux via inhibiting the fusion of autophagosomes and lysosomes». Toxicology Letters. 321: 32–43. doi:10.1016/j.toxlet.2019.12.019. ISSN 0378-4274. PMID 31862506. S2CID 209435190.
- ^ Nguyen, An; Gonzalez, Christina M; Sinelnikov, Regina; Newman, W; Sun, Sarah; Lockwood, Ross; Veinot, Jonathan G C; Meldrum, Al (2016-02-10). «Detection of nitroaromatics in the solid, solution, and vapor phases using silicon quantum dot sensors». Nanotechnology. 27 (10): 105501. Bibcode:2016Nanot..27j5501N. doi:10.1088/0957-4484/27/10/105501. ISSN 0957-4484. PMID 26863492. S2CID 24292648.
- ^ Gonzalez, Christina M.; Veinot, Jonathan G. C. (2016-06-02). «Silicon nanocrystals for the development of sensing platforms». Journal of Materials Chemistry C. 4 (22): 4836–4846. doi:10.1039/C6TC01159D. ISSN 2050-7534.
- ^ Yue, Zhao; Lisdat, Fred; Parak, Wolfgang J.; Hickey, Stephen G.; Tu, Liping; Sabir, Nadeem; Dorfs, Dirk; Bigall, Nadja C. (2013-04-24). «Quantum-Dot-Based Photoelectrochemical Sensors for Chemical and Biological Detection». ACS Applied Materials & Interfaces. 5 (8): 2800–2814. doi:10.1021/am3028662. ISSN 1944-8244. PMID 23547912.
- ^ Kim, Sang Gyu; Kim, Ki Woo; Park, Eun Woo; Choi, Doil (2002). «Silicon-Induced Cell Wall Fortification of Rice Leaves: A Possible Cellular Mechanism of Enhanced Host Resistance to Blast». Phytopathology. 92 (10): 1095–103. doi:10.1094/PHYTO.2002.92.10.1095. PMID 18944220.
- ^ a b c Epstein, Emanuel (1999). «SILICON». Annual Review of Plant Physiology and Plant Molecular Biology. 50: 641–664. doi:10.1146/annurev.arplant.50.1.641. PMID 15012222.
- ^ Leroy, Nicolas; de Tombeur, Felix; Walgraffe, Yseult; Cornelis, Jean-Thomas; Verheggen, Francois (23 October 2019). «Silicon and plant natural defenses against insect pests: impact on plant volatile organic compounds and cascade effects on multitrophic interactions». Plants. 8 (444): 444. doi:10.3390/plants8110444. PMC 6918431. PMID 31652861.
- ^ Rahman, Atta-ur- (2008). «Silicon». Studies in Natural Products Chemistry. Vol. 35. p. 856. ISBN 978-0-444-53181-0.
- ^ Exley, C. (1998). «Silicon in life:A bioinorganic solution to bioorganic essentiality». Journal of Inorganic Biochemistry. 69 (3): 139–144. doi:10.1016/S0162-0134(97)10010-1.
- ^ Aguilera Mochón, Juan Antonio (2016). La vida no terrestre [The non-terrestrial life] (in Spanish). RBA. pp. 43–45. ISBN 978-84-473-8665-9.
- ^ Bidle, Kay D.; Manganelli, Maura; Azam, Farooq (2002-12-06). «Regulation of Oceanic Silicon and Carbon Preservation by Temperature Control on Bacteria». Science. 298 (5600): 1980–1984. Bibcode:2002Sci…298.1980B. doi:10.1126/science.1076076. ISSN 0036-8075. PMID 12471255. S2CID 216994.
- ^ Durkin, Colleen A.; Koester, Julie A.; Bender, Sara J.; Armbrust, E. Virginia (2016). «The evolution of silicon transporters in diatoms». Journal of Phycology. 52 (5): 716–731. doi:10.1111/jpy.12441. ISSN 1529-8817. PMC 5129515. PMID 27335204.
- ^ a b Dugdale, R. C.; Wilkerson, F. P. (2001-12-30). «Sources and fates of silicon in the ocean: the role of diatoms in the climate and glacial cycles». Scientia Marina. 65 (S2): 141–152. doi:10.3989/scimar.2001.65s2141. ISSN 1886-8134.
- ^ Baines, Stephen B.; Twining, Benjamin S.; Brzezinski, Mark A.; Krause, Jeffrey W.; Vogt, Stefan; Assael, Dylan; McDaniel, Hannah (December 2012). «Significant silicon accumulation by marine picocyanobacteria». Nature Geoscience. 5 (12): 886–891. Bibcode:2012NatGe…5..886B. doi:10.1038/ngeo1641. ISSN 1752-0908.
- ^ Turner, Jefferson T. (January 2015). «Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump». Progress in Oceanography. 130: 205–248. Bibcode:2015PrOce.130..205T. doi:10.1016/j.pocean.2014.08.005. ISSN 0079-6611.
- ^ Yool, Andrew; Tyrrell, Toby (2003). «Role of diatoms in regulating the ocean’s silicon cycle». Global Biogeochemical Cycles. 17 (4): n/a. Bibcode:2003GBioC..17.1103Y. CiteSeerX 10.1.1.394.3912. doi:10.1029/2002GB002018. ISSN 1944-9224. S2CID 16849373.
- ^ Marinov, I.; Gnanadesikan, A.; Toggweiler, J. R.; Sarmiento, J. L. (June 2006). «The Southern Ocean biogeochemical divide». Nature. 441 (7096): 964–967. Bibcode:2006Natur.441..964M. doi:10.1038/nature04883. PMID 16791191. S2CID 4428683.
- ^ Martin, Keith R. (2013). «Chapter 14. Silicon: The Health Benefits of a Metalloid». In Astrid Sigel; Helmut Sigel; Roland K.O. Sigel (eds.). Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. Vol. 13. Springer. pp. 451–473. doi:10.1007/978-94-007-7500-8_14. ISBN 978-94-007-7499-5. PMID 24470100.
- ^ Jugdaohsingh, R. (Mar–Apr 2007). «Silicon and bone health». The Journal of Nutrition, Health and Aging. 11 (2): 99–110. PMC 2658806. PMID 17435952.
- ^ Loeper, J.; Fragny, M. (1978). The Physiological Role of the Silicon and its AntiAtheromatous Action. Biochemistry of Silicon and Related Problems. pp. 281–296. doi:10.1007/978-1-4613-4018-8_13. ISBN 978-1-4613-4020-1.
- ^ Nielsen, Forrest H. (1984). «Ultratrace Elements in Nutrition». Annual Review of Nutrition. 4: 21–41. doi:10.1146/annurev.nu.04.070184.000321. PMID 6087860.
- ^ Lippard, Stephen J.; Jeremy M. Berg (1994). Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books. p. 411. ISBN 978-0-935702-72-9.
- ^ Muhammad Ansar Farooq; Karl-Josef Dietz (2015). «Silicon as Versatile Player in Plant and Human Biology: Overlooked and Poorly Understood Muhammad Ansar Farooq and Karl-J». Front. Plant Sci. 6 (994): 994. doi:10.3389/fpls.2015.00994. PMC 4641902. PMID 26617630.
- ^ «AAPFCO Board of Directors 2006 Mid-Year Meeting» (PDF). Association of American Plant Food Control Officials. Archived from the original (PDF) on 6 January 2012. Retrieved 2011-07-18.
A presentation was made for Excell Minerals to recognize Silicon as a recognized plant nutrient
- ^ Miranda, Stephen R.; Barker, Bruce (August 4, 2009). «Silicon: Summary of Extraction Methods». Harsco Minerals. Archived from the original on November 12, 2012. Retrieved 2011-07-18.
- ^ Science Lab.com. «Material Safety Data Sheet: Silicon MSDS». sciencelab.com. Archived from the original on 23 March 2018. Retrieved 11 March 2018.
- ^ «CDC – NIOSH Pocket Guide to Chemical Hazards – Silicon». www.cdc.gov. Retrieved 2015-11-21.
- ^ Jane A. Plant; Nick Voulvoulis; K. Vala Ragnarsdottir (2012). Pollutants, Human Health and the Environment: A Risk Based Approach. Applied Geochemistry. Vol. 26. John Wiley & Sons. p. 273. Bibcode:2011ApGC…26S.238P. doi:10.1016/j.apgeochem.2011.03.113. ISBN 978-0-470-74261-7. Retrieved 24 August 2012.
Bibliography[edit]
- Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford University Press. ISBN 978-0-19-927029-3.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- King, R. Bruce (1995). Inorganic Chemistry of Main Group Elements. Wiley-VCH. ISBN 978-0-471-18602-1.
- Zulehner, Werner; Neuer, Bernd; Rau, Gerhard. «Silicon». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_721.
- Kamal, Kamal Y. (2022). «The Silicon Age: Trends in Semiconductor Devices Industry» (PDF). Journal of Engineering Science and Technology Review. 15 (1): 110–5. doi:10.25103/jestr.151.14. S2CID 249074588.
External links[edit]
- «Silicon Video — The Periodic Table of Videos — University of Nottingham». www.periodicvideos.com. Retrieved 2021-06-08.
- «CDC — NIOSH Pocket Guide to Chemical Hazards — Silicon». www.cdc.gov. Retrieved 2021-06-08.
- «Physical properties of Silicon (Si)». www.ioffe.ru. Retrieved 2021-06-08.
- The Story of Solar-Grade Silicon. Asianometry. 30 November 2022.
Not to be confused with the silicon-containing synthetic polymer silicone.
| Silicon | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allotropes | see Allotropes of silicon | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | crystalline, reflective with bluish-tinged faces | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Si) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Silicon in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 14 (carbon group) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1687 K (1414 °C, 2577 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3538 K (3265 °C, 5909 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 2.3290 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 2.57 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 50.21 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 383 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 19.789 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −4, −3, −2, −1, 0,[2] +1,[3] +2, +3, +4 (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 111 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 111 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 210 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of silicon |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered diamond-cubic
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 8433 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 2.6 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 149 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 2.3×103 Ω⋅m (at 20 °C)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Band gap | 1.12 eV (at 300 K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −3.9×10−6 cm3/mol (298 K)[6] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 130–188 GPa[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 51–80 GPa[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 97.6 GPa[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.064–0.28[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-21-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Latin silex or silicis, meaning ‘flint’ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prediction | Antoine Lavoisier (1787) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Jöns Jacob Berzelius[8][9] (1823) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Named by | Thomas Thomson (1817) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of silicon
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive.
Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron.
Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth’s crust. It is widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide (silica) or silicates. More than 90% of the Earth’s crust is composed of silicate minerals, making silicon the second most abundant element in the Earth’s crust (about 28% by mass), after oxygen.
Most silicon is used commercially without being separated, often with very little processing of the natural minerals. Such use includes industrial construction with clays, silica sand, and stone. Silicates are used in Portland cement for mortar and stucco, and mixed with silica sand and gravel to make concrete for walkways, foundations, and roads. They are also used in whiteware ceramics such as porcelain, and in traditional silicate-based soda-lime glass and many other specialty glasses. Silicon compounds such as silicon carbide are used as abrasives and components of high-strength ceramics. Silicon is the basis of the widely used synthetic polymers called silicones.
The late 20th century to early 21st century has been described as the Silicon Age (also known as the Digital Age or Information Age) because of the large impact that elemental silicon has on the modern world economy. The small portion of very highly purified elemental silicon used in semiconductor electronics (<10%[citation needed]) is essential to the transistors and integrated circuit chips used in most modern technology such as smartphones and other computers. In 2019, 32.4% of the semiconductor market segment was for networks and communications devices, and the semiconductors industry is projected to reach $726.73 billion by 2027. [10]
Silicon is an essential element in biology. Only traces are required by most animals, but some sea sponges and microorganisms, such as diatoms and radiolaria, secrete skeletal structures made of silica. Silica is deposited in many plant tissues.[11]
History[edit]
Owing to the abundance of silicon in the Earth’s crust, natural silicon-based materials have been used for thousands of years. Silicon rock crystals were familiar to various ancient civilizations, such as the predynastic Egyptians who used it for beads and small vases, as well as the ancient Chinese. Glass containing silica was manufactured by the Egyptians since at least 1500 BC, as well as by the ancient Phoenicians. Natural silicate compounds were also used in various types of mortar for construction of early human dwellings.[12]
Discovery[edit]
In 1787, Antoine Lavoisier suspected that silica might be an oxide of a fundamental chemical element,[13] but the chemical affinity of silicon for oxygen is high enough that he had no means to reduce the oxide and isolate the element.[14] After an attempt to isolate silicon in 1808, Sir Humphry Davy proposed the name «silicium» for silicon, from the Latin silex, silicis for flint, and adding the «-ium» ending because he believed it to be a metal.[15] Most other languages use transliterated forms of Davy’s name, sometimes adapted to local phonology (e.g. German Silizium, Turkish silisyum, Catalan silici, Armenian Սիլիցիում or Silitzioum). A few others use instead a calque of the Latin root (e.g. Russian кремний, from кремень «flint»; Greek πυρίτιο from πυρ «fire»; Finnish pii from piikivi «flint», Czech křemík from křemen «quartz», «flint»).[16]
Gay-Lussac and Thénard are thought to have prepared impure amorphous silicon in 1811, through the heating of recently isolated potassium metal with silicon tetrafluoride, but they did not purify and characterize the product, nor identify it as a new element.[17] Silicon was given its present name in 1817 by Scottish chemist Thomas Thomson. He retained part of Davy’s name but added «-on» because he believed that silicon was a nonmetal similar to boron and carbon.[18] In 1824, Jöns Jacob Berzelius prepared amorphous silicon using approximately the same method as Gay-Lussac (reducing potassium fluorosilicate with molten potassium metal), but purifying the product to a brown powder by repeatedly washing it.[19] As a result, he is usually given credit for the element’s discovery.[20][21] The same year, Berzelius became the first to prepare silicon tetrachloride; silicon tetrafluoride had already been prepared long before in 1771 by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid.[14] In 1823 for the first time Jacob Berzelius discovered silicon tetrachloride (SiCl4).[22] In 1846 Von Ebelman’s had synthesized Tetraethyl orthosilicate (Si(OC2H5)4).[23][22]
Silicon in its more common crystalline form was not prepared until 31 years later, by Deville.[24][25] By electrolyzing a mixture of sodium chloride and aluminium chloride containing approximately 10% silicon, he was able to obtain a slightly impure allotrope of silicon in 1854.[26] Later, more cost-effective methods have been developed to isolate several allotrope forms, the most recent being silicene in 2010.[27][28] Meanwhile, research on the chemistry of silicon continued; Friedrich Wöhler discovered the first volatile hydrides of silicon, synthesising trichlorosilane in 1857 and silane itself in 1858, but a detailed investigation of the silanes was only carried out in the early 20th century by Alfred Stock, despite early speculation on the matter dating as far back as the beginnings of synthetic organic chemistry in the 1830s.[29][30] Similarly, the first organosilicon compound, tetraethylsilane, was synthesised by Charles Friedel and James Crafts in 1863, but detailed characterisation of organosilicon chemistry was only done in the early 20th century by Frederic Kipping.[14]
Starting in the 1920s, the work of William Lawrence Bragg on X-ray crystallography elucidated the compositions of the silicates, which had previously been known from analytical chemistry but had not yet been understood, together with Linus Pauling’s development of crystal chemistry and Victor Goldschmidt’s development of geochemistry. The middle of the 20th century saw the development of the chemistry and industrial use of siloxanes and the growing use of silicone polymers, elastomers, and resins. In the late 20th century, the complexity of the crystal chemistry of silicides was mapped, along with the solid-state physics of doped semiconductors.[14]
Silicon semiconductors[edit]
The first semiconductor devices did not use silicon, but used galena, including German physicist Ferdinand Braun’s crystal detector in 1874 and Indian physicist Jagadish Chandra Bose’s radio crystal detector in 1901.[31][32] The first silicon semiconductor device was a silicon radio crystal detector, developed by American engineer Greenleaf Whittier Pickard in 1906.[32]
In 1940, Russell Ohl discovered the p–n junction and photovoltaic effects in silicon. In 1941, techniques for producing high-purity germanium and silicon crystals were developed for radar microwave detector crystals during World War II.[31] In 1947, physicist William Shockley theorized a field-effect amplifier made from germanium and silicon, but he failed to build a working device, before eventually working with germanium instead. The first working transistor was a point-contact transistor built by John Bardeen and Walter Brattain later that year while working under Shockley.[33] In 1954, physical chemist Morris Tanenbaum fabricated the first silicon junction transistor at Bell Labs.[34] In 1955, Carl Frosch and Lincoln Derick at Bell Labs accidentally discovered that silicon dioxide (SiO
2) could be grown on silicon,[35] and they later proposed this could mask silicon surfaces during diffusion processes in 1958.[36]
Silicon Age[edit]
The «Silicon Age» refers to the late 20th century to early 21st century.[37][38][39] This is due to silicon being the dominant material of the Silicon Age (also known as the Digital Age or Information Age), similar to how the Stone Age, Bronze Age and Iron Age were defined by the dominant materials during their respective ages of civilization.[37]
Because silicon is an important element in high-technology semiconductor devices, many places in the world bear its name. For example, Santa Clara Valley in California acquired the nickname Silicon Valley, as the element is the base material in the semiconductor industry there. Since then, many other places have been dubbed similarly, including Silicon Wadi in Israel, Silicon Forest in Oregon, Silicon Hills in Austin, Texas, Silicon Slopes in Salt Lake City, Utah, Silicon Saxony in Germany, Silicon Valley in India, Silicon Border in Mexicali, Mexico, Silicon Fen in Cambridge, England, Silicon Roundabout in London, Silicon Glen in Scotland, Silicon Gorge in Bristol, England, Silicon Alley in New York, New York and Silicon Beach in Los Angeles, California.[40]
Characteristics[edit]
Physical and atomic[edit]
A silicon atom has fourteen electrons. In the ground state, they are arranged in the electron configuration [Ne]3s23p2. Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. Like the other members of its group, the lighter carbon and the heavier germanium, tin, and lead, it has the same number of valence electrons as valence orbitals: hence, it can complete its octet and obtain the stable noble gas configuration of argon by forming sp3 hybrid orbitals, forming tetrahedral SiX
4 derivatives where the central silicon atom shares an electron pair with each of the four atoms it is bonded to.[42] The first four ionisation energies of silicon are 786.3, 1576.5, 3228.3, and 4354.4 kJ/mol respectively; these figures are high enough to preclude the possibility of simple cationic chemistry for the element. Following periodic trends, its single-bond covalent radius of 117.6 pm is intermediate between those of carbon (77.2 pm) and germanium (122.3 pm). The hexacoordinate ionic radius of silicon may be considered to be 40 pm, although this must be taken as a purely notional figure given the lack of a simple Si4+
cation in reality.[43]
Electrical[edit]
At standard temperature and pressure, silicon is a shiny semiconductor with a bluish-grey metallic lustre; as typical for semiconductors, its resistivity drops as temperature rises. This arises because silicon has a small energy gap (band gap) between its highest occupied energy levels (the valence band) and the lowest unoccupied ones (the conduction band). The Fermi level is about halfway between the valence and conduction bands and is the energy at which a state is as likely to be occupied by an electron as not. Hence pure silicon is effectively an insulator at room temperature. However, doping silicon with a pnictogen such as phosphorus, arsenic, or antimony introduces one extra electron per dopant and these may then be excited into the conduction band either thermally or photolytically, creating an n-type semiconductor. Similarly, doping silicon with a group 13 element such as boron, aluminium, or gallium results in the introduction of acceptor levels that trap electrons that may be excited from the filled valence band, creating a p-type semiconductor.[44] Joining n-type silicon to p-type silicon creates a p–n junction with a common Fermi level; electrons flow from n to p, while holes flow from p to n, creating a voltage drop. This p–n junction thus acts as a diode that can rectify alternating current that allows current to pass more easily one way than the other. A transistor is an n–p–n junction, with a thin layer of weakly p-type silicon between two n-type regions. Biasing the emitter through a small forward voltage and the collector through a large reverse voltage allows the transistor to act as a triode amplifier.[44]
Crystal structure[edit]
Silicon crystallises in a giant covalent structure at standard conditions, specifically in a diamond cubic lattice (space group 227). It thus has a high melting point of 1414 °C, as a lot of energy is required to break the strong covalent bonds and melt the solid. Upon melting silicon contracts as the long-range tetrahedral network of bonds breaks up and the voids in that network are filled in, similar to water ice when hydrogen bonds are broken upon melting. It does not have any thermodynamically stable allotropes at standard pressure, but several other crystal structures are known at higher pressures. The general trend is one of increasing coordination number with pressure, culminating in a hexagonal close-packed allotrope at about 40 gigapascals known as Si–VII (the standard modification being Si–I). An allotrope called BC8 (or bc8), having a body-centred cubic lattice with eight atoms per primitive unit cell (space group 206), can be created at high pressure and remains metastable at low pressure. Its properties have been studied in detail.[45]
Silicon boils at 3265 °C: this, while high, is still lower than the temperature at which its lighter congener carbon sublimes (3642 °C) and silicon similarly has a lower heat of vaporisation than carbon, consistent with the fact that the Si–Si bond is weaker than the C–C bond.[44]
It is also possible to construct silicene layers analogous to graphene.[27][28]
Isotopes[edit]
Naturally occurring silicon is composed of three stable isotopes, 28Si (92.23%), 29Si (4.67%), and 30Si (3.10%).[46] Out of these, only 29Si is of use in NMR and EPR spectroscopy,[47] as it is the only one with a nuclear spin (I =1/2).[29] All three are produced in Type Ia supernovae[48][49] through the oxygen-burning process, with 28Si being made as part of the alpha process and hence the most abundant. The fusion of 28Si with alpha particles by photodisintegration rearrangement in stars is known as the silicon-burning process; it is the last stage of stellar nucleosynthesis before the rapid collapse and violent explosion of the star in question in a type II supernova.[50]
Twenty radioisotopes have been characterized, the two stablest being 32Si with a half-life of about 150 years, and 31Si with a half-life of 2.62 hours.[46] All the remaining radioactive isotopes have half-lives that are less than seven seconds, and the majority of these have half-lives that are less than one tenth of a second.[46] Silicon has one known nuclear isomer, 34mSi, with a half-life less than 210 nanoseconds.[46] 32Si undergoes low-energy beta decay to 32P and then stable 32S. 31Si may be produced by the neutron activation of natural silicon and is thus useful for quantitative analysis; it can be easily detected by its characteristic beta decay to stable 31P, in which the emitted electron carries up to 1.48 MeV of energy.[29]
The known isotopes of silicon range in mass number from 22 to 44.[46] The most common decay mode of the isotopes with mass numbers lower than the three stable isotopes is inverse beta decay, primarily forming aluminium isotopes (13 protons) as decay products.[46] The most common decay mode for the heavier unstable isotopes is beta decay, primarily forming phosphorus isotopes (15 protons) as decay products.[46]
Silicon can enter the oceans through groundwater and riverine transport. Large fluxes of groundwater input have an isotopic composition which is distinct from riverine silicon inputs. Isotopic variations in groundwater and riverine transports contribute to variations in oceanic 30Si values. Currently, there are substantial differences in the isotopic values of deep water in the world’s ocean basins. Between the Atlantic and Pacific oceans, there is a deep water 30Si gradient of greater than 0.3 parts per thousand. 30Si is most commonly associated with productivity in the oceans.[51]
Chemistry and compounds[edit]
| X = | C | Si | H | F | Cl | Br | I | O– | N< |
|---|---|---|---|---|---|---|---|---|---|
| C–X | 368 | 360 | 435 | 453 | 351 | 293 | 216 | ~360 | ~305 |
| Si–X | 360 | 340 | 393 | 565 | 381 | 310 | 234 | 452 | 322 |
Crystalline bulk silicon is rather inert, but becomes more reactive at high temperatures. Like its neighbour aluminium, silicon forms a thin, continuous surface layer of silicon dioxide (SiO
2) that protects the metal from oxidation. Thus silicon does not measurably react with the air below 900 °C, but formation of the vitreous dioxide rapidly increases between 950 °C and 1160 °C and when 1400 °C is reached, atmospheric nitrogen also reacts to give the nitrides SiN and Si
3N
4. Silicon reacts with gaseous sulfur at 600 °C and gaseous phosphorus at 1000 °C. This oxide layer nevertheless does not prevent reaction with the halogens; fluorine attacks silicon vigorously at room temperature, chlorine does so at about 300 °C, and bromine and iodine at about 500 °C. Silicon does not react with most aqueous acids, but is oxidised and complexed by hydrofluoric acid mixtures containing either chlorine or nitric acid to form hexafluorosilicates. It readily dissolves in hot aqueous alkali to form silicates.[52] At high temperatures, silicon also reacts with alkyl halides; this reaction may be catalysed by copper to directly synthesise organosilicon chlorides as precursors to silicone polymers. Upon melting, silicon becomes extremely reactive, alloying with most metals to form silicides, and reducing most metal oxides because the heat of formation of silicon dioxide is so large. In fact, molten silicon reacts virtually with every known kind of crucible material (except its own oxide, SiO
2).[53]: 13 This happens due to silicon’s high binding forces for the light elements and to its high dissolving power for most elements.[53]: 13 As a result, containers for liquid silicon must be made of refractory, unreactive materials such as zirconium dioxide or group 4, 5, and 6 borides.[44][54]
Tetrahedral coordination is a major structural motif in silicon chemistry just as it is for carbon chemistry. However, the 3p subshell is rather more diffuse than the 2p subshell and does not hybridise so well with the 3s subshell. As a result, the chemistry of silicon and its heavier congeners shows significant differences from that of carbon,[55] and thus octahedral coordination is also significant.[44] For example, the electronegativity of silicon (1.90) is much less than that of carbon (2.55), because the valence electrons of silicon are further from the nucleus than those of carbon and hence experience smaller electrostatic forces of attraction from the nucleus. The poor overlap of 3p orbitals also results in a much lower tendency toward catenation (formation of Si–Si bonds) for silicon than for carbon, due to the concomitant weakening of the Si–Si bond compared to the C–C bond:[56] the average Si–Si bond energy is approximately 226 kJ/mol, compared to a value of 356 kJ/mol for the C–C bond.[57] This results in multiply bonded silicon compounds generally being much less stable than their carbon counterparts, an example of the double bond rule. On the other hand, the presence of radial nodes in the 3p orbitals of silicon suggests the possibility of hypervalence, as seen in five and six-coordinate derivatives of silicon such as SiX−
5 and SiF2−
6.[58][56] Lastly, because of the increasing energy gap between the valence s and p orbitals as the group is descended, the divalent state grows in importance from carbon to lead, so that a few unstable divalent compounds are known for silicon; this lowering of the main oxidation state, in tandem with increasing atomic radii, results in an increase of metallic character down the group. Silicon already shows some incipient metallic behavior, particularly in the behavior of its oxide compounds and its reaction with acids as well as bases (though this takes some effort), and is hence often referred to as a metalloid rather than a nonmetal.[56] However, metallicity does not become clear in group 14 until germanium and dominant until tin, with the growing importance of the lower +2 oxidation state.[14]
Silicon shows clear differences from carbon. For example, organic chemistry has very few analogies with silicon chemistry, while silicate minerals have a structural complexity unseen in oxocarbons.[59] Silicon tends to resemble germanium far more than it does carbon, and this resemblance is enhanced by the d-block contraction, resulting in the size of the germanium atom being much closer to that of the silicon atom than periodic trends would predict.[60] Nevertheless, there are still some differences because of the growing importance of the divalent state in germanium compared to silicon, which result in germanium being significantly more metallic than silicon. Additionally, the lower Ge–O bond strength compared to the Si–O bond strength results in the absence of «germanone» polymers that would be analogous to silicone polymers.[57]
Silicides[edit]
Phase diagram of the Fe–Si system
Many metal silicides are known, most of which have formulae that cannot be explained through simple appeals to valence: their bonding ranges from metallic to ionic and covalent. Some known stoichiometries are M
6Si, M
5Si, M
4Si, M
15Si
4, M
3Si, M
5Si
2, M
2Si, M
5Si
3, M
3Si
2, MSi, M
2Si
3, MSi
2, MSi
3, and MSi
6. They are structurally more similar to the borides than the carbides, in keeping with the diagonal relationship between boron and silicon, although the larger size of silicon than boron means that exact structural analogies are few and far between. The heats of formation of the silicides are usually similar to those of the borides and carbides of the same elements, but they usually melt at lower temperatures.[61] Silicides are known for all stable elements in groups 1–10, with the exception of beryllium: in particular, uranium and the transition metals of groups 4–10 show the widest range of stoichiometries. Except for copper, the metals in groups 11–15 do not form silicides. Instead, most form eutectic mixtures, although the heaviest post-transition metals mercury, thallium, lead, and bismuth are completely immiscible with liquid silicon.[44]
Usually, silicides are prepared by direct reaction of the elements. For example, the alkali metals and alkaline earth metals react with silicon or silicon oxide to give silicides. Nevertheless, even with these highly electropositive elements true silicon anions are not obtainable, and most of these compounds are semiconductors. For example, the alkali metal silicides (M+
)
4(Si4−
4) contain pyramidal tricoordinate silicon in the Si4−
4 anion, isoelectronic with white phosphorus, P
4.[44][62] Metal-rich silicides tend to have isolated silicon atoms (e. g. Cu
5Si); with increasing silicon content, catenation increases, resulting in isolated clusters of two (e. g. U
3Si
2) or four silicon atoms (e. g. [K+
]
4[Si
4]4−
) at first, followed by chains (e. g. CaSi), layers (e. g. CaSi
2), or three-dimensional networks of silicon atoms spanning space (e. g. α-ThSi
2) as the silicon content rises even higher.[44]
The silicides of the group 1 and 2 metals usually are more reactive than the transition metal silicides. The latter usually do not react with aqueous reagents, except for hydrofluoric acid; however, they do react with much more aggressive reagents such as liquid potassium hydroxide, or gaseous fluorine or chlorine when red-hot. The pre-transition metal silicides instead readily react with water and aqueous acids, usually producing hydrogen or silanes:[44]
- Na
2Si + 3 H2O → Na
2SiO
3 + 3 H
2 - Mg
2Si + 2 H
2SO
4 → 2 MgSO
4 + SiH
4
Products often vary with the stoichiometry of the silicide reactant. For example, Ca
2Si is polar and non-conducting and has the anti-PbCl
2 structure with single isolated silicon atoms, and reacts with water to produce calcium hydroxide, hydrated silicon dioxide, and hydrogen gas. CaSi with its zigzag chains of silicon atoms instead reacts to give silanes and polymeric SiH
2, while CaSi
2 with its puckered layers of silicon atoms does not react with water, but will react with dilute hydrochloric acid: the product is a yellow polymeric solid with stoichiometry Si
2H
2O.[44]
Silanes[edit]
Speculation on silicon hydride chemistry started in the 1830s, contemporary with the development of synthetic organic chemistry. Silane itself, as well as trichlorosilane, were first synthesised by Friedrich Wöhler and Heinrich Buff in 1857 by reacting aluminium–silicon alloys with hydrochloric acid, and characterised as SiH
4 and SiHCl
3 by Charles Friedel and Albert Ladenburg in 1867. Disilane (Si
2H
6) followed in 1902, when it was first made by Henri Moissan and Samuel Smiles by the protonolysis of magnesium silicides. Further investigation had to wait until 1916 because of the great reactivity and thermal instability of the silanes; it was then that Alfred Stock began to study silicon hydrides in earnest with new greaseless vacuum techniques, as they were found as contaminants of his focus, the boron hydrides. The names silanes and boranes are his, based on analogy with the alkanes.[29][63][64] The Moissan and Smiles method of preparation of silanes and silane derivatives via protonolysis of metal silicides is still used, although the yield is lowered by the hydrolysis of the products that occurs simultaneously, so that the preferred route today is to treat substituted silanes with hydride reducing agents such as lithium aluminium hydride in etheric solutions at low temperatures. Direct reaction of HX or RX with silicon, possibly with a catalyst such as copper, is also a viable method of producing substituted silanes.[29]
The silanes comprise a homologous series of silicon hydrides with a general formula of Si
nH
2n + 2. They are all strong reducing agents. Unbranched and branched chains are known up to n=8, and the cycles Si
5H
10 and Si
6H
12 are also known. The first two, silane and disilane, are colourless gases; the heavier members of the series are volatile liquids. All silanes are very reactive and catch fire or explode spontaneously in air. They become less thermally stable with room temperature, so that only silane is indefinitely stable at room temperature, although disilane does not decompose very quickly (only 2.5% of a sample decomposes after the passage of eight months).[29] They decompose to form polymeric polysilicon hydride and hydrogen gas.[65][66] As expected from the difference in atomic weight, the silanes are less volatile than the corresponding alkanes and boranes, but more so than the corresponding germanes. They are much more reactive than the corresponding alkanes, because of the larger radius of silicon compared to carbon facilitating nucleophilic attack at the silicon, the greater polarity of the Si–H bond compared to the C–H bond, and the ability of silicon to expand its octet and hence form adducts and lower the reaction’s activation energy.[29]
Silane pyrolysis gives polymeric species and finally elemental silicon and hydrogen; indeed ultrapure silicon is commercially produced by the pyrolysis of silane. While the thermal decomposition of alkanes starts by the breaking of a C–H or C–C bond and the formation of radical intermediates, polysilanes decompose by eliminating silylenes :SiH
2 or :SiHR, as the activation energy of this process (~210 kJ/mol) is much less than the Si–Si and Si–H bond energies. While pure silanes do not react with pure water or dilute acids, traces of alkali catalyse immediate hydrolysis to hydrated silicon dioxide. If the reaction is carried out in methanol, controlled solvolysis results in the products SiH
2(OMe)
2, SiH(OMe)
3, and Si(OMe)
4. The Si–H bond also adds to alkenes, a reaction which proceeds slowly and speeds up with increasing substitution of the silane involved. At 450 °C, silane participates in an addition reaction with acetone, as well as a ring-opening reaction with ethylene oxide. Direct reaction of the silanes with chlorine or bromine results in explosions at room temperature, but the reaction of silane with bromine at −80 °C is controlled and yields bromosilane and dibromosilane. The monohalosilanes may be formed by reacting silane with the appropriate hydrogen halide with an Al
2X
6 catalyst, or by reacting silane with a solid silver halide in a heated flow reactor:[29]
- SiH
4 + 2 AgCl 260 °C→ SiH
3Cl + HCl + 2 Ag
Among the derivatives of silane, iodosilane (SiH
3I) and potassium silanide (KSiH
3) are very useful synthetic intermediates in the production of more complicated silicon-containing compounds: the latter is a colourless crystalline ionic solid containing K+ cations and SiH−
3 anions in the NaCl structure, and is made by the reduction of silane by potassium metal.[67] Additionally, the reactive hypervalent species SiH−
5 is also known.[29] With suitable organic substituents it is possible to produce stable polysilanes: they have surprisingly high electric conductivities, arising from sigma delocalisation of the electrons in the chain.[68]
Halides[edit]
Silicon and silicon carbide readily react with all four stable halogens, forming the colourless, reactive, and volatile silicon tetrahalides[69] Silicon tetrafluoride also may be made by fluorinating the other silicon halides, and is produced by the attack of hydrofluoric acid on glass.[70] Heating two different tetrahalides together also produces a random mixture of mixed halides, which may also be produced by halogen exchange reactions. The melting and boiling points of these species usually rise with increasing atomic weight, though there are many exceptions: for example, the melting and boiling points drop as one passes from SiFBr
3 through SiFClBr
2 to SiFCl
2Br. The shift from the hypoelectronic elements in Group 13 and earlier to the Group 14 elements is illustrated by the change from an infinite ionic structure in aluminium fluoride to a lattice of simple covalent silicon tetrafluoride molecules, as dictated by the lower electronegativity of aluminium than silicon, the stoichiometry (the +4 oxidation state being too high for true ionicity), and the smaller size of the silicon atom compared to the aluminium atom.[69]
Silicon tetrachloride is manufactured on a huge scale as a precursor to the production of pure silicon, silicon dioxide, and some silicon esters.[69] The silicon tetrahalides hydrolyse readily in water, unlike the carbon tetrahalides, again because of the larger size of the silicon atom rendering it more open to nucleophilic attack and the ability of the silicon atom to expand its octet which carbon lacks.[70] The reaction of silicon tetrafluoride with excess hydrofluoric acid produces the octahedral hexafluorosilicate anion SiF2−
6.[70]
Analogous to the silanes, halopolysilanes Si
nX
2n + 2 also are known. While catenation in carbon compounds is maximised in the hydrogen compounds rather than the halides, the opposite is true for silicon, so that the halopolysilanes are known up to at least Si
14F
30, Si
6Cl
14, and Si
4Br
10. A suggested explanation for this phenomenon is the compensation for the electron loss of silicon to the more electronegative halogen atoms by pi backbonding from the filled pπ orbitals on the halogen atoms to the empty dπ orbitals on silicon: this is similar to the situation of carbon monoxide in metal carbonyl complexes and explains their stability. These halopolysilanes may be produced by comproportionation of silicon tetrahalides with elemental silicon, or by condensation of lighter halopolysilanes (trimethylammonium being a useful catalyst for this reaction).[69]
Silica[edit]
Silicon dioxide (SiO
2), also known as silica, is one of the best-studied compounds, second only to water. Twelve different crystal modifications of silica are known, the most common being α-quartz, a major constituent of many rocks such as granite and sandstone. It also is known to occur in a pure form as rock crystal; impure forms are known as rose quartz, smoky quartz, morion, amethyst, and citrine. Some poorly crystalline forms of quartz are also known, such as chalcedony, chrysoprase, carnelian, agate, onyx, jasper, heliotrope, and flint. Other modifications of silicon dioxide are known in some other minerals such as tridymite and cristobalite, as well as the much less common coesite and stishovite. Biologically generated forms are also known as kieselguhr and diatomaceous earth. Vitreous silicon dioxide is known as tektites, and obsidian, and rarely as lechatelierite. Some synthetic forms are known as keatite. Opals are composed of complicated crystalline aggregates of partially hydrated silicon dioxide.[71]
-
Quartz
-
Agate
-
Tridymite
-
Cristobalite
-
Coesite
Most crystalline forms of silica are made of infinite arrangements of SiO tetrahedra (with Si at the center) connected at their corners, with each oxygen atom linked to two silicon atoms. In the thermodynamically stable room-temperature form, α-quartz, these tetrahedra are linked in intertwined helical chains with two different Si–O distances (159.7 and 161.7 pm) with a Si–O–Si angle of 144°. These helices can be either left- or right-handed, so that individual α-quartz crystals are optically active. At 537 °C, this transforms quickly and reversibly into the similar β-quartz, with a change of the Si–O–Si angle to 155° but a retention of handedness. Further heating to 867 °C results in another reversible phase transition to β-tridymite, in which some Si–O bonds are broken to allow for the arrangement of the SiO tetrahedra into a more open and less dense hexagonal structure. This transition is slow and hence tridymite occurs as a metastable mineral even below this transition temperature; when cooled to about 120 °C it quickly and reversibly transforms by slight displacements of individual silicon and oxygen atoms to α-tridymite, similarly to the transition from α-quartz to β-quartz. β-tridymite slowly transforms to cubic β-cristobalite at about 1470 °C, which once again exists metastably below this transition temperature and transforms at 200–280 °C to α-cristobalite via small atomic displacements. β-cristobalite melts at 1713 °C; the freezing of silica from the melt is quite slow and vitrification, or the formation of a glass, is likely to occur instead. In vitreous silica, the SiO tetrahedra remain corner-connected, but the symmetry and periodicity of the crystalline forms are lost. Because of the slow conversions between these three forms, it is possible upon rapid heating to melt β-quartz (1550 °C) or β-tridymite (1703 °C). Silica boils at approximately 2800 °C. Other high-pressure forms of silica are known, such as coesite and stishovite: these are known in nature, formed under the shock pressure of a meteorite impact and then rapidly quenched to preserve the crystal structure. Similar melting and cooling of silica occurs following lightning strikes, forming glassy lechatelierite. W-silica is an unstable low-density form involving SiO tetrahedra sharing opposite edges instead of corners, forming parallel chains similarly to silicon disulfide (SiS
2) and silicon diselenide (SiSe
2): it quickly returns to forming amorphous silica with heat or traces of water[72]
Condensed polysilicic acid
Silica is rather inert chemically. It is not attacked by any acids other than hydrofluoric acid. However, it slowly dissolves in hot concentrated alkalis, and does so rather quickly in fused metal hydroxides or carbonates, to give metal silicates. Among the elements, it is attacked only by fluorine at room temperature to form silicon tetrafluoride: hydrogen and carbon also react, but require temperatures over 1000 °C to do so. Silica nevertheless reacts with many metal and metalloid oxides to form a wide variety of compounds important in the glass and ceramic industries above all, but also have many other uses: for example, sodium silicate is often used in detergents due to its buffering, saponifying, and emulsifying properties[72]
Silicic acids[edit]
Adding water to silica drops its melting point by around 800 °C due to the breaking of the structure by replacing Si–O–Si linkages with terminating Si–OH groups. Increasing water concentration results in the formation of hydrated silica gels and colloidal silica dispersions. Many hydrates and silicic acids exist in the most dilute of aqueous solutions, but these are rather insoluble and quickly precipitate and condense and cross-link to form various polysilicic acids of variable combinations following the formula [SiO
x(OH)
4−2x]
n, similar to the behaviour of boron, aluminium, and iron, among other elements. Hence, although some simple silicic acids have been identified in dilute solutions, such as orthosilicic acid Si(OH)
4 and metasilicic acid SiO(OH)
2, none of these are likely to exist in the solid state.[72]
Silicate minerals[edit]
| CN 4 | LiI (59) |
BeII (27) | AlIII (39) | SiIV (26) | |
|---|---|---|---|---|---|
| CN 6 | NaI (102) | MgII (72) | AlIII (54) | TiIV (61) | FeII (78) |
| CN 8 | KI (151) | CaII (112) | |||
| CN 12 | KI (164) |
About 95% of the Earth’s crustal rocks are made of silica or silicate and aluminosilicate minerals, as reflected in oxygen, silicon, and aluminium being the three most common elements in the crust (in that order).[73] Measured by mass, silicon makes up 27.7% of the Earth’s crust.[74] Pure silicon crystals are very rarely found in nature, but notable exceptions are crystals as large as to 0.3 mm across found during sampling gases from the Kudriavy volcano on Iturup, one of the Kuril Islands.[75][76]
Silicate and aluminosilicate minerals have many different structures and varying stoichiometry, but they may be classified following some general principles. Tetrahedral SiO units are common to almost all these compounds, either as discrete structures, or combined into larger units by the sharing of corner oxygen atoms. These may be divided into neso-silicates (discrete SiO units) sharing no oxygen atoms, soro-silicates (discrete Si units) sharing one, cyclo-silicates (closed ring structures) and ino-silicates (continuous chain or ribbon structures) both sharing two, phyllo-silicates (continuous sheets) sharing three, and tecto-silicates (continuous three-dimensional frameworks) sharing four. The lattice of oxygen atoms that results is usually close-packed, or close to it, with the charge being balanced by other cations in various different polyhedral sites according to size.[77]
The orthosilicates MII
2SiO
4 (M = Be, Mg, Mn, Fe, Zn) and ZrSiO
4 are neso-silicates. Be
2SiO
4 (phenacite) is unusual as both BeII and SiIV occupy tetrahedral four-coordinated sites; the other divalent cations instead occupy six-coordinated octahedral sites and often isomorphously replace each other as in olivine, (Mg,Fe,Mn)
2SiO
4. Zircon, ZrSiO
4, demands eight-coordination of the ZrIV cations due to stoichiometry and because of their larger ionic radius (84 pm). Also significant are the garnets, [MII
3MIII
2(SiO
4)
3], in which the divalent cations (e.g. Ca, Mg, Fe) are eight-coordinated and the trivalent ones are six-coordinated (e.g. Al, Cr, Fe). Regular coordination is not always present: for example, it is not found in Ca
2SiO
4, which mixes six- and eight-coordinate sites for CaII. Soro-silicates, involving discrete double or triple tetrahedral units, are quite rare: metasilicates involving cyclic «[(SiOn
3)]2n−» units of corner-abutting tetrahedra forming a polygonal ring are also known.[73]
Chain metasilicates, {SiO2−
3}
∞, form by corner-sharing of an indefinite chain of linked SiO tetrahedra. Many differences arise due to the differing repeat distances of conformation across the line of tetrahedra. A repeat distance of two is most common, as in most pyroxene minerals, but repeat distances of one, three, four, five, six, seven, nine, and twelve are also known. These chains may then link across each other to form double chains and ribbons, as in the asbestos minerals, involving repeated chains of cyclic tetrahedron rings.[73]
A typical zeolite structure
Layer silicates, such as the clay minerals and the micas, are very common, and often are formed by horizontal cross-linking of metasilicate chains or planar condensation of smaller units. An example is kaolinite [Al
2(OH)
4Si
2O
5]; in many of these minerals cation and anion replacement is common, so that for example tetrahedral SiIV may be replaced by AlIII, octahedral AlIII by MgII, and OH−
by F−
. Three-dimensional framework aluminosilicates are structurally very complex; they may be conceived of as starting from the SiO
2 structure, but having replaced up to one-half of the SiIV atoms with AlIII, they require more cations to be included in the structure to balance charge. Examples include feldspars (the most abundant minerals on the Earth), zeolites, and ultramarines. Many feldspars can be thought of as forming part of the ternary system NaAlSi
3O
8–KAlSi
3O
8–CaAl
2Si
2O
8. Their lattice is destroyed by high pressure prompting AlIII to undergo six-coordination rather than four-coordination, and this reaction destroying feldspars may be a reason for the Mohorovičić discontinuity, which would imply that the crust and mantle have the same chemical composition, but different lattices, although this is not a universally held view. Zeolites have many polyhedral cavities in their frameworks (truncated cuboctahedra being most common, but other polyhedra also are known as zeolite cavities), allowing them to include loosely bound molecules such as water in their structure. Ultramarines alternate silicon and aluminium atoms and include a variety of other anions such as Cl−, SO2−
4, and S2−
2, but are otherwise similar to the feldspars.[73]
Other inorganic compounds[edit]
Silicon disulfide (SiS
2) is formed by burning silicon in gaseous sulfur at 100 °C; sublimation of the resulting compound in nitrogen results in white, flexible long fibers reminiscent of asbestos with a structure similar to W-silica. This melts at 1090 °C and sublimes at 1250 °C; at high temperature and pressure this transforms to a crystal structure analogous to cristobalite. However, SiS
2 lacks the variety of structures of SiO
2, and quickly hydrolyses to silica and hydrogen sulfide. It is also ammonolysed quickly and completely by liquid ammonia as follows to form an imide:[60]
- SiS
2 + 4 NH
3 → Si(NH)
2 + 2 NH
4SH
It reacts with the sulfides of sodium, magnesium, aluminium, and iron to form metal thiosilicates: reaction with ethanol results in tetraethylsilicate Si(OEt)
4 and hydrogen sulfide. Ethylsilicate is useful as its controlled hydrolysis produces adhesive or film-like forms of silica. Reacting hydrogen sulfide with silicon tetrahalides yields silicon thiohalides such as S(SiCl)
3, cyclic Cl
2Si(μ-S)
2SiCl
2, and crystalline (SiSCl
2)
4. Despite the double bond rule, stable organosilanethiones RR’Si=S have been made thanks to the stabilising mechanism of intermolecular coordination via an amine group.[78]
Silicon nitride, Si
3N
4, may be formed by directly reacting silicon with nitrogen above 1300 °C, but a more economical means of production is by heating silica and coke in a stream of nitrogen and hydrogen gas at 1500 °C. It would make a promising ceramic if not for the difficulty of working with and sintering it: chemically, it is near-totally inert, and even above 1000 °C it keeps its strength, shape, and continues to be resistant to wear and corrosion. It is very hard (9 on the Mohs hardness scale), dissociates only at 1900 °C at 1 atm, and is quite dense (density 3.185 g/cm3), because of its compact structure similar to that of phenacite (Be
2SiO
4). A similar refractory material is Si
2N
2O, formed by heating silicon and silica at 1450 °C in an argon stream containing 5% nitrogen gas, involving 4-coordinate silicon and 3-coordinate nitrogen alternating in puckered hexagonal tilings interlinked by non-linear Si–O–Si linkages to each other.[78]
Reacting silyl halides with ammonia or alkylammonia derivatives in the gaseous phase or in ethanolic solution produces various volatile silylamides, which are silicon analogues of the amines:[78]
- 3 SiH
3Cl + 4 NH
3 → N(SiH
3)
3 + 3 NH
4Cl - SiH
3Br + 2 Me
2NH → SiH
3NMe
2 + Me
2NH
2Br - 4 SiH
3I + 5 N
2H
4 → (SiH
3)
2NN(SiH
3)
2 + 4 N
2H
5I
Many such compounds have been prepared, the only known restriction being that the nitrogen is always tertiary, and species containing the SiH–NH group are unstable at room temperature. The stoichiometry around the nitrogen atom in compounds such as N(SiH
3)
3 is planar. Similarly, trisilylamines are weaker as ligands than their carbon analogues, the tertiary amines, although substitution of some SiH
3 groups by CH
3 groups mitigates this weakness. For example, N(SiH
3)
3 does not form an adduct with BH
3 at all, while MeN(SiH
3)
2 and Me
2NSiH
3 form adducts at low temperatures that decompose upon warming. Some silicon analogues of imines, with a Si=N double bond, are known: the first found was But2Si=N–SiBut3, which was discovered in 1986.[78]
Silicon carbide (SiC) was first made by Edward Goodrich Acheson in 1891, who named it carborundum to reference its intermediate hardness and abrasive power between diamond (an allotrope of carbon) and corundum (aluminium oxide). He soon founded a company to manufacture it, and today about one million tonnes are produced each year.[79] Silicon carbide exists in about 250 crystalline forms.[80] The polymorphism of SiC is characterized by a large family of similar crystalline structures called polytypes. They are variations of the same chemical compound that are identical in two dimensions and differ in the third. Thus they can be viewed as layers stacked in a certain sequence.[81] It is made industrially by reduction of quartz sand with excess coke or anthracite at 2000–2500 °C in an electric furnace:[79]
- SiO
2 + 2 C → Si + 2 CO - Si + C → SiC
It is the most thermally stable binary silicon compound, only decomposing through loss of silicon starting from around 2700 °C. It is resistant to most aqueous acids, phosphoric acid being an exception. It forms a protective layer of silicon dioxide on the surface and hence only oxidises appreciably in air above 1000 °C; removal of this layer by molten hydroxides or carbonates leads to quick oxidation. Silicon carbide is rapidly attacked by chlorine gas, which forms SiCl
4 and carbon at 100 °C and SiCl
4 and CCl
4 at 1000 °C. It is mostly used as an abrasive and a refractory material, as it is chemically stable and very strong, and it fractures to form a very sharp cutting edge. It is also useful as an intrinsic semiconductor, as well as an extrinsic semiconductor upon being doped.[79] In its diamond-like behavior it serves as an illustration of the chemical similarity between carbon and silicon.[82]
Organosilicon compounds[edit]
A hydrosilylation reaction, in which Si–H is added to an unsaturated substrate
Because the Si–C bond is close in strength to the C–C bond, organosilicon compounds tend to be markedly thermally and chemically stable. For example, tetraphenylsilane (SiPh
4) may be distilled in air even at its boiling point of 428 °C, and so may its substituted derivatives Ph
3SiCl and Ph
2SiCl
2, which boil at 378 °C and 305 °C respectively. Furthermore, since carbon and silicon are chemical congeners, organosilicon chemistry shows some significant similarities with carbon chemistry, for example in the propensity of such compounds for catenation and forming multiple bonds.[82] However, significant differences also arise: since silicon is more electropositive than carbon, bonds to more electronegative elements are generally stronger with silicon than with carbon, and vice versa. Thus the Si–F bond is significantly stronger than even the C–F bond and is one of the strongest single bonds, while the Si–H bond is much weaker than the C–H bond and is readily broken. Furthermore, the ability of silicon to expand its octet is not shared by carbon, and hence some organosilicon reactions have no organic analogues. For example, nucleophilic attack on silicon does not proceed by the SN2 or SN1 processes, but instead goes through a negatively charged true pentacoordinate intermediate and appears like a substitution at a hindered tertiary atom. This works for silicon, unlike for carbon, because the long Si–C bonds reduce the steric hindrance and there are no geometric constraints for nucleophilic attack, unlike for example a C–O σ* antibonding orbital. Nevertheless, despite these differences, the mechanism is still often called «SN2 at silicon» for simplicity.[83]
One of the most useful silicon-containing groups is trimethylsilyl, Me
3Si–. The Si–C bond connecting it to the rest of the molecule is reasonably strong, allowing it to remain while the rest of the molecule undergoes reactions, but is not so strong that it cannot be removed specifically when needed, for example by the fluoride ion, which is a very weak nucleophile for carbon compounds but a very strong one for organosilicon compounds. It may be compared to acidic protons; while trisilylmethyl is removed by hard nucleophiles instead of bases, both removals usually promote elimination. As a general rule, while saturated carbon is best attacked by nucleophiles that are neutral compounds, those based on nonmetals far down on the periodic table (e.g. sulfur, selenium, or iodine), or even both, silicon is best attacked by charged nucleophiles, particularly those involving such highly electronegative nonmetals as oxygen, fluorine, or chlorine. For example, enolates react at the carbon in haloalkanes, but at the oxygen in silyl chlorides; and when trimethylsilyl is removed from an organic molecule using hydroxide as a nucleophile, the product of the reaction is not the silanol as one would expect from using carbon chemistry as an analogy, because the siloxide is strongly nucleophilic and attacks the original molecule to yield the silyl ether hexamethyldisiloxane, (Me
3Si)
2O. Conversely, while the SN2 reaction is mostly unaffected by the presence of a partial positive charge (δ+) at the carbon, the analogous «SN2″ reaction at silicon is so affected. Thus, for example, the silyl triflates are so electrophilic that they react 108 to 109 times faster than silyl chlorides with oxygen-containing nucleophiles. Trimethylsilyl triflate is in particular a very good Lewis acid and is used to convert carbonyl compounds to acetals and silyl enol ethers, reacting them together analogously to the aldol reaction.[83]
Si–C bonds are commonly formed in three ways. In the laboratory, preparation is often carried out in small quantities by reacting tetrachlorosilane (silicon tetrachloride) with organolithium, Grignard, or organoaluminium reagents, or by catalytic addition of Si–H across C=C double bonds. The second route has the drawback of not being applicable to the most important silanes, the methyl and phenyl silanes. Organosilanes are made industrially by directly reacting alkyl or aryl halides with silicon with 10% by weight metallic copper as a catalyst. Standard organic reactions suffice to produce many derivatives; the resulting organosilanes are often significantly more reactive than their carbon congeners, readily undergoing hydrolysis, ammonolysis, alcoholysis, and condensation to form cyclic oligomers or linear polymers.[82]
Silicone polymers[edit]
The word «silicone» was first used by Frederic Kipping in 1901. He invented the word to illustrate the similarity of chemical formulae between Ph
2SiO and benzophenone, Ph
2CO, although he also stressed the lack of chemical resemblance due to the polymeric structure of Ph
2SiO, which is not shared by Ph
2CO.[82]
Silicones may be considered analogous to mineral silicates, in which the methyl groups of the silicones correspond to the isoelectronic <O−
of the silicates.[82] They are quite stable to extreme temperatures, oxidation, and water, and have useful dielectric, antistick, and antifoam properties. Furthermore, they are resistant over long periods of time to ultraviolet radiation and weathering, and are inert physiologically. They are fairly unreactive, but do react with concentrated solutions bearing the hydroxide ion and fluorinating agents, and occasionally, may even be used as mild reagents for selective syntheses. For example, (Me
3Si)
2O is valuable for the preparation of derivatives of molybdenum and tungsten oxyhalides, converting a tungsten hexachloride suspension in dichloroethane solution quantitatively to WOCl
4 in under an hour at room temperature, and then to yellow WO
2C
2 at 100 °C in light petroleum at a yield of 95% overnight.[82]
Occurrence[edit]
Silicon is the eighth most abundant element in the universe, coming after hydrogen, helium, carbon, nitrogen, oxygen, iron, and neon. These abundances are not replicated well on Earth due to substantial separation of the elements taking place during the formation of the Solar System. Silicon makes up 27.2% of the Earth’s crust by weight, second only to oxygen at 45.5%, with which it always is associated in nature. Further fractionation took place in the formation of the Earth by planetary differentiation: Earth’s core, which makes up 31.5% of the mass of the Earth, has approximate composition Fe
25Ni
2Co
0.1S
3; the mantle makes up 68.1% of the Earth’s mass and is composed mostly of denser oxides and silicates, an example being olivine, (Mg,Fe)
2SiO
4; while the lighter siliceous minerals such as aluminosilicates rise to the surface and form the crust, making up 0.4% of the Earth’s mass.[84][85]
The crystallisation of igneous rocks from magma depends on a number of factors; among them are the chemical composition of the magma, the cooling rate, and some properties of the individual minerals to be formed, such as lattice energy, melting point, and complexity of their crystal structure. As magma is cooled, olivine appears first, followed by pyroxene, amphibole, biotite mica, orthoclase feldspar, muscovite mica, quartz, zeolites, and finally, hydrothermal minerals. This sequence shows a trend toward increasingly complex silicate units with cooling, and the introduction of hydroxide and fluoride anions in addition to oxides. Many metals may substitute for silicon. After these igneous rocks undergo weathering, transport, and deposition, sedimentary rocks like clay, shale, and sandstone are formed. Metamorphism also may occur at high temperatures and pressures, creating an even vaster variety of minerals.[84]
There are four sources for silicon fluxes into the ocean include chemical weathering of continental rocks, river transport, dissolution of continental terrigenous silicates, and through the reaction between submarine basalts and hydrothermal fluid which release dissolved silicon. All four of these fluxes are interconnected in the ocean’s biogeochemical cycle as they all were initially formed from the weathering of Earth’s crust.[86]
Approximately 300–900 megatonnes of Aeolian dust is deposited into the world’s oceans each year. Of that value, 80–240 megatonnes are in the form of particulate silicon. The total amount of particulate silicon deposition into the ocean is still less than the amount of silicon influx into the ocean via riverine transportation.[87] Aeolian inputs of particulate lithogenic silicon into the North Atlantic and Western North Pacific oceans are the result of dust settling on the oceans from the Sahara and Gobi Desert, respectively.[86] Riverine transports are the major source of silicon influx into the ocean in coastal regions, while silicon deposition in the open ocean is greatly influenced by the settling of Aeolian dust.[87]
Production[edit]
Silicon of 96–99% purity is made by reducing quartzite or sand with highly pure coke. The reduction is carried out in an electric arc furnace, with an excess of SiO
2 used to stop silicon carbide (SiC) from accumulating:[29]
- SiO
2 + 2 C → Si + 2 CO - 2 SiC + SiO
2 → 3 Si + 2 CO
This reaction, known as carbothermal reduction of silicon dioxide, usually is conducted in the presence of scrap iron with low amounts of phosphorus and sulfur, producing ferrosilicon.[29] Ferrosilicon, an iron-silicon alloy that contains varying ratios of elemental silicon and iron, accounts for about 80% of the world’s production of elemental silicon, with China, the leading supplier of elemental silicon, providing 4.6 million tonnes (or 2/3rds of world output) of silicon, most of it in the form of ferrosilicon. It is followed by Russia (610,000 t), Norway (330,000 t), Brazil (240,000 t), and the United States (170,000 t).[88] Ferrosilicon is primarily used by the iron and steel industry (see below) with primary use as alloying addition in iron or steel and for de-oxidation of steel in integrated steel plants.[29]
Another reaction, sometimes used, is aluminothermal reduction of silicon dioxide, as follows:[89]
- 3 SiO
2 + 4 Al → 3 Si + 2 Al
2O
3
Leaching powdered 96–97% pure silicon with water results in ~98.5% pure silicon, which is used in the chemical industry. However, even greater purity is needed for semiconductor applications, and this is produced from the reduction of tetrachlorosilane (silicon tetrachloride) or trichlorosilane. The former is made by chlorinating scrap silicon and the latter is a byproduct of silicone production. These compounds are volatile and hence can be purified by repeated fractional distillation, followed by reduction to elemental silicon with very pure zinc metal as the reducing agent. The spongy pieces of silicon thus produced are melted and then grown to form cylindrical single crystals, before being purified by zone refining. Other routes use the thermal decomposition of silane or tetraiodosilane (SiI
4). Another process used is the reduction of sodium hexafluorosilicate, a common waste product of the phosphate fertilizer industry, by metallic sodium: this is highly exothermic and hence requires no outside energy source. Hyperfine silicon is made at a higher purity than almost any other material: transistor production requires impurity levels in silicon crystals less than 1 part per 1010, and in special cases impurity levels below 1 part per 1012 are needed and attained.[29]
Silicon nanostructures can directly be produced from silica sand using conventional metalothermic processes, or the combustion synthesis approach. Such nanostructured silicon materials can be used in various functional applications including the anode of lithium ion batteries (LIBs) or phorocatalytic applications.[90]
Applications[edit]
Compounds[edit]
Most silicon is used industrially without being purified, and indeed, often with comparatively little processing from its natural form. More than 90% of the Earth’s crust is composed of silicate minerals, which are compounds of silicon and oxygen, often with metallic ions when negatively charged silicate anions require cations to balance the charge. Many of these have direct commercial uses, such as clays, silica sand, and most kinds of building stone. Thus, the vast majority of uses for silicon are as structural compounds, either as the silicate minerals or silica (crude silicon dioxide). Silicates are used in making Portland cement (made mostly of calcium silicates) which is used in building mortar and modern stucco, but more importantly, combined with silica sand, and gravel (usually containing silicate minerals such as granite), to make the concrete that is the basis of most of the very largest industrial building projects of the modern world.[91]
Silica is used to make fire brick, a type of ceramic. Silicate minerals are also in whiteware ceramics, an important class of products usually containing various types of fired clay minerals (natural aluminium phyllosilicates). An example is porcelain, which is based on the silicate mineral kaolinite. Traditional glass (silica-based soda-lime glass) also functions in many of the same ways, and also is used for windows and containers. In addition, specialty silica based glass fibers are used for optical fiber, as well as to produce fiberglass for structural support and glass wool for thermal insulation.
Silicones often are used in waterproofing treatments, molding compounds, mold-release agents, mechanical seals, high temperature greases and waxes, and caulking compounds. Silicone is also sometimes used in breast implants, contact lenses, explosives and pyrotechnics.[92] Silly Putty was originally made by adding boric acid to silicone oil.[93] Other silicon compounds function as high-technology abrasives and new high-strength ceramics based upon silicon carbide. Silicon is a component of some superalloys.
Alloys[edit]
Elemental silicon is added to molten cast iron as ferrosilicon or silicocalcium alloys to improve performance in casting thin sections and to prevent the formation of cementite where exposed to outside air. The presence of elemental silicon in molten iron acts as a sink for oxygen, so that the steel carbon content, which must be kept within narrow limits for each type of steel, can be more closely controlled. Ferrosilicon production and use is a monitor of the steel industry, and although this form of elemental silicon is grossly impure, it accounts for 80% of the world’s use of free silicon. Silicon is an important constituent of electrical steel, modifying its resistivity and ferromagnetic properties.
The properties of silicon may be used to modify alloys with metals other than iron. «Metallurgical grade» silicon is silicon of 95–99% purity. About 55% of the world consumption of metallurgical purity silicon goes for production of aluminium-silicon alloys (silumin alloys) for aluminium part casts, mainly for use in the automotive industry. Silicon’s importance in aluminium casting is that a significantly high amount (12%) of silicon in aluminium forms a eutectic mixture which solidifies with very little thermal contraction. This greatly reduces tearing and cracks formed from stress as casting alloys cool to solidity. Silicon also significantly improves the hardness and thus wear-resistance of aluminium.[94][95]
Electronics[edit]
Silicon wafer with mirror finish
Most elemental silicon produced remains as a ferrosilicon alloy, and only approximately 20% is refined to metallurgical grade purity (a total of 1.3–1.5 million metric tons/year). An estimated 15% of the world production of metallurgical grade silicon is further refined to semiconductor purity.[95] This typically is the «nine-9» or 99.9999999% purity,[96] nearly defect-free single crystalline material.[97]
Monocrystalline silicon of such purity is usually produced by the Czochralski process, and is used to produce silicon wafers used in the semiconductor industry, in electronics, and in some high-cost and high-efficiency photovoltaic applications.[98] Pure silicon is an intrinsic semiconductor, which means that unlike metals, it conducts electron holes and electrons released from atoms by heat; silicon’s electrical conductivity increases with higher temperatures. Pure silicon has too low a conductivity (i.e., too high a resistivity) to be used as a circuit element in electronics. In practice, pure silicon is doped with small concentrations of certain other elements, which greatly increase its conductivity and adjust its electrical response by controlling the number and charge (positive or negative) of activated carriers. Such control is necessary for transistors, solar cells, semiconductor detectors, and other semiconductor devices used in the computer industry and other technical applications.[99] In silicon photonics, silicon may be used as a continuous wave Raman laser medium to produce coherent light.[100]
In common integrated circuits, a wafer of monocrystalline silicon serves as a mechanical support for the circuits, which are created by doping and insulated from each other by thin layers of silicon oxide, an insulator that is easily produced on Si surfaces by processes of thermal oxidation or local oxidation (LOCOS), which involve exposing the element to oxygen under the proper conditions that can be predicted by the Deal–Grove model. Silicon has become the most popular material for both high power semiconductors and integrated circuits because it can withstand the highest temperatures and greatest electrical activity without suffering avalanche breakdown (an electron avalanche is created when heat produces free electrons and holes, which in turn pass more current, which produces more heat). In addition, the insulating oxide of silicon is not soluble in water, which gives it an advantage over germanium (an element with similar properties which can also be used in semiconductor devices) in certain fabrication techniques.[101]
Monocrystalline silicon is expensive to produce, and is usually justified only in production of integrated circuits, where tiny crystal imperfections can interfere with tiny circuit paths. For other uses, other types of pure silicon may be employed. These include hydrogenated amorphous silicon and upgraded metallurgical-grade silicon (UMG-Si) used in the production of low-cost, large-area electronics in applications such as liquid crystal displays and of large-area, low-cost, thin-film solar cells. Such semiconductor grades of silicon are either slightly less pure or polycrystalline rather than monocrystalline, and are produced in comparable quantities as the monocrystalline silicon: 75,000 to 150,000 metric tons per year. The market for the lesser grade is growing more quickly than for monocrystalline silicon. By 2013, polycrystalline silicon production, used mostly in solar cells, was projected to reach 200,000 metric tons per year, while monocrystalline semiconductor grade silicon was expected to remain less than 50,000 tons per year.[95]
Quantum dots[edit]
Silicon quantum dots are created through the thermal processing of hydrogen silsesquioxane into nanocrystals ranging from a few nanometers to a few microns, displaying size dependent luminescent properties.[102][103] The nanocrystals display large Stokes shifts converting photons in the ultra-violet range to photons in the visible or infrared, depending on the particle size, allowing for applications in quantum dot displays and luminescent solar concentrators due to their limited self absorption. A benefit of using silicon based quantum dots over cadmium or indium is the non-toxic, metal-free nature of silicon.[104][105]
Another application of silicon quantum dots is for sensing of hazardous materials. The sensors take advantage of the luminescent properties of the quantum dots through quenching of the photoluminescence in the presence of the hazardous substance.[106] There are many methods used for hazardous chemical sensing with a few being electron transfer, fluorescence resonance energy transfer, and photocurrent generation.[107] Electron transfer quenching occurs when the lowest unoccupied molecular orbital (LUMO) is slightly lower in energy than the conduction band of the quantum dot, allowing for the transfer electrons between the two, preventing recombination of the holes and electrons within the nanocrystals. The effect can also be achieved in reverse with a donor molecule having its highest occupied molecular orbital (HOMO) slightly higher than a valence band edge of the quantum dot, allowing electrons to transfer between them, filling the holes and preventing recombination. Fluorescence resonance energy transfer occurs when a complex forms between the quantum dot and a quencher molecule. The complex will continue to absorb light but when the energy is converted to the ground state it does not release a photon, quenching the material. The third method uses different approach by measuring the photocurrent emitted by the quantum dots instead of monitoring the photoluminescent display. If the concentration of the desired chemical increases then the photocurrent given off by the nanocrystals will change in response.[108]
Biological role[edit]
A diatom, enclosed in a silica cell wall
Although silicon is readily available in the form of silicates, very few organisms use it directly. Diatoms, radiolaria, and siliceous sponges use biogenic silica as a structural material for their skeletons. Some plants accumulate silica in their tissues and require silicon for their growth, for example rice. Silicon may be taken up by plants as orthosilicic acid (also known as monosilicic acid) and transported through the xylem, where it forms amorphous complexes with components of the cell wall. This has been shown to improve cell wall strength and structural integrity in some plants, thereby reducing insect herbivory and pathogenic infections. In certain plants, silicon may also upregulate the production of volatile organic compounds and phytohormones which play a significant role in plant defense mechanisms.[109][110][111] In more advanced plants, the silica phytoliths (opal phytoliths) are rigid microscopic bodies occurring in the cell.[112][113][110]
Several horticultural crops are known to protect themselves against fungal plant pathogens with silica, to such a degree that fungicide application may fail unless accompanied by sufficient silicon nutrition. Silicaceous plant defense molecules activate some phytoalexins, meaning some of them are signalling substances producing acquired immunity. When deprived, some plants will substitute with increased production of other defensive substances.[110]
Life on Earth is largely composed of carbon, but astrobiology considers that extraterrestrial life may have other hypothetical types of biochemistry. Silicon is considered an alternative to carbon, as it can create complex and stable molecules with four covalent bonds, required for a DNA-analog, and it is available in large quantities.[114]
Marine microbial influences[edit]
Diatoms uses silicon in the biogenic silica (BSIO
2) form,[115] which is taken up by the silicon transport protein (SIT) to be predominantly used in the cell wall structure as frustules.[116] Silicon enters the ocean in a dissolved form such as silicic acid or silicate.[117] Since diatoms are one of the main users of these forms of silicon, they contribute greatly to the concentration of silicon throughout the ocean. Silicon forms a nutrient-like profile in the ocean due to the diatom productivity in shallow depths.[117] Therefore, less concentration of silicon in the upper ocean and more concentrations of silicon in the deep/lower ocean.
Diatom productivity in the upper ocean contribute to the amount of silicon exported to the lower ocean.[118] When diatom cells are lysed in the upper ocean, their nutrients like, iron, zinc, and silicon, are brought to the lower ocean through a process called marine snow. Marine snow involves the downward transfer of particulate organic matter by vertical mixing of dissolved organic matter.[119] It has been suggested that silicon is considered crucial to diatom productivity and as long as there is silicic acid available for diatoms to use, the diatoms can contribute to other important nutrient concentrations in the deep ocean as well.[120]
In coastal zones, diatoms serve as the major phytoplanktonic organisms and greatly contribute to biogenic silica production. In the open ocean, however, diatoms have a reduced role in global annual silica production. Diatoms in North Atlantic and North Pacific subtropical gyres only contribute about 5-7% of global annual marine silica production. The Southern Ocean produces about one-third of global marine biogenic silica.[86] The Southern Ocean is referred to as having a «biogeochemical divide»[121] since only minuscule amounts of silicon are transported out of this region.
Human nutrition[edit]
There is some evidence that silicon is important to human health for their nail, hair, bone, and skin tissues,[122] for example, in studies that demonstrate that premenopausal women with higher dietary silicon intake have higher bone density, and that silicon supplementation can increase bone volume and density in patients with osteoporosis.[123] Silicon is needed for synthesis of elastin and collagen, of which the aorta contains the greatest quantity in the human body,[124] and has been considered an essential element;[125] nevertheless, it is difficult to prove its essentiality, because silicon is very common, and hence, deficiency symptoms are difficult to reproduce.[126][127]
Silicon is currently under consideration for elevation to the status of a «plant beneficial substance by the Association of American Plant Food Control Officials (AAPFCO).»[128][129]
Safety[edit]
People may be exposed to elemental silicon in the workplace by breathing it in, swallowing it, or having contact with the skin or eye. In the latter two cases, silicon poses a slight hazard as an irritant. It is hazardous if inhaled.[130] The Occupational Safety and Health Administration (OSHA) has set the legal limit for silicon exposure in the workplace as 15 mg/m3 total exposure and 5 mg/m3 respiratory exposure over an eight-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 10 mg/m3 total exposure and 5 mg/m3 respiratory exposure over an eight-hour workday.[131] Inhalation of crystalline silica dust may lead to silicosis, an occupational lung disease marked by inflammation and scarring in the form of nodular lesions in the upper lobes of the lungs.[132]
See also[edit]
- Amorphous silicon
- Black silicon
- Covalent superconductors
- List of countries by silicon production
- List of silicon producers
- Monocrystalline silicon
- Silicon Nanowires (SiNWs)
- Polycrystalline silicon
- Printed silicon electronics
- Silicon tombac
- Silicon Valley
- Silicene
- Transistor
References[edit]
- ^ «Standard Atomic Weights: Silicon». CIAAW. 2009.
- ^ «New Type of Zero-Valent Tin Compound». Chemistry Europe. 27 August 2016.
- ^ Ram, R. S.; et al. (1998). «Fourier Transform Emission Spectroscopy of the A2D–X2P Transition of SiH and SiD» (PDF). J. Mol. Spectr. 190 (2): 341–352. doi:10.1006/jmsp.1998.7582. PMID 9668026.
- ^ Eranna, Golla (2014). Crystal Growth and Evaluation of Silicon for VLSI and ULSI. CRC Press. p. 7. ISBN 978-1-4822-3281-3.
- ^ Magnetic susceptibility of the elements and inorganic compounds, in Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ a b c d Hopcroft, Matthew A.; Nix, William D.; Kenny, Thomas W. (2010). «What is the Young’s Modulus of Silicon?». Journal of Microelectromechanical Systems. 19 (2): 229. doi:10.1109/JMEMS.2009.2039697.
- ^ Weeks, Mary Elvira (1932). «The discovery of the elements: XII. Other elements isolated with the aid of potassium and sodium: beryllium, boron, silicon, and aluminum». Journal of Chemical Education. 9 (8): 1386–1412. Bibcode:1932JChEd…9.1386W. doi:10.1021/ed009p1386.
- ^ Voronkov, M. G. (2007). «Silicon era». Russian Journal of Applied Chemistry. 80 (12): 2190. doi:10.1134/S1070427207120397.
- ^ Kamal 2022
- ^ Cutter, Elizabeth G. (1978). Plant Anatomy. Part 1 Cells and Tissues (2nd ed.). London: Edward Arnold. ISBN 978-0-7131-2639-6.
- ^ «Silicon». Encyclopedia Britannica. Retrieved 22 August 2019.
- ^ In his table of the elements, Lavoisier listed five «salifiable earths» (i.e., ores that could be made to react with acids to produce salts (salis = salt, in Latin): chaux (calcium oxide), magnésie (magnesia, magnesium oxide), baryte (barium sulfate), alumine (alumina, aluminium oxide), and silice (silica, silicon dioxide). About these «elements», Lavoisier speculates: «We are probably only acquainted as yet with a part of the metallic substances existing in nature, as all those which have a stronger affinity to oxygen than carbon possesses, are incapable, hitherto, of being reduced to a metallic state, and consequently, being only presented to our observation under the form of oxyds, are confounded with earths. It is extremely probable that barytes, which we have just now arranged with earths, is in this situation; for in many experiments it exhibits properties nearly approaching to those of metallic bodies. It is even possible that all the substances we call earths may be only metallic oxyds, irreducible by any hitherto known process.» – from Lavoisier (1799). Elements of Chemistry. Translated by Robert Kerr (4 ed.). Edinburgh, Scotland: William Creec. p. 218. (The original passage appears in: Lavoisier (1789). Traité Élémentaire de Chimie. Vol. 1. Paris, France: Cuchet. p. 174.
- ^ a b c d e Greenwood & Earnshaw 1997, p. 328.
- ^ Davy, Humphry (1808). «Electro chemical researches, on the decomposition of the earths; with observations on the metals obtained from the alkaline earths, and on the amalgam procured from ammonia». Philosophical Transactions of the Royal Society of London. W. Bowyer and J. Nichols. 98: 333–370.. On p. 353 Davy coins the name «silicium» : «Had I been so fortunate as to have obtained more certain evidences on this subject, and to have procured the metallic substances I was in search of, I should have proposed for them the names of silicium [silicon], alumium [aluminium], zirconium, and glucium [beryllium].»
- ^ «14 Silicon». Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Gay-Lussac, Joseph Louis; Thénard, Louis Jacques baron (1811). Recherches physico-chimiques, faites sur la pile: sur la préparation chimique et les propriétés du potassium et du sodium; sur la décomposition de l’acide boracique; sur les acides fluorique, muriatique et muriatique oxigéné; sur l’action chimique de la lumière; sur l’analyse végétale et animale, etc (in French). Deterville. pp. 313–314; vol. 2, pp. 55–65.
- ^ Thomson, Thomas; Baldwin, Charles; Blackwood, William; Baldwin, Cradock; Bell & Bradfute, bookseller; Hodges & McArthur, bookseller (1817). A system of chemistry : in four volumes. University of Wisconsin — Madison. London : Printed for Baldwin, Craddock, and Joy, Paternoster-Row; William Blackwood, and Bell and Bradfute, Edinburgh; and Hodges and Macarthur, Dublin. p. 252.: «The base of silica has been usually considered as a metal, and called silicium. But as there is not the smallest evidence for its metallic nature, and as it bears a close resemblance to boron and carbon, it is better to class it along with these bodies, and to give it the name of silicon.»
- ^ See
- Berzelius announced his discovery of silicon («silicium») in: Berzelius, J. (presented: 1823; published: 1824) «Undersökning af flusspatssyran och dess märkvärdigaste föreningar» (Investigation of hydrofluoric acid and of its most noteworthy compounds), Kongliga Vetenskaps-Academiens Handlingar [Proceedings of the Royal Science Academy], 12 : 46–98. The isolation of silicon and its characterization are detailed in the section titled «Flussspatssyrad kisseljords sönderdelning med kalium,» pp. 46–68.
- The above article was printed in German in: J.J. Berzelius (1824) «II. Untersuchungen über Flussspathsäure und deren merkwürdigsten Verbindungen» (II. Investigations of hydrofluoric acid and its most noteworthy compounds), Annalen der Physik, 77: 169–230. The isolation of silicon is detailed in the section titled: «Zersetzung der flussspaths. Kieselerde durch Kalium» (Decomposition of silicate fluoride by potassium), pp. 204–210.
- The above article was reprinted in French in: Berzelius (1824) «Décomposition du fluate de silice par le potassium» (Decomposition of silica fluoride by potassium), Annales de Chimie et de Physique, 27: 337–359.
- Reprinted in English in: «On the mode of obtaining silicium, and on the characters and properties of that substance». The Philosophical Magazine and Journal: Comprehending Various Branches of Science, the Liberal and Fine Arts, Agriculture, Manufactures, and Commerce. Richard Taylor and Company. 65: 254–267. 1825.
- ^ Weeks, Mary Elvira (1932). «The discovery of the elements: XII. Other elements isolated with the aid of potassium and sodium: beryllium, boron, silicon, and aluminum». Journal of Chemical Education. 9 (8): 1386–1412. Bibcode:1932JChEd…9.1386W. doi:10.1021/ed009p1386.
- ^ Voronkov, M.G. (2007). «Silicon era». Russian Journal of Applied Chemistry. 80 (12): 2190. doi:10.1134/S1070427207120397. S2CID 195240638.
- ^ a b Kipping, Frederic Stanley (1937-03-01). «The bakerian lecture organic derivatives of silicon». Proceedings of the Royal Society of London. Series A — Mathematical and Physical Sciences. 159 (896): 139–148. doi:10.1098/rspa.1937.0063.
- ^ Muller, Richard (January 1965). «One hundred years of organosilicon chemistry». Journal of Chemical Education. 42 (1): 41. doi:10.1021/ed042p41. ISSN 0021-9584.
- ^ In 1854, Deville was trying to prepare aluminium metal from aluminium chloride that was heavily contaminated with silicon chloride. Deville used two methods to prepare aluminium: heating aluminium chloride with sodium metal in an inert atmosphere (of hydrogen); and melting aluminum chloride with sodium chloride and then electrolyzing the mixture. In both cases, pure silicon was produced: the silicon dissolved in the molten aluminium, but crystallized upon cooling. Dissolving the crude aluminum in hydrochloric acid revealed flakes of crystallized silicon. See: Henri Sainte-Claire Deville (1854) «Note sur deux procédés de préparation de l’aluminium et sur une nouvelle forme du silicium» (Note on two procedures for the preparation of aluminium and on a new form of silicon), Comptes rendus, 39: 321–326.
Subsequently, Deville obtained crystalline silicon by heating the chloride or fluoride of silicon with sodium metal, isolating the amorphous silicon, then melting the amorphous form with salt and heating the mixture until most of the salt evaporated. See: Sainte-Claire Deville, H. (1855). «Du silicium et du titane (On silicon and titanium)». Comptes rendus. 40: 1034–1036. - ^ «Information on silicon – history, thermodynamic, chemical, physical and electronic properties». Etacude. Retrieved 2021-06-08.
- ^ «Silicon: History». Nautilus.fis.uc.pt. 2011-07-27. Archived from the original on July 27, 2011.
- ^ a b Aufray, B.; Kara, A.; Vizzini, S. B.; Oughaddou, H.; LéAndri, C.; Ealet, B.; Le Lay, G. (2010). «Graphene-like silicon nanoribbons on Ag(110): A possible formation of silicene». Applied Physics Letters. 96 (18): 183102. Bibcode:2010ApPhL..96r3102A. doi:10.1063/1.3419932.
- ^ a b Lalmi, B.; Oughaddou, H.; Enriquez, H.; Kara, A.; Vizzini, S. B.; Ealet, B. N.; Aufray, B. (2010). «Epitaxial growth of a silicene sheet». Applied Physics Letters. 97 (22): 223109. arXiv:1204.0523. Bibcode:2010ApPhL..97v3109L. doi:10.1063/1.3524215. S2CID 118490651.
- ^ a b c d e f g h i j k l m n Greenwood & Earnshaw 1997, p. 330.
- ^ Greenwood & Earnshaw 1997, pp. 337–340
- ^ a b «Timeline». The Silicon Engine. Computer History Museum. Retrieved 22 August 2019.
- ^ a b «1901: Semiconductor Rectifiers Patented as «Cat’s Whisker» Detectors». The Silicon Engine. Computer History Museum. Retrieved 23 August 2019.
- ^ «1947: Invention of the Point-Contact Transistor». The Silicon Engine. Computer History Museum. Retrieved 23 August 2019.
- ^ «1954: Morris Tanenbaum fabricates the first silicon transistor at Bell Labs». The Silicon Engine. Computer History Museum. Retrieved 23 August 2019.
- ^ Bassett, Ross Knox (2007). To the Digital Age: Research Labs, Start-up Companies, and the Rise of MOS Technology. Johns Hopkins University Press. pp. 22–23. ISBN 978-0-8018-8639-3.
- ^ Saxena, A. (2009). Invention of integrated circuits: untold important facts. International series on advances in solid state electronics and technology. World Scientific. pp. 96–97. ISBN 978-981-281-445-6.
- ^ a b Feldman, Leonard C. (2001). «Introduction». Fundamental Aspects of Silicon Oxidation. Springer Science & Business Media. pp. 1–11. ISBN 978-3-540-41682-1.
- ^ Dabrowski, Jarek; Müssig, Hans-Joachim (2000). «1.2. The Silicon Age». Silicon Surfaces and Formation of Interfaces: Basic Science in the Industrial World. World Scientific. pp. 3–13. ISBN 978-981-02-3286-3.
- ^ Siffert, Paul; Krimmel, Eberhard (2013). «Preface». Silicon: Evolution and Future of a Technology. Springer Science & Business Media. ISBN 978-3-662-09897-4.
- ^ Uskali, T.; Nordfors, D. (23 May 2007). «The role of journalism in creating the metaphor of Silicon Valley» (PDF). Innovation Journalism 4 Conference, Stanford University. Archived from the original (PDF) on 2012-09-07. Retrieved 2016-08-08.
- ^ «Silicon and Germanium». hyperphysics.phy-astr.gsu.edu. Retrieved 2021-06-07.
- ^ King 1995, pp. xiii–xviii
- ^ Greenwood & Earnshaw 1997, pp. 372.
- ^ a b c d e f g h i j Greenwood & Earnshaw 1997, p. 331.
- ^ Vladimir E. Dmitrienko and Viacheslav A. Chizhikov (2020). «An infinite family of bc8-like metastable phases in silicon» (PDF). Phys. Rev. B. 101 (24): 245203. arXiv:1912.10672. Bibcode:2020PhRvB.101x5203D. doi:10.1103/PhysRevB.101.245203. S2CID 209444444.
- ^ a b c d e f g Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). «The NUBASE2016 evaluation of nuclear properties» (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- ^ Jerschow, Alexej. «Interactive NMR Frequency Map». New York University. Retrieved 2011-10-20.
- ^ Seitenzahl, Ivo Rolf; Townsley, Dean M. (2017). Nucleosynthesis in Thermonuclear Supernovae. Handbook of Supernovae. pp. 1955–1978. arXiv:1704.00415. Bibcode:2017hsn..book.1955S. doi:10.1007/978-3-319-21846-5_87. ISBN 978-3-319-21845-8. S2CID 118993185.
- ^ Khokhlov, A. M.; Oran, E. S.; Wheeler, J. C. (April 1997). «Deflagration‐to‐Detonation Transition in Thermonuclear Supernovae». The Astrophysical Journal. 478 (2): 678–688. arXiv:astro-ph/9612226. Bibcode:1997ApJ…478..678K. doi:10.1086/303815. S2CID 53486905.
- ^ Cameron, A.G.W. (1973). «Abundance of the Elements in the Solar System» (PDF). Space Science Reviews. 15 (1): 121–146. Bibcode:1973SSRv…15..121C. doi:10.1007/BF00172440. S2CID 120201972. Archived from the original (PDF) on 2011-10-21.
- ^ Reynolds, B. C. (June 2009). «Modeling the modern marine δ 30 Si distribution: MODELING THE MODERN MARINE δ 30 Si DISTRIBUTION». Global Biogeochemical Cycles. 23 (2): 1–13. doi:10.1029/2008GB003266. S2CID 128652214.
- ^ Stapf, André; Gondek, Christoph; Kroke, Edwin; Roewer, Gerhard (2019), Yang, Deren (ed.), «Wafer Cleaning, Etching, and Texturization», Handbook of Photovoltaic Silicon, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 311–358, doi:10.1007/978-3-662-56472-1_17, ISBN 978-3-662-56471-4, S2CID 226945433, retrieved 2021-03-07
- ^ a b Grabmaier, J. (1982). Silicon Chemical Etching. Berlin, Heidelberg: Springer Berlin Heidelberg. ISBN 978-3-642-68765-5. OCLC 840294227.
- ^ Greenwood & Earnshaw 1997, pp. 331–5
- ^ Kaupp, Martin (1 December 2006). «The role of radial nodes of atomic orbitals for chemical bonding and the periodic table» (PDF). Journal of Computational Chemistry. 28 (1): 320–325. doi:10.1002/jcc.20522. PMID 17143872. S2CID 12677737. Retrieved 14 October 2016.
- ^ a b c King 1995, pp. 43–44
- ^ a b Greenwood & Earnshaw 1997, pp. 374.
- ^ Kaupp, Martin (2007). «The role of radial nodes of atomic orbitals for chemical bonding and the periodic table». Journal of Computational Chemistry. 28 (1): 320–325. doi:10.1002/jcc.20522. ISSN 0192-8651. PMID 17143872.
- ^ Greenwood & Earnshaw 1997, pp. 327–328.
- ^ a b Greenwood & Earnshaw 1997, pp. 359–361.
- ^ Greenwood & Earnshaw 1997, p. 335-337.
- ^ King 1995, pp. 45–47
- ^ Wiber, E. (1977). «Alfred Stock and the Renaissance of Inorganic Chemistry» (PDF). Pure Appl. Chem. 49 (6): 691–700. doi:10.1351/pac197749060691. S2CID 53313463.
- ^ Mellor, J.W. (1947). A Comprehensive Treatise on Inorganic and Theoretical Chemistry. Vol. VI, [C(Part II), Si, Silicates]. Longman, Green and Co. pp. 223–7. OCLC 1044702591.
- ^ Porterfield, W.W. (2013) [1993]. «4.8 Bonding in Elements». Inorganic Chemistry: A Unified Approach (2nd ed.). Elsevier. p. 219. ISBN 978-0-323-13894-9.
- ^ Wiberg, N.; Wiberg, E.; Holleman, A.F. (2001). «2.2.3 Higher Saturated Silanes». Inorganic Chemistry. Academic Press. p. 844. ISBN 0-12-352651-5.
- ^ King 1995, p. 47
- ^ Miller, R.D.; Michl, J. (1989). «Polysilane high polymers». Chemical Reviews. 89 (6): 1359. doi:10.1021/cr00096a006.
- ^ a b c d Greenwood & Earnshaw 1997, pp. 340.
- ^ a b c King 1995, p. 48
- ^ Greenwood & Earnshaw 1997, p. 342-347.
- ^ a b c Greenwood & Earnshaw 1997, p. 342.
- ^ a b c d e Greenwood & Earnshaw 1997, p. 347.
- ^ Geological Survey (U.S.) (1975). Geological Survey professional paper.
- ^ Korzhinsky, M.A.; Tkachenko, S.I.; Shmulovich, K.I.; Steinberg, G.S. (1995). «Native AI and Si formation» (PDF). Nature. 375 (6532): 544. Bibcode:1995Natur.375..544K. doi:10.1038/375544a0. ISSN 0028-0836. S2CID 39954119.
- ^ Cordua, Courtesy of Dr Bill (1998-01-10), English: PDF file entitled: «Silicon, Silica, Silicates and Silicone» (PDF), archived from the original (PDF) on 2016-04-18, retrieved 2016-03-29
- ^ Greenwood & Earnshaw 1997, p. 347-359.
- ^ a b c d Greenwood & Earnshaw 1997, p. 359.
- ^ a b c Greenwood & Earnshaw 1997, p. 334.
- ^ Cheung, Rebecca (2006). Silicon carbide microelectromechanical systems for harsh environments. Imperial College Press. p. 3. ISBN 978-1-86094-624-0.
- ^ Morkoç, H.; Strite, S.; Gao, G.B.; Lin, M.E.; Sverdlov, B.; Burns, M. (1994). «Large-band-gap SiC, III–V nitride, and II–VI ZnSe-based semiconductor device technologies». Journal of Applied Physics. 76 (3): 1363. Bibcode:1994JAP….76.1363M. doi:10.1063/1.358463.
- ^ a b c d e f Greenwood & Earnshaw 1997, p. 361.
- ^ a b Clayden, pp. 668–77
- ^ a b Greenwood & Earnshaw 1997, p. 329.
- ^ Greenwood & Earnshaw 1997, pp. 329–330
- ^ a b c Tréguer, Paul J.; De La Rocha, Christina L. (3 January 2013). «The World Ocean Silica Cycle». Annual Review of Marine Science. 5 (1): 477–501. doi:10.1146/annurev-marine-121211-172346. PMID 22809182.
- ^ a b Tegen, Ina; Kohfeld, Karen (2006). Atmospheric transport of silicon. Island Press. pp. 81–91. ISBN 1-59726-115-7.
- ^ «Silicon Commodities Report 2011» (PDF). USGS. Retrieved 2011-10-20.
- ^ Zulehner, Neuer & Rau, p. 574
- ^ Kamali, A.R. (2019). «Ultra-fast shock-wave combustion synthesis of nanostructured silicon from sand with excellent Li storage performance». Sustainable Energy Fuels. 3 (6): 1396–1405. doi:10.1039/C9SE00046A. S2CID 139986478.
- ^ Greenwood & Earnshaw 1997, p. 356.
- ^ Koch, E.C.; Clement, D. (2007). «Special Materials in Pyrotechnics: VI. Silicon – An Old Fuel with New Perspectives». Propellants, Explosives, Pyrotechnics. 32 (3): 205. doi:10.1002/prep.200700021.
- ^ Walsh, Tim (2005). «Silly Putty». Timeless toys: classic toys and the playmakers who created them. Andrews McMeel Publishing. ISBN 978-0-7407-5571-2.
- ^ Apelian, D. (2009). «Aluminum Cast Alloys: Enabling Tools for Improved Performance» (PDF). Wheeling, Illinois: North American Die Casting Association. Archived from the original (PDF) on 2012-01-06.
- ^ a b c Corathers, Lisa A. 2009 Minerals Yearbook. USGS
- ^ «Semi» SemiSource 2006: A supplement to Semiconductor International. December 2005. Reference Section: How to Make a Chip. Adapted from Design News. Reed Electronics Group.
- ^ SemiSource 2006: A supplement to Semiconductor International. December 2005. Reference Section: How to Make a Chip. Adapted from Design News. Reed Electronics Group.
- ^ Zulehner, Neuer & Rau, p. 590
- ^ Zulehner, Neuer & Rau, p. 573
- ^ Dekker, R; Usechak, N; Först, M; Driessen, A (2008). «Ultrafast nonlinear all-optical processes in silicon-on-insulator waveguides». Journal of Physics D. 40 (14): R249–R271. Bibcode:2007JPhD…40..249D. doi:10.1088/0022-3727/40/14/r01. S2CID 123008652.
- ^ Semiconductors Without the Quantum Physics. Electropaedia
- ^ Clark, Rhett J.; Aghajamali, Maryam; Gonzalez, Christina M.; Hadidi, Lida; Islam, Muhammad Amirul; Javadi, Morteza; Mobarok, Md Hosnay; Purkait, Tapas K.; Robidillo, Christopher Jay T.; Sinelnikov, Regina; Thiessen, Alyxandra N. (2017-01-10). «From Hydrogen Silsesquioxane to Functionalized Silicon Nanocrystals». Chemistry of Materials. 29 (1): 80–89. doi:10.1021/acs.chemmater.6b02667. ISSN 0897-4756.
- ^ Hessel, Colin M.; Henderson, Eric J.; Veinot, Jonathan G. C. (2007). «Hydrogen Silsesquioxane: A Molecular Precursor for Nanocrystalline Si—SiO2 Composites and Freestanding Hydride-Surface-Terminated Silicon Nanoparticles». ChemInform. 38 (10). doi:10.1002/chin.200710014. ISSN 1522-2667.
- ^ Lim, Cheol Hong; Han, Jeong-Hee; Cho, Hae-Won; Kang, Mingu (2014). «Studies on the Toxicity and Distribution of Indium Compounds According to Particle Size in Sprague-Dawley Rats». Toxicological Research. 30 (1): 55–63. doi:10.5487/TR.2014.30.1.055. ISSN 1976-8257. PMC 4007045. PMID 24795801.
- ^ Zou, Hui; Wang, Tao; Yuan, Junzhao; Sun, Jian; Yuan, Yan asdf; Gu, Jianhong; Liu, Xuezhong; Bian, Jianchun; Liu, Zongping (2020-03-15). «Cadmium-induced cytotoxicity in mouse liver cells is associated with the disruption of autophagic flux via inhibiting the fusion of autophagosomes and lysosomes». Toxicology Letters. 321: 32–43. doi:10.1016/j.toxlet.2019.12.019. ISSN 0378-4274. PMID 31862506. S2CID 209435190.
- ^ Nguyen, An; Gonzalez, Christina M; Sinelnikov, Regina; Newman, W; Sun, Sarah; Lockwood, Ross; Veinot, Jonathan G C; Meldrum, Al (2016-02-10). «Detection of nitroaromatics in the solid, solution, and vapor phases using silicon quantum dot sensors». Nanotechnology. 27 (10): 105501. Bibcode:2016Nanot..27j5501N. doi:10.1088/0957-4484/27/10/105501. ISSN 0957-4484. PMID 26863492. S2CID 24292648.
- ^ Gonzalez, Christina M.; Veinot, Jonathan G. C. (2016-06-02). «Silicon nanocrystals for the development of sensing platforms». Journal of Materials Chemistry C. 4 (22): 4836–4846. doi:10.1039/C6TC01159D. ISSN 2050-7534.
- ^ Yue, Zhao; Lisdat, Fred; Parak, Wolfgang J.; Hickey, Stephen G.; Tu, Liping; Sabir, Nadeem; Dorfs, Dirk; Bigall, Nadja C. (2013-04-24). «Quantum-Dot-Based Photoelectrochemical Sensors for Chemical and Biological Detection». ACS Applied Materials & Interfaces. 5 (8): 2800–2814. doi:10.1021/am3028662. ISSN 1944-8244. PMID 23547912.
- ^ Kim, Sang Gyu; Kim, Ki Woo; Park, Eun Woo; Choi, Doil (2002). «Silicon-Induced Cell Wall Fortification of Rice Leaves: A Possible Cellular Mechanism of Enhanced Host Resistance to Blast». Phytopathology. 92 (10): 1095–103. doi:10.1094/PHYTO.2002.92.10.1095. PMID 18944220.
- ^ a b c Epstein, Emanuel (1999). «SILICON». Annual Review of Plant Physiology and Plant Molecular Biology. 50: 641–664. doi:10.1146/annurev.arplant.50.1.641. PMID 15012222.
- ^ Leroy, Nicolas; de Tombeur, Felix; Walgraffe, Yseult; Cornelis, Jean-Thomas; Verheggen, Francois (23 October 2019). «Silicon and plant natural defenses against insect pests: impact on plant volatile organic compounds and cascade effects on multitrophic interactions». Plants. 8 (444): 444. doi:10.3390/plants8110444. PMC 6918431. PMID 31652861.
- ^ Rahman, Atta-ur- (2008). «Silicon». Studies in Natural Products Chemistry. Vol. 35. p. 856. ISBN 978-0-444-53181-0.
- ^ Exley, C. (1998). «Silicon in life:A bioinorganic solution to bioorganic essentiality». Journal of Inorganic Biochemistry. 69 (3): 139–144. doi:10.1016/S0162-0134(97)10010-1.
- ^ Aguilera Mochón, Juan Antonio (2016). La vida no terrestre [The non-terrestrial life] (in Spanish). RBA. pp. 43–45. ISBN 978-84-473-8665-9.
- ^ Bidle, Kay D.; Manganelli, Maura; Azam, Farooq (2002-12-06). «Regulation of Oceanic Silicon and Carbon Preservation by Temperature Control on Bacteria». Science. 298 (5600): 1980–1984. Bibcode:2002Sci…298.1980B. doi:10.1126/science.1076076. ISSN 0036-8075. PMID 12471255. S2CID 216994.
- ^ Durkin, Colleen A.; Koester, Julie A.; Bender, Sara J.; Armbrust, E. Virginia (2016). «The evolution of silicon transporters in diatoms». Journal of Phycology. 52 (5): 716–731. doi:10.1111/jpy.12441. ISSN 1529-8817. PMC 5129515. PMID 27335204.
- ^ a b Dugdale, R. C.; Wilkerson, F. P. (2001-12-30). «Sources and fates of silicon in the ocean: the role of diatoms in the climate and glacial cycles». Scientia Marina. 65 (S2): 141–152. doi:10.3989/scimar.2001.65s2141. ISSN 1886-8134.
- ^ Baines, Stephen B.; Twining, Benjamin S.; Brzezinski, Mark A.; Krause, Jeffrey W.; Vogt, Stefan; Assael, Dylan; McDaniel, Hannah (December 2012). «Significant silicon accumulation by marine picocyanobacteria». Nature Geoscience. 5 (12): 886–891. Bibcode:2012NatGe…5..886B. doi:10.1038/ngeo1641. ISSN 1752-0908.
- ^ Turner, Jefferson T. (January 2015). «Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump». Progress in Oceanography. 130: 205–248. Bibcode:2015PrOce.130..205T. doi:10.1016/j.pocean.2014.08.005. ISSN 0079-6611.
- ^ Yool, Andrew; Tyrrell, Toby (2003). «Role of diatoms in regulating the ocean’s silicon cycle». Global Biogeochemical Cycles. 17 (4): n/a. Bibcode:2003GBioC..17.1103Y. CiteSeerX 10.1.1.394.3912. doi:10.1029/2002GB002018. ISSN 1944-9224. S2CID 16849373.
- ^ Marinov, I.; Gnanadesikan, A.; Toggweiler, J. R.; Sarmiento, J. L. (June 2006). «The Southern Ocean biogeochemical divide». Nature. 441 (7096): 964–967. Bibcode:2006Natur.441..964M. doi:10.1038/nature04883. PMID 16791191. S2CID 4428683.
- ^ Martin, Keith R. (2013). «Chapter 14. Silicon: The Health Benefits of a Metalloid». In Astrid Sigel; Helmut Sigel; Roland K.O. Sigel (eds.). Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. Vol. 13. Springer. pp. 451–473. doi:10.1007/978-94-007-7500-8_14. ISBN 978-94-007-7499-5. PMID 24470100.
- ^ Jugdaohsingh, R. (Mar–Apr 2007). «Silicon and bone health». The Journal of Nutrition, Health and Aging. 11 (2): 99–110. PMC 2658806. PMID 17435952.
- ^ Loeper, J.; Fragny, M. (1978). The Physiological Role of the Silicon and its AntiAtheromatous Action. Biochemistry of Silicon and Related Problems. pp. 281–296. doi:10.1007/978-1-4613-4018-8_13. ISBN 978-1-4613-4020-1.
- ^ Nielsen, Forrest H. (1984). «Ultratrace Elements in Nutrition». Annual Review of Nutrition. 4: 21–41. doi:10.1146/annurev.nu.04.070184.000321. PMID 6087860.
- ^ Lippard, Stephen J.; Jeremy M. Berg (1994). Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books. p. 411. ISBN 978-0-935702-72-9.
- ^ Muhammad Ansar Farooq; Karl-Josef Dietz (2015). «Silicon as Versatile Player in Plant and Human Biology: Overlooked and Poorly Understood Muhammad Ansar Farooq and Karl-J». Front. Plant Sci. 6 (994): 994. doi:10.3389/fpls.2015.00994. PMC 4641902. PMID 26617630.
- ^ «AAPFCO Board of Directors 2006 Mid-Year Meeting» (PDF). Association of American Plant Food Control Officials. Archived from the original (PDF) on 6 January 2012. Retrieved 2011-07-18.
A presentation was made for Excell Minerals to recognize Silicon as a recognized plant nutrient
- ^ Miranda, Stephen R.; Barker, Bruce (August 4, 2009). «Silicon: Summary of Extraction Methods». Harsco Minerals. Archived from the original on November 12, 2012. Retrieved 2011-07-18.
- ^ Science Lab.com. «Material Safety Data Sheet: Silicon MSDS». sciencelab.com. Archived from the original on 23 March 2018. Retrieved 11 March 2018.
- ^ «CDC – NIOSH Pocket Guide to Chemical Hazards – Silicon». www.cdc.gov. Retrieved 2015-11-21.
- ^ Jane A. Plant; Nick Voulvoulis; K. Vala Ragnarsdottir (2012). Pollutants, Human Health and the Environment: A Risk Based Approach. Applied Geochemistry. Vol. 26. John Wiley & Sons. p. 273. Bibcode:2011ApGC…26S.238P. doi:10.1016/j.apgeochem.2011.03.113. ISBN 978-0-470-74261-7. Retrieved 24 August 2012.
Bibliography[edit]
- Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford University Press. ISBN 978-0-19-927029-3.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- King, R. Bruce (1995). Inorganic Chemistry of Main Group Elements. Wiley-VCH. ISBN 978-0-471-18602-1.
- Zulehner, Werner; Neuer, Bernd; Rau, Gerhard. «Silicon». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_721.
- Kamal, Kamal Y. (2022). «The Silicon Age: Trends in Semiconductor Devices Industry» (PDF). Journal of Engineering Science and Technology Review. 15 (1): 110–5. doi:10.25103/jestr.151.14. S2CID 249074588.
External links[edit]
- «Silicon Video — The Periodic Table of Videos — University of Nottingham». www.periodicvideos.com. Retrieved 2021-06-08.
- «CDC — NIOSH Pocket Guide to Chemical Hazards — Silicon». www.cdc.gov. Retrieved 2021-06-08.
- «Physical properties of Silicon (Si)». www.ioffe.ru. Retrieved 2021-06-08.
- The Story of Solar-Grade Silicon. Asianometry. 30 November 2022.
1. Положение кремния в периодической системе химических элементов
2. Электронное строение кремния
3. Физические свойства и нахождение в природе кремния
4. Качественные реакции на силикаты
5. Основные соединения кремния
6. Способы получения кремния
7. Химические свойства кремния
7.1. Взаимодействие с простыми веществами
7.1.1. Взаимодействие с галогенами
7.1.2. Взаимодействие с серой и углеродом
7.1.3. Взаимодействие с водородом
7.1.4. Взаимодействие с азотом
7.1.5. Взаимодействие с активными металлами
7.1.6. Горение
7.2. Взаимодействие со сложными веществами
7.2.1. Взаимодействие с щелочами
7.2.2. Взаимодействие с кислотами
7.2.3. Взаимодействие с азотной кислотой
Бинарные соединения кремния — силициды, силан и др.
Оксид кремния (IV)
1. Физические свойства и нахождение в природе
2. Химические свойства
2.1. Взаимодействие с щелочами и основными оксидами
2.2. Взаимодействие с водой
2.3. Взаимодействие с карбонатами
2.4. Взаимодействие с кислотами
2.5. Взаимодействие с металлами
2.6. Взаимодействие с неметаллами
Кремниевая кислота
1. Строение молекулы и физические свойства
2. Способы получения
3. Химические свойства
Силикаты
Кремний
Положение в периодической системе химических элементов
Кремний расположен в главной подгруппе IV группы (или в 14 группе в современной форме ПСХЭ) и в третьем периоде периодической системы химических элементов Д.И. Менделеева.
Электронное строение кремния
Электронная конфигурация кремния в основном состоянии:
+14Si 1s22s22p63s23p2
Электронная конфигурация кремния в возбужденном состоянии:
+14Si* 1s22s22p63s13p3
Атом кремния содержит на внешнем энергетическом уровне 2 неспаренных электрона и 1 неподеленную электронную пару в основном энергетическом состоянии и 4 неспаренных электрона в возбужденном энергетическом состоянии.
Степени окисления атома кремния — от -4 до +4. Характерные степени окисления -4, 0, +2, +4.
Физические свойства, способы получения и нахождение в природе кремния
Кремний — второй по распространенности элемент на Земле после кислорода. Встречается только в виде соединений. Оксид кремния SiO2 образует большое количество природных веществ – горный хрусталь, кварц, кремнезем.
Простое вещество кремний – атомный кристалл темно-серого цвета с металлическим блеском, довольно хрупок. Температура плавления 1415 °C, плотность 2,33 г/см3. Полупроводник.
Качественные реакции
Качественная реакция на силикат-ионы SiO32- — взаимодействие солей-силикатов с сильными кислотами. Кремниевая кислота – слабая. Она легко выделяется из растворов солей кремниевой кислоты при действии на них более сильными кислотами.
Например, если к раствору силиката натрия прилить сильно разбавленный раствор соляной кислоты, то кремниевая кислота выделится не в виде осадка, а в виде геля. Раствор помутнеет и «застынет».
Na2SiO3 + 2HCl = H2SiO3 + 2 NaCl
Видеоопыт взаимодействия силиката натрия с соляной кислоты (получение кремниевой кислоты) можно посмотреть здесь.
Соединения кремния
Основные степени окисления кремния +4, 0 и -4.
Наиболее типичные соединения кремния:
| Степень окисления | Типичные соединения |
| +4 | оксид кремния (IV) SiO2
кремниевая кислота H2SiO3 силикаты MeSiO3 бинарные соединения с неметаллами (карбид кремния SiC) |
| -4 | силан SiH4
силициды металлов (силицид натрия Na4Si) |
Способы получения кремния
В свободном состоянии кремний был получен Берцелиусом в 1822 г. Его латинское название «силиций» произошло от латинского слова «sileх», что означает «кремень». Аморфный кремний в лаборатории можно получить при прокаливании смеси металлического магния с диоксидом кремния. Для опыта диоксид кремния следует тщательно измельчить. При нагревании смеси начинается бурная реакция. Одним из продуктов этой реакции является аморфный кремний.
SiO2 + 2Mg → Si + 2MgO
Видеоопыт взаимодействия оксида кремния (IV) с магнием можно посмотреть здесь.
Еще один способ получения кремния в лаборатории — восстановление из оксида алюминием:
3SiO2 + 4Al → 3Si + 2Al2O3
В промышленности использовать дорогие алюминий и магний неэффективно, поэтому используют другие, более дешевые способы:
1. Восстановление из оксида коксом в электрических печах:
SiO2 + 2C → Si + 2CO
Однако в таком процессе образующийся кремний загрязнен примесями карбидов кремния, и для производства, например, микросхем уже не подходит.
2. Наиболее чистый кремний получают восстановлением тетрахлорида кремния водородом при 1200 °С:
SiCl4 +2H2 → Si + 4HCl
или цинком:
SiCl4 + 2Zn → Si + 2ZnCl2
3. Также чистый кремний получается при разложении силана:
SiH4 → Si + 2H2
Химические свойства
При нормальных условиях кремний существует в виде атомного кристалла, поэтому химическая активность кремния крайне невысокая.
1. Кремний проявляет свойства окислителя (при взаимодействии с элементами, которые расположены ниже и левее в Периодической системе) и свойства восстановителя (при взаимодействии с элементами, расположенными выше и правее). Поэтому кремний реагирует и с металлами, и с неметаллами.
1.1. При обычных условиях кремний реагирует с фтором с образованием фторида кремния (IV):
Si + 2F2 → SiF4
При нагревании кремний реагирует с хлором, бромом, йодом:
Si + 2Cl2 → SiCl4
Si + 2Br2 → SiBr4
1.2. При сильном нагревании (около 2000оС) кремний реагирует с углеродом с образованием бинарного соединения карбида кремния (карборунда):
C + Si → SiC
При температуре выше 600°С взаимодействует с серой:
Si + 2S → SiS2
1.3. Кремний не взаимодействует с водородом.
1.4. С азотом кремний реагирует в очень жестких условиях:
3Si + 2N2 → Si3N4
1.5. В реакциях с активными металлами кремний проявляет свойства окислителя. При этом образуются силициды:
2Ca + Si → Ca2Si
Si + 2Mg → Mg2Si
1.6. При нагревании выше 400°С кремний взаимодействует с кислородом:
Si + O2 → SiO2
2. Кремний взаимодействует со сложными веществами:
2.1. В водных растворах щелочей кремний растворяется с образованием солей кремниевой кислоты. При этом щелочь окисляет кремний.
Si + 2NaOH + H2O → Na2SiO3 + 2H2
Видеоопыт взаимодействия кремния с раствором щелочи можно посмотреть здесь.
2.2. Кремний не взаимодействует с водными растворами кислот, но аморфный кремний растворяется в плавиковой кислоте с образованием гексафторкремниевой кислоты:
Si + 6HF → H2[SiF6] + 2H2
При обработке кремния безводным фтороводородом комплекс не образуется:
Si(тв.) + 4HF(г.) = SiF4 + 2H2
С хлороводородом кремний реагирует при 300 °С, с бромоводородом – при 500 °С.
2.3. Кремний растворяется в смеси концентрированных азотной и плавиковой кислот:
3Si + 4HNO3 + 12HF → 3SiF4 + 4NO + 8H2O
Бинарные соединения кремния
Силициды металлов
Силициды – это бинарные соединения кремния с металлами, в которых кремний имеет степень окисления -4. Химическая связь в силицидах металлов — ионная.
Силициды, как правило, легко гидролизуются в воде или в кислой среде.
Например, силицид магния разлагается водой на гидроксид магния и силан:
Mg2Si + 4H2O → 2Mg(OH)2 + SiH4
Соляная кислота легко разлагает силицид магния:
Mg2Si + 4HCl → 2MgCl2 + SiH4
Получают силициды сплавлением простых веществ или восстановлением смеси оксидов коксом в электропечах:
2Mg + Si → Mg2Si
2MgO + SiO2 + 4C → Mg2Si + 4CO
Силан
Силан – это бинарное соединение кремния с водородом SiH4, ядовитый бесцветный газ.
Если поместить порошок силицида магния в очень слабый раствор соляной кислоты, то на поверхности раствора образуются пузырьки газа. Они лопаются и загораются на воздухе. Это горит силан. Он образуется при взаимодействии кислоты с силицидом магния:
Mg2Si + 4HCl → 2MgCl2 + SiH4
Видеоопыт получения силана из силицида магния можно посмотреть здесь.
На воздухе силан горит с образованием SiO2 и H2O:
SiH4 + 2O2 → SiO2 + 2H2O
Видеоопыт сгорания силана можно посмотреть здесь.
Силан разлагается водой разлагается с выделением водорода:
SiH4 + 2H2O → SiO2 + 4H2
Силан разлагается (окисляется) щелочами:
SiH4 + 2NaOH + H2O → Na2SiO3 + 4H2
Силан при нагревании разлагается:
SiH4 → Si + 2H2
Карбид кремния
В соединениях кремния с неметаллами — ковалентная связь.
Рассмотрим карбид кремния – карборунд Si+4C-4. Это вещество с атомной кристаллической решеткой. Он имеет структуру, подобную структуре алмаза и характеризуется высокой твердостью и температурой плавления, а также высокой химической устойчивостью.
Карборунд окисляется кислородом при высокой температуре:
SiC +2O2 → SiO2 + CO2
Карборунд окисляется кислородом в расплаве щелочи:
SiC + 2O2 + 4NaOH → Na2SiO3 + Na2CO3 + 2H2O
Галогениды кремния
Хлорид и фторид кремния – галогенангидриды кремниевой кислоты.
SiCl4.
Получают галогениды кремния действием хлора на сплав оксида кремния с углем:
SiO2 + C + Cl2 → SiCl4 + CO
Галогениды кремния разлагаются водой до кремниевой кислоты и хлороводорода:
SiCl4 + 3H2O → H2SiO3↓ + 4HCl
Хлорид кремния (IV) восстанавливается водородом:
SiCl4 + 2H2 → Si + 4HCl
Оксид кремния (IV)
Физические свойства и нахождение в природе
Оксид кремния (IV) – это твердое вещество с атомной кристаллической решеткой. В природе встречается в виде кварца, речного песка, кремнезема и прочих модификаций:
Химические свойства
Оксид кремния (IV) – типичный кислотный оксид. За счет кремния со степенью окисления +4 проявляет слабые окислительные свойства.
1. Как кислотный оксид, диоксид кремния (IV) взаимодействует с растворами и расплавами щелочей и в расплаве с основными оксидами. При этом образуются силикаты.
Например, диоксид кремния взаимодействует с гидроксидом калия:
SiO2 + 2KOH → K2SiO3 + H2O
Еще пример: диоксид кремния взаимодействует с оксидом кальция.
SiO2 + CaO → CaSiO3
2. Оксид кремния (IV) не взаимодействует с водой, т.к. кремниевая кислота нерастворима.
3. Оксид кремния (IV) реагирует при сплавлении с карбонатами щелочных металлов. При этом работает правило: менее летучий оксид вытесняет более летучий оксид из солей при сплавлении.
Например, оксид кремния (IV) взаимодействует с карбонатом калия. При этом образуется силикат калия и углекислый газ:
SiO2 + K2CO3 → K2SiO3 + CO2
4. Из кислот диоксид кремния реагирует только с плавиковой или с газообразным фтороводородом:
SiO2 + 4HF(г) = SiF4 + 2H2O
SiO2 + 6HF(р-р) → H2[SiF6] + 2H2O
5. При температуре выше 1000 °С оксид кремния реагирует с активными металлами, при этом образуется кремний.
Например, оксид кремния взаимодействует с магнием с образованием кремния и оксида магния:
SiO2 + 2Mg → Si + 2MgO
Видеоопыт взаимодействия оксида кремния (IV) с магнием можно посмотреть здесь.
При избытке восстановителя образуются силициды:
SiO2 + 4Mg → Mg2Si + 2MgO
6. Оксид кремния (IV) взаимодействует с неметаллами.
Например, оксид кремния (IV) реагирует с водородом в жестких условиях. При этом оксид кремния проявляет окислительные свойства:
SiO2 + 2Н2 → Si + 2Н2O
Еще пример: оксид кремния взаимодействует с углеродом. При этом образуется карборунд и угарный газ:
SiO2 + 3С → SiС + 2СО
При сплавлении оксид кремния взаимодействует с фосфатом кальция и углем:
3SiO2 + Ca3(PO4)2 + 5C → 3CaSiO3 + 5CO + 2P
Кремниевая кислота
Строение молекулы и физические свойства
Кремниевые кислоты — очень слабые, малорастворимые в воде соединения общей формулы nSiO2•mH2O. Образует коллоидный раствор в воде.
Метакремниевая H2SiO3 существует в растворе в виде полимера:
Способы получения
Кремниевая кислота образуется при действии сильных кислот на растворимые силикаты (силикаты щелочных металлов).
Например, при действии соляной кислоты на силикат натрия:
Na2SiO3 + 2HCl → H2SiO3 + 2 NaCl
Видеоопыт получения кремниевой кислоты из силиката натрия можно посмотреть здесь.
Даже слабая угольная кислота вытесняет кремниевую кислоту из солей:
Na2SiO3 + 2Н2O + 2CO2 → 2NaHCO3 + H2SiO3
Химические свойства
1. Кремниевая кислота — нерастворимая. Кислотные свойства выражены очень слабо, поэтому кислота реагирует только с сильными основаниями и их оксидами:
Например, кремниевая кислота реагирует с концентрированным гидроксидом калия:
H2SiO3 + 2KOH → K2SiO3 + 2H2O
2. При нагревании кремниевая кислота разлагается на оксид и воду:
H2SiO3 → SiO2 + H2O
Силикаты
Силикаты — это соли кремниевой кислоты. Большинство силикатов нерастворимо в воде, кроме силикатов натрия и калия, их называют «жидким стеклом».
Способы получения силикатов:
1. Растворение кремния, кремниевой кислоты или оксида в щелочи:
H2SiO3 + 2KOH → K2SiO3 + 2H2O
Si + 2NaOH + H2O → Na2SiO3 + 2H2
SiO2 + 2KOH → K2SiO3 + H2O
2. Сплавление с основными оксидами:
СаО + SiO2 → CaSiO3
3. Взаимодействие растворимых силикатов с солями:
K2SiO3 + CaCl2 → CaSiO3 + 2KCl
Оконное стекло (натриевое стекло) — силикат натрия и кальция: Na2O·CaO·6SiO2.
Стекло получают при сплавлении в специальных печах смеси соды Na2CO3, известняка CaCO3 и белого песка SiO2:
6SiO2 + Na2CO3 + CaCO3 → Na2O·CaO·6SiO2 + 2CO2
Для получения специального стекла вводят различные добавки, так стекло содержащее ионы Pb2+ – хрусталь; Cr3+ – имеет зеленую окраску, Fe3+ – коричневое бутылочное стекло, Co2+ – дает синий цвет, Mn2+ – красновато-лиловый.
Кремний — химический элемент № (14). Он расположен в IVА группе третьем периоде Периодической системы.
Si14+14)2e)8e)4e
На внешнем слое атома кремния содержатся четыре валентных электрона. До его завершения не хватает четырёх электронов. Поэтому в соединениях с металлами кремнию характерна степень окисления (–4), а при взаимодействии с более электроотрицательными неметаллами он проявляет положительные степени окисления ( +2) или (+4).
По содержанию в земной коре кремний занимает второе место после кислорода. Земная кора более чем наполовину образована соединениями кремния. Распространены оксид кремния(IV)
SiO2
, силикаты и алюмосиликаты. Песок, кварц, горный хрусталь, аметист состоят из оксида. Гранит, полевой шпат, глина представляют собой силикаты и алюмосиликаты.
Входит кремний и в состав живых организмов. Его соединения придают прочность стеблям растений, содержатся в наружных покровах животных, образуют раковины и скелеты некоторых обитателей водной среды. У человека кремний присутствует в волосах и ногтях.
Рис. (1). Скелеты радиолярий
Кремний имеет атомную кристаллическую решётку, похожую на решётку алмаза. Каждый атом кремния в его кристаллах связан четырьмя ковалентными связями с соседними атомами. Благодаря такому строению у него высокая твёрдость.
Радиус атома кремния больше радиуса атома углерода, поэтому в его кристаллах электроны более свободны по сравнению с алмазом. Кремний проводит электрический ток, а его электропроводность увеличивается с повышением температуры или при освещении. Такие вещества относятся к полупроводникам.
В отличие от алмаза кремний представляет собой чёрно-серое непрозрачное вещество. У него высокая температура плавления ((1428) °С).
Рис. (2). Кремний
Получают кремний восстановлением его оксида коксом в электропечах:
В химических реакциях кремний может проявлять и окислительные, и восстановительные свойства. Окислительные свойства кремния выражены слабее, чем у остальных неметаллов.
- Взаимодействие с металлами.
При высокой температуре кремний реагирует с металлами с образованием силицидов:
В этой реакции кремний — окислитель.
- С водородом не реагирует.
С водородом кремний практически не реагирует по причине неустойчивости водородного соединения силана
SiH4
. Силан можно получить при гидролизе силицидов:
.
Он самовоспламеняется на воздухе и сгорает с образованием оксида кремния((IV)) и воды:
- Взаимодействие с кислородом.
Кремний горит в кислороде и проявляет в этой реакции восстановительные свойства:
- Взаимодействие с оксидами металлов.
Кремний способен восстанавливать некоторые металлы из их оксидов:
- Взаимодействие со щелочами.
В отличие от углерода кремний растворяется в концентрированных растворах щелочей c образованием силикатов и выделением водорода:
.
Применение кремния
-
используется в производстве полупроводников для электронной промышленности;
-
применяется для изготовления солнечных батарей;
-
входит в состав жаропрочных и кислотоустойчивых сплавов.
Рис. (3).Солнечные батареи
Источники:
Рис. 1. Скелеты радиолярий https://upload.wikimedia.org/wikipedia/commons/thumb/6/6e/Haeckel_Stephoidea.jpg/800px-Haeckel_Stephoidea.jpg
Рис. 2. Кремний https://upload.wikimedia.org/wikipedia/commons/e/e9/SiliconCroda.jpg
Рис. 3. Солнечные батареи https://cdn.pixabay.com/photo/2017/08/21/17/25/solar-photovoltaic-2666105_960_720.jpg
|
|||
| Внешний вид простого вещества | |||
|---|---|---|---|
|
|
|||
| Свойства атома | |||
| Имя, символ, номер |
Кремний/Silicium (Si), 14 |
||
| Атомная масса (молярная масса) |
28,0855 а. е. м. (г/моль) |
||
| Электронная конфигурация |
[Ne] 3s2 3p2; в соед. [Ne] 3s 3p3 (гибридизация) |
||
| Радиус атома |
132 пм |
||
| Химические свойства | |||
| Ковалентный радиус |
111 пм |
||
| Радиус иона |
42 (+4e) 271 (-4e) пм |
||
| Электроотрицательность |
1,90 (шкала Полинга) |
||
| Электродный потенциал |
0 |
||
| Степени окисления |
+4, +2, 0, −4 |
||
| Энергия ионизации (первый электрон) |
786,0(8,15) кДж/моль (эВ) |
||
| Термодинамические свойства простого вещества | |||
| Плотность (при н. у.) |
2,33 г/см³ |
||
| Температура плавления |
1688 K |
||
| Температура кипения |
2623 K |
||
| Теплота плавления |
50,6 кДж/моль |
||
| Теплота испарения |
383 кДж/моль |
||
| Молярная теплоёмкость |
20,16[1] Дж/(K·моль) |
||
| Молярный объём |
12,1 см³/моль |
||
| Кристаллическая решётка простого вещества | |||
| Структура решётки |
кубическая, алмазная |
||
| Параметры решётки |
5,4307 Å |
||
| Температура Дебая |
625 K |
||
| Прочие характеристики | |||
| Теплопроводность |
(300 K) 149 Вт/(м·К) |
Кремний — элемент главной подгруппы четвёртой группы третьего периода периодической системы химических элементов Д. И. Менделеева, с атомным номером 14. Обозначается символом Si (лат. Silicium).
Содержание
- 1 История
- 2 Происхождение названия
- 3 Нахождение в природе
- 4 Получение
- 5 Физические свойства
- 5.1 Электрофизические свойства
- 6 Химические свойства
- 7 Применение
- 8 Биологическая роль
- 9 См. также
- 10 Примечания
- 11 Литература
- 12 Ссылки
История
В чистом виде кре́мний был выделен в 1811 году французскими учёными Жозефом Луи Гей-Люссаком и Луи Жаком Тенаром.
Происхождение названия
В 1825 году шведский химик Йёнс Якоб Берцелиус действием металлического калия на фтористый кремний SiF4 получил чистый элементарный кремний. Новому элементу было дано название «силиций» (от лат. silex — кремень). Русское название «кремний» введено в 1834 году российским химиком Германом Ивановичем Гессом. В переводе c др.-греч. κρημνός — «утёс, гора».
Нахождение в природе
Содержание кремния в земной коре составляет по разным данным 27,6—29,5 % по массе. Таким образом по распространённости в земной коре кремний занимает второе место после кислорода. Концентрация в морской воде 3 мг/л[2].
Чаще всего в природе кремний встречается в виде кремнезёма — соединений на основе диоксида кремния (IV) SiO2 (около 12 % массы земной коры). Основные минералы и горные породы, образуемые диоксидом кремния — это песок (речной и кварцевый), кварц и кварциты, кремень, полевые шпаты. Вторую по распространённости в природе группу соединений кремния составляют силикаты и алюмосиликаты.
Отмечены единичные факты нахождения чистого кремния в самородном виде[3].
Получение
«Свободный кремний можно получить прокаливанием с магнием мелкого белого песка, который представляет собой диоксид кремния:
При этом образуется бурый порошок аморфного кремния.»[4]
В промышленности кремний технической чистоты получают, восстанавливая расплав SiO2 коксом при температуре около 1800 °C в руднотермических печах шахтного типа. Чистота полученного таким образом кремния может достигать 99,9 % (основные примеси — углерод, металлы).
Возможна дальнейшая очистка кремния от примесей.
- Очистка в лабораторных условиях может быть проведена путём предварительного получения силицида магния Mg2Si. Далее из силицида магния с помощью соляной или уксусной кислот получают газообразный моносилан SiH4. Моносилан очищают ректификацией, сорбционными и др. методами, а затем разлагают на кремний и водород при температуре около 1000 °C.
- Очистка кремния в промышленных масштабах осуществляется путём непосредственного хлорирования кремния. При этом образуются соединения состава SiCl4 и SiCl3H. Эти хлориды различными способами очищают от примесей (как правило перегонкой и диспропорционированием) и на заключительном этапе восстанавливают чистым водородом при температурах от 900 до 1100 °C.
- Разрабатываются более дешёвые, чистые и эффективные промышленные технологии очистки кремния. На 2010 г. к таковым можно отнести технологии очистки кремния с использованием фтора (вместо хлора); технологии предусматривающие дистилляцию монооксида кремния; технологии, основанные на вытравливании примесей, концентрирующихся на межкристаллитных границах.
Содержание примесей в доочищенном кремнии может быть снижено до 10−8—10−6% по массе. Более подробно вопросы получения сверхчистого кремния рассмотрены в статье Поликристаллический кремний
Способ получения кремния в чистом виде разработан Николаем Николаевичем Бекетовым.
В России технический кремний производится «ОК Русал» на заводах в г. Каменск-Уральский (Свердловская область) и г. Шелехов (Иркутская область); доочищенный по хлоридной технологии кремний производит группа «Nitol Solar» на заводе в г. Усолье-Сибирское.
Физические свойства
Кристаллическая структура кремния.
Кристаллическая решётка кремния кубическая гранецентрированная типа алмаза, параметр а = 0,54307 нм (при высоких давлениях получены и другие полиморфные модификации кремния), но из-за большей длины связи между атомами Si—Si по сравнению с длиной связи С—С твёрдость кремния значительно меньше, чем алмаза. Кремний хрупок, только при нагревании выше 800 °C он становится пластичным веществом. Интересно, что кремний прозрачен для инфракрасного излучения начиная с длины волны 1,1 мкм. Собственная концентрация носителей заряда — 5,81·1015 м−3 (для температуры 300 K).
Схематическое изображение зонной структуры кремния[5]
Электрофизические свойства
Элементарный кремний в монокристаллической форме является непрямозонным полупроводником. Ширина запрещённой зоны при комнатной температуре составляет 1,12 эВ, а при Т = 0 К составляет 1,21 эВ[6]. Концентрация собственных носителей заряда в кремнии при нормальных условиях составляет порядка 1,5·1010 см−3[7].
На электрофизические свойства кристаллического кремния большое влияние оказывают содержащиеся в нём примеси. Для получения кристаллов кремния с дырочной проводимостью в кремний вводят атомы элементов III-й группы, таких, как бор, алюминий, галлий, индий. Для получения кристаллов кремния с электронной проводимостью в кремний вводят атомы элементов V-й группы, таких, как фосфор, мышьяк, сурьма.
При создании электронных приборов на основе кремния задействуется преимущественно приповерхностный слой материала (до десятков микрон), поэтому качество поверхности кристалла может оказывать существенное влияние на электрофизические свойства кремния и, соответственно, на свойства готового прибора. При создании некоторых приборов используются приёмы, связанные с модификацией поверхности, например, обработка поверхности кремния различными химическими агентами.
- Диэлектрическая проницаемость: 12[1]
- Подвижность электронов: 1200—1450 см²/(В·c).
- Подвижность дырок: 500 см²/(В·c).
- Ширина запрещённой зоны 1,205-2,84·10−4·T
- Продолжительность жизни электрона: 5 нс — 10 мс
- Длина свободного пробега электрона: порядка 0,1 см
- Длина свободного пробега дырки: порядка 0,02 — 0,06 см
Все значения приведены для нормальных условий.
Химические свойства
Подобно атомам углерода, для атомов кремния является характерным состояние sp3-гибридизации орбиталей. В связи с гибридизацией чистый кристаллический кремний образует алмазоподобную решётку, в которой кремний четырёхвалентен. В соединениях кремний обычно также проявляет себя как четырёхвалентный элемент со степенью окисления +4 или −4. Встречаются двухвалентные соединения кремния, например, оксид кремния (II) SiO.
При нормальных условиях кремний химически малоактивен и активно реагирует только с газообразным фтором, при этом образуется летучий тетрафторид кремния SiF4. Такая «неактивность» кремния связана с пассивацией поверхности наноразмерным слоем диоксида кремния, немедленно образующегося в присутствии кислорода, воздуха или воды (водяных паров).
При нагревании до температуры свыше 400—500 °C кремний реагирует с кислородом с образованием диоксида SiO2, процесс сопровождается увеличением толщины слоя диоксида на поверхности, скорость процесса окисления лимитируется диффузией атомарного кислорода сквозь плёнку диоксида.
При нагревании до температуры свыше 400—500 °C кремний реагирует с хлором, бромом и иодом — с образованием соответствующих легко летучих тетрагалогенидов SiHalogen4 и, возможно, галогенидов более сложного состава.
С водородом кремний непосредственно не реагирует, соединения кремния с водородом — силаны с общей формулой SinH2n+2 — получают косвенным путем. Моносилан SiH4 (его часто называют просто силаном) выделяется при взаимодействии силицидов металлов с растворами кислот, например:
Образующийся в этой реакции силан SiH4 содержит примесь и других силанов, в частности, дисилана Si2H6 и трисилана Si3H8, в которых имеется цепочка из атомов кремния, связанных между собой одинарными связями (—Si—Si—Si—).
С азотом кремний при температуре около 1000 °C образует нитрид Si3N4, с бором — термически и химически стойкие бориды SiB3, SiB6 и SiB12.
При температурах свыше 1000С °C можно получить соединение кремния и его ближайшего аналога по таблице Менделеева — углерода — карбид кремния SiC (карборунд), который характеризуется высокой твёрдостью и низкой химической активностью. Карборунд широко используется как абразивный материал. При этом, что интересно, расплав кремния (1415 °C) может длительное время контактировать с углеродом в виде крупных кусков плотноспечённого мелкозернистого графита изостатического прессования, практически не растворяя и никак не взаимодействуя с последним.
Нижележащие элементы 4-й группы (Ge, Sn, Pb) неограниченно растворимы в кремнии, как и большинство других металлов. При нагревании кремния с металлами могут образовываться силициды. Силициды можно подразделить на две группы: ионно-ковалентные (силициды щелочных, щелочноземельных металлов и магния типа Ca2Si, Mg2Si и др.) и металлоподобные (силициды переходных металлов). Силициды активных металлов разлагаются под действием кислот, силициды переходных металлов химически стойки и под действием кислот не разлагаются. Металлоподобные силициды имеют высокие температуры плавления (до 2000 °C). Наиболее часто образуются металлоподобные силициды составов MeSi, Me3Si2, Me2Si3, Me5Si3 и MeSi2. Металлоподобные силициды химически инертны, устойчивы к действию кислорода даже при высоких температурах.
Особо следует отметить, что с железом кремний образует эвтектическую смесь, что позволяет спекать (сплавлять) эти материалы для образования ферросилициевой керамики при температурах заметно меньших, чем температуры плавления железа и кремния.
При восстановлении SiO2 кремнием при температурах свыше 1200 °C образуется оксид кремния (II) — SiO. Этот процесс постоянно наблюдается при производстве кристаллов кремния методами Чохральского, направленной кристаллизации, потому что в них используются контейнеры из диоксида кремния, как наименее загрязняющего кремний материала.
Для кремния характерно образование кремнийорганических соединений, в которых атомы кремния соединены в длинные цепочки за счет мостиковых атомов кислорода —О—, а к каждому атому кремния, кроме двух атомов О, присоединены ещё два органических радикала R1 и R2 = CH3, C2H5, C6H5, CH2CH2CF3 и др.
Для травления кремния наиболее широко используют смесь плавиковой и азотной кислот. Некоторые специальные травители предусматривают добавку хромового ангидрида и иных веществ. При травлении кислотный травильный раствор быстро разогревается до температуры кипения, при этом скорость травления многократно возрастает.
- Si+2HNO3=SiO2+NO+NO2+H2O
- SiO2+4HF=SiF4+2H2O
- 3SiF4+3H2O=2H2SiF6+↓H2SiO3
Для травления кремния могут использоваться водные растворы щёлочей. Травление кремния в щелочных растворах начинается при температуре раствора более 60 °C.
- Si+2KOH+H2O=K2SiO3+2H2↑
- K2SiO3+2H2O↔H2SiO3+2KOH
Применение
Технический кремний находит следующие применения:
- сырьё для металлургических производств: компонент сплава (бронзы, силумин); раскислитель (при выплавке чугуна); модификатор свойств металлов или легирующий элемент (например, добавка определённого количества кремния при производстве трансформаторных сталей уменьшает коэрцитивную силу готового продукта) и т. п.;
- сырьё для производства более чистого поликристаллического кремния и очищенного металлургического кремния (в литературе «umg-Si»);
- сырьё для производства кремнийорганических материалов, силанов;
- иногда кремний технической чистоты и его сплав с железом (ферросилиций) используется для производства водорода в полевых условиях;
- для производства солнечных батарей.
Монокристалл кремния, выращенный по методу Чохральского
Cверхчистый кремний преимущественно используется для производства одиночных электронных приборов (нелинейные пассивные элементы электрических схем) и однокристальных микросхем. Чистый кремний, отходы сверхчистого кремния, очищенный металлургический кремний в виде кристаллического кремния являются основным сырьевым материалом для солнечной энергетики.
Монокристаллический кремний — помимо электроники и солнечной энергетики используется для изготовления зеркал газовых лазеров.
Соединения металлов с кремнием — силициды — являются широкоупотребляемыми в промышленности (например, электронной и атомной) материалами с широким спектром полезных химических, электрических и ядерных свойств (устойчивость к окислению, нейтронам и др.). Силициды ряда элементов являются важными термоэлектрическими материалами.
Соединения кремния служат основой для производства стекла и цемента. Производством стекла и цемента занимается силикатная промышленность. Она также выпускает силикатную керамику — кирпич, фарфор, фаянс и изделия из них.
Широко известен силикатный клей, применяемый в строительстве как сиккатив, а в пиротехнике и в быту для склеивания бумаги.
Получили широкое распространение силиконовые масла и силиконы — материалы на основе кремнийорганических соединений.
Биологическая роль
Для некоторых организмов кремний является важным биогенным элементом. Он входит в состав опорных образований у растений и скелетных — у животных. В больших количествах кремний концентрируют морские организмы — диатомовые водоросли, радиолярии, губки. Большие количества кремния концентрируют хвощи и злаки, в первую очередь — подсемейства Бамбуков и Рисовидных, в том числе — рис посевной. Мышечная ткань человека содержит (1-2)·10−2% кремния, костная ткань — 17·10−4%, кровь — 3,9 мг/л. С пищей в организм человека ежедневно поступает до 1 г кремния.
Соединения кремния относительно нетоксичны. Но очень опасно вдыхание высокодисперсных частиц как силикатов, так и диоксида кремния, образующихся, например, при взрывных работах, при долблении пород в шахтах, при работе пескоструйных аппаратов, при обработке кремнийсодержащих материалов угловой шлифовальной машиной («болгаркой») и т. д. Микрочастицы SiO2, попавшие в лёгкие, кристаллизуются в них, а возникающие кристаллики разрушают лёгочную ткань и вызывают тяжёлую болезнь — силикоз. Чтобы не допустить попадания в лёгкие опасной пыли, следует использовать для защиты органов дыхания респиратор.
См. также
- Категория:Соединения кремния
- Поликристаллический кремний (вопросы, связанные со свойствами, промышленным получением и очисткой кремния как исходного сырья для других производств)
- Кристаллический кремний (вопросы, связанные со свойствами и производством кристаллов кремния для нужд электронной промышленности и солнечной энергетики)
- Метод Чохральского (основной метод переработки кремния для нужд электронной промышленности и солнечной энергетики)
- Бестигельная зонная плавка (основной метод производства сверхчистых монокристаллов кремния)
- Пористый кремний
- Германий
- Кремнийорганические соединения
Примечания
- ↑ 1 2 Химическая энциклопедия: в 5-ти тт. / Редкол.:Кнунянц И. Л. (гл. ред.). — Москва: Советская энциклопедия, 1990. — Т. 2. — С. 508. — 671 с. — 100 000 экз.
- ↑ J.P. Riley and Skirrow G. Chemical Oceanography V. 1, 1965
- ↑ Металлический кремний в ийолитах Горячегорского массива, Петрология обыкновенных хондритов
- ↑ Глинка Н.Л. Общая химия. — 24-е изд., испр. — Л.: Химия, 1985. — С. 492. — 702 с.
- ↑ Р Смит., Полупроводники: Пер. с англ. — М.: Мир, 1982. — 560 с, ил.
- ↑ Зи С., Физика полупроводниковых приборов: В 2-х книгах. Кн. 1. Пер. с англ. — М.: Мир, 1984. — 456 с, ил.
- ↑ Коледов Л. А. Технологии и конструкции микросхем, микропроцессоров и микросборок: Учебное пособие//2-е изд., испр. и доп. — СПб.:Издательство «Лань», 2007. — С. 200—201. — ISBN 978-5-8114-0766-8
Литература
- Самсонов. Г. В. Силициды и их использование в технике. — Киев, Изд-во АН УССР, 1959. — 204 с. с илл.
Ссылки
| Кремний на Викискладе? |
- Кремний на Webelements
- Кремний в Популярной библиотеке химических элементов
| Периодическая система химических элементов Д. И. Менделеева | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||||||
| 1 | H | He | ||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo |
|
|
Соединения кремния |
|---|
|
Гексафторосиликат натрия (Na2[SiF6]) • Гексафторосиликат(IV) калия (K2[SiF6]) • Карбид кремния (SiC) • Кремнефтористоводородная кислота (H2[SiF6]) • Кремниевые кислоты (SiO2•n H2О) • Метасиликат калия (K2SiO3) • Метасиликат натрия (Na2SiO3) • Метилсилан (CH3-SiH3) • Муассанит • Нитрид кремния (Si3N4) • Оксид кремния(II) (SiO) • Оксид кремния(IV) (SiO2) • Ортосиликат натрия (Na4SiO4) • Полевые шпаты • Силаны (SinH2n+2) • Силикагель (n SiO2•m H2O) • Силицид сурьмы (Si3Sb4) • Силиконовое масло • Силиконы ([R2SiO]n) • Силицид ванадия (V3Si) • Силицид висмута (Si3Bi4) • Силицид кальция (CaSi2) • Силицид лития (Li6Si2) • Силицид магния (Mg2Si) • Силицид молибдена (MoSi2) • Силицид полония (SiPo2) • Силицид рения (ReSi) • Сульфид кремния (SiS2) • Тетрабромид кремния (SiBr4) • Тетраиодид кремния (SiI4) • Тетрасиликат калия (K2Si4O9•H2O) • Тетрафторид кремния (SiF4) • Трихлорсилан (SiHCl3) • Хлорид кремния(IV) (SiCl4) • Хлориды кремния |
Содержание:
Кремний — химический элемент и простое вещество
В периодической системе химических элементов кремний Si расположен в третьем периоде в IVА-группе. Чем же он отличается от углерода? Познакомимся со свойствами этого химического элемента и образуемого им простого вещества подробнее.
Кремний в природе
Кремний после кислорода — самый распространенный элемент в земной коре (массовая доля 27,6 %). Земная кора в основном состоит из соединений кремния с кислородом, в состав которых включаются и другие элементы. В природе кремний встречается преимущественно в виде оксида кремния(IV)
Кремний один из самых распространенных элементов во всей Вселенной. Основной компонент марсианской почвы — кремнезем SiO3, а в лунном грунте на долю этого вещества приходится 41 %. Силикаты металлов обнаружены на Венере и других планетах.
Кремний является важным элементом для нормального существования всех живых организмов. Повышенным содержанием кремния характеризуются морские организмы — диатомовые водоросли, радиолярии, губки (рис. 108). Большое количество кремния накапливают хвощи и злаки, в том числе рис.
Строение атома
В атоме кремния 14 электронов, которые располагаются на трех электронных слоях:
Так же как и у атомов углерода, у атомов кремния на внешнем электронном слое находится по 4 электрона и до его завершения не хватает тоже 4 электрона. Поэтому в своих соединениях кремний проявляет отрицательную степень окисления, равную –4, например в силициде магния 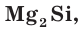
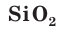
Строение и физические свойства простого вещества
Кристаллическая решетка кремния аналогична кристаллической решетке алмаза (см. рис. 90). В кристалле кремния каждый его атом соединен с другими атомами четырьмя прочными ковалентными связями. В настоящее время получают кремний 99,9999999 %-й чистоты. Это означает, что среди миллиарда атомов кремния может быть лишь один атом другого элемента.
В отличие от алмаза кремний обладает способностью при определенных условиях проводить электрический ток. Электропроводность кремния возрастает при нагревании или освещении. Именно поэтому он используется в полупроводниковой технике, в том числе для преобразования энергии солнечного излучения в электрическую энергию в солнечных батареях.
Химические свойства кремния
Так же как и углерод, кремний реагирует с другими веществами, как правило, при нагревании.
Взаимодействуя с атомами менее электроотрицательных элементов (металлов), атомы кремния принимают электроны (восстанавливаются), приобретая при этом отрицательные степени окисления:
При этом простое вещество кремний проявляет окислительные свойства.
Взаимодействуя с атомами более электроотрицательных элементов, атомы кремния могут отдавать электроны (окисляться), приобретая положительные степени окисления:
При этом простое вещество кремний проявляет восстановительные свойства.
При очень высоких температурах кремний взаимодействует с углеродом, образуя карбид кремния (карборунд):
В этой реакции кремний выступает в качестве восстановителя, а углерод — в качестве окислителя. В качестве восстановителя кремний применяют также при промышленном получении металлов из руд.
Структура простого вещества кремния аналогична структуре алмаза.
При взаимодействии с другими веществами кремний может проявлять как восстановительные, так и окислительные свойства.
Оксид кремния(IV). Кремниевая кислота и ее соли
Среди кислородсодержащих соединений кремния наибольшее значение имеют оксид кремния(IV), кремниевая кислота и ее соли — силикаты.
Оксид кремния(IV)
Оксид кремния(IV) представляет собой твердое тугоплавкое вещество (температура плавления 1713 °С), нерастворимое в воде. Высокая температура плавления этого вещества свидетельствует о том, что оно имеет немолекулярное строение.
В кристаллах оксида кремния(IV) атомы кремния и кислорода связаны между собой ковалентными связями (рис. 109). Для описания состава таких веществ, как вы помните, пользуются формульными единицами. Состав формульной единицы 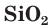
В природе оксид кремния(IV) образует речной песок, горный хрусталь и распространенный на территории Беларуси кремень. Часто в природе минералы на основе SiO3 содержат примеси оксидов железа, алюминия, хрома и других элементов, придающих им определенную окраску. Они используются в качестве поделочных и драгоценных камней (например, цитрин, аметист, яшма, агат и др.).
Оксид кремния(IV) — химически неактивное вещество. Он не растворяется в воде и не взаимодействует с ней. Но как кислотный оксид SiO2 реагирует с основными оксидами, щелочами и некоторыми солями, например карбонатами, при нагревании или сплавлении с образованием солей слабой кремниевой кислоты — силикатов:
Чистый кристаллический оксид кремния(IV) прозрачен, бесцветен, как вода, и в связи с этим применяется для изготовления оптических приборов. Из расплавленного SiO2 получают так называемое кварцевое стекло. Оно выдерживает нагревание до 1000—1200 °С и устойчиво к резкому перепаду температур. У кварцевого стекла есть еще одно важное достоинство: оно пропускает ультрафиолетовые лучи, что позволяет использовать его в производстве медицинской, научно-исследовательской и промышленной аппаратуры.
Кремниевая кислота
Кремниевую кислоту получают, действуя более сильными кислотами на растворы ее солей. Она образует студенистый осадок, содержащий воду (рис. 110). Кремниевая кислота имеет сложный состав, который условно можно выразить простейшей формулой 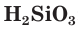
Кремниевая кислота мало растворяется в воде. Она является непрочным соединением — при нагревании или длительном хранении постепенно разлагается на воду и оксид кремния(IV):
Кремниевая кислота очень слабая.
При обезвоживании осадка кремниевой кислоты образуется пористый аморфный оксид кремния(IV) — силикагель. Он имеет развитую поверхность, поэтому отлично поглощает влагу. В химических лабораториях силикагель используют для осушения газов.
Соли кремниевой кислоты
Из солей кремниевой кислоты растворимы только силикаты щелочных металлов. Силикаты калия и натрия называют растворимыми стеклами, а их концентрированные водные растворы — жидким стеклом. Раньше жидкое стекло широко использовалось в качестве силикатного клея. Концентрированный раствор силиката натрия применяется для пропитки деревянных изделий и тканей для придания им огнеупорных свойств. Приготовим две одинаковые полоски бумаги. Одну из них покроем тонким слоем жидкого стекла и высушим на воздухе. Затем одновременно внесем полоски в пламя спиртовки (рис. 111). Что при этом наблюдается?
Если в разбавленный раствор силиката натрия поместить несколько кристаллов окрашенных солей, то через некоторое время в растворе появятся длинные цветные нити в виде веточек. Получается силикатный «сад» (рис. 112). С особенностями протекания этого процесса вы можете познакомиться, если прочитаете дополнительную литературу.
Природные кремнеземы, силикаты и глина являются сырьем для силикатной промышленности.
В составе силикатов часто встречается третий по распространенности в земной коре после кислорода и кремния элемент алюминий. В этом случае они называются алюмосиликатами. Их состав часто записывают в виде соединения оксидов. Например, состав калиевого полевого шпата выражается формулой 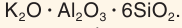

- Оксид кремния(IV) SiO2 является кислотным оксидом. При нагревании или сплавлении SiO2 реагирует с основными оксидами, щелочами и некоторыми солями.
- Кремниевую кислоту H2SiO3 можно получить, действуя более сильными кислотами на растворы ее солей.
- Соли кремниевой кислоты называются силикатами.
- Концентрированные водные растворы силикатов калия и натрия называют жидким стеклом.
Строительные материалы на основе природных оксидов и солей
С древних времен человек старался использовать окружавшие его воду, растительный и животный мир, почву, камни. Именно из камня он сделал первые орудия труда и охоты. На заре возникновения цивилизации появились нехитрые каменные сооружения. Камни надо было скреплять между собой, чтобы сооружение не разваливалось. В связи с этим в обиходе появились вяжущие вещества. Человек научился пользоваться глиной, делать из нее различные изделия. Еще позже возникло производство кирпича и стекла. Так зарождалось строительное ремесло.
Современная строительная индустрия использует неорганические соединения в самом разнообразном виде: материалы из силикатных расплавов (стекло), керамические изделия, вяжущие вещества. Основным источником сырья для производства строительных материалов являются природные соединения: песок, известняк, силикаты, алюмосиликаты, глина.
Керамические материалы
Слово «керамика» происходит от греческого слова керамос — глина, глиняная посуда. Основным сырьем для производства керамики служит глина, которая способна образовывать с водой пластичную массу. Влажной глине можно придать любую форму. При высокой температуре она необратимо твердеет, что и используется в производстве керамических изделий. После обжига керамические изделия получаются пористыми и водопроницаемыми. Поэтому керамику часто покрывают глазурью — легкоплавкими смесями, которые после специальной термической обработки образуют на поверхности изделий стекловидную массу (рис. 113).
Керамика представляет собой один из древнейших искусственных материалов. Керамические изделия были известны человеку с эпохи неолита. Первыми керамическими материалами были кирпич, плитка, посуда и самые разнообразные емкости (см. рис.).
К керамическим изделиям относятся строительный кирпич, черепица, огнеупорные и облицовочные материалы, сантехническое оборудование (ванны, раковины и др.).
Вяжущие строительные материалы
Вяжущие строительные материалы представляют собой вещества или смеси веществ, способные при смешивании с водой образовывать вязкую массу, которая постепенно затвердевает.
Одним из древнейших строительных материалов является известь. Различают негашеную известь CaO и гашеную известь 
Оксид кальция CaO (негашеную известь) переводят в гидроксид («гасят» водой) и получают гашеную известь
Эта реакция протекает с выделением большого количества теплоты, что приводит к сильному разогреванию смеси (рис. 114). В результате образуется облако водяного пара, как при гашении костра водой. Поэтому данная реакция и называется «гашение извести». Гашеную известь в смеси с песком используют в качестве вяжущего строительного материала.
Другим примером вяжущих строительных материалов является цемент. Если его смешать с водой, то образуется тестообразная масса, которая через некоторое время затвердевает. Это свойство цемента и используется в строительном деле для скрепления, например, кирпичей при сооружении стен. В Беларуси его производят на нескольких предприятиях, крупнейшими из которых являются ОАО «Красносельскстройматериалы» и ОАО «Кричевцементошифер».
Из смеси цемента, песка и воды с добавлением мелкого щебня или гравия получают бетон. Если в бетон ввести каркас из железных стержней, то получается железобетон. Бетон и железобетон широко применяются в строительстве. Введение в бетон химических веществ определенного состава позволяет получать пенобетон, отличающийся легкостью, высокими тепло- и звукоизоляционными свойствами. Важнейшим отличием бетона от известкового раствора является то, что при его затвердевании происходит поглощение воды.
Недавно ученые на основе фосфата магния разработали биобетон. Внешние панели из биобетона после постройки тут же начинают накапливать дождевую воду, становясь идеальной средой для развития лишайников и мхов. Это позволяет создавать вертикальные сады на стенах жилых зданий, реализуя концепцию экогородов (см. рис. вверху). Кроме того, бетон активно используется как дизайнерский материал для изготовления садовой скульптуры (см. рис. внизу), предметов мебели и интерьера.
В качестве вяжущего материала используют также алебастр, который часто называют полуводным гипсом. Его формулу записывают следующим образом 
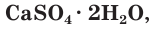
Стекло
Чаще всего мы сталкиваемся со стеклами, полученными на основе различных силикатов, поэтому в быту слово «стекло» употребляется для обозначения именно силикатных стекол.
Кроме красивого внешнего вида, стекло обладает низкой теплопроводностью и высокой прозрачностью, что позволяет использовать его для изготовления оконных стеклопакетов. При нагревании стекло легко вытягивается в тонкие, длинные нити, из которых изготавливают стекловату, стекловолокно и стеклоткани. Стекловата и стекловолокно используются в качестве звуко- и теплоизоляторов. Крупнейшим производителем стеклонитей и стеклотканей различного назначения на территории Беларуси является ОАО «Полоцк-Стекловолокно».
Стекольная промышленность Беларуси имеет давнюю историю. В 1717 г. в деревнях Налибоки и Янковичи (ныне Столбцовский район) по образцу Дрезденской мануфактуры Радзивиллы основали Налибокскую стеклянную мануфактуру, где изготавливались зеркала, подсвечники, художественная и бытовая посуда. В 1737 г. в деревне Уречье (ныне Любанский район) была основана Уречская стеклянная мануфактура, которая выпускала изделия из бесцветного и цветного стекла. Эти мануфактуры существовали до середины XVIII в. В 1883 г. помещик Зенон Ленский построил в поселке Березовка (ныне Лидский район) мануфактуру, которая позже, в 1908 г., стала стеклозаводом «Неман», работающим и по сей день.
Стекло не является индивидуальным соединением, а представляет собой сплав нескольких веществ. Для получения стекла (как говорят на производстве, при «варке» стекла) в качестве исходных материалов используют 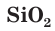
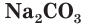
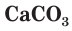
Часто при варке стекла для придания ему специфических свойств и окраски в исходную смесь добавляют разнообразные соли и оксиды.
- Стекло является экологически чистым материалом. Оно может подвергаться вторичной переработке, не загрязняя при этом окружающую среду.
- Основным источником сырья для производства строительных материалов являются природные соединения: песок, глина, известняк, силикаты и алюмосиликаты.
- К строительным материалам относятся стекло, керамика, вяжущие материалы.
Понятие о выходе продукта химической реакции
На практике при проведении химических реакций обычно получается несколько меньшее количество продукта, чем рассчитанное теоретически в соответствии с уравнением реакции. Это может происходить по нескольким причинам.
Многие химические реакции обратимы, т. е. протекают не до конца. Потери веществ могут быть также обусловлены их испарением, частичным растворением (ведь абсолютно нерастворимых веществ нет), потерями при упаривании или фильтровании растворов и т. п. Немаловажное значение имеет оборудование, с помощью которого осуществляется химическая реакция. Негерметичность оборудования, в котором проходят химические процессы, всегда приводит к потерям газообразных веществ. И наконец, часть веществ может не вступить в реакцию или образовать при взаимодействии побочные продукты.
Для оценки полноты протекания процесса пользуются понятием выход продукта химической реакции. Оно подобно понятию «коэффициент полезного действия», которое применяется в физике для характеристики процессов преобразования и использования энергии, работы различных двигателей и механизмов.
Выход продукта химической реакции обозначается буквой греческого алфавита η (эта). Он представляет собой величину, равную отношению реально полученной, т. е. практической массы вещества 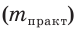
Выход продукта химической реакции — безразмерная величина, например: η(СаО) = 0,75, или 75 %.
Поскольку масса вещества пропорциональна его химическому количеству, то выход продукта реакции можно определять и как отношение соответствующих химических количеств вещества или объемов (для газов):
Например, если известно, что в реакции синтеза аммиака
выход продукта составляет 0,75 (η = 0,75, или 75 %), то это означает, что из азота массой 28 г (объемом 22,4 дм3 , химическим количеством 1 моль) мы получим аммиак массой не 34 г (объемом 44,8 дм3 , химическим количеством 2 моль), а массой 34 г
Таким образом, выход продукта реакции — это величина, равная отношению реально полученной массы (химического количества, объема) вещества к массе (химическому количеству, объему) этого вещества, рассчитанной по уравнению реакции.
Величина выхода продукта реакции не может превышать 100 %. Если выход равен 100 %, то говорят, что реакция протекает количественно. В этом случае
Кроме выражения «выход продукта химической реакции», часто используют и более краткие формы этого понятия: «выход продукта», «выход реакции», «реакция протекает с 90 %-м выходом».
На практике часто приходится рассчитывать химическое количество, массу или объем продукта реакции, если его выход отличается от 100 %, или, наоборот, определять выход продукта реакции. Рассмотрим типы расчетов с использованием этого понятия.
Тип 1. Даны массы (объемы, химические количества) исходного вещества и продукта реакции. Требуется определить выход продукта реакции.
Пример:
При прокаливании гидроксида алюминия 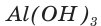
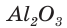
Дано:
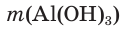
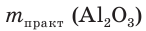
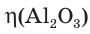
Решение
1. Определяем молярные массы гидроксида и оксида алюминия:
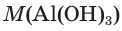
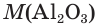
2. Находим химические количества гидроксида и оксида алюминия:
3. Записываем уравнение реакции разложения гидроксида алюминия и производим расчет теоретического химического количества (х) и теоретической массы полученного оксида алюминия:
откуда получим: х = 0,6 моль. Это —
Тогда теоретическая масса оксида алюминия составит:
4. Определяем выход продукта реакции (двумя способами):
а)
б)
Ответ: выход продукта реакции равен 85 %.
Тип 2. Даны масса (объем, химическое количество) исходного вещества и выход продукта реакции. Требуется определить массу (объем, химическое количество) продукта реакции.
Пример:
Рассчитайте массу нитрата аммония 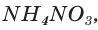
Дано:
V(NH3) = 4,48 м3 = 4480 дм3
η (NH4NO3) = 90 %

Решение 1.
Найдем химическое количество аммиака:
2. Составим уравнение реакции и рассчитаем теоретическое химическое количество
3. Находим теоретическую массу
4. Из формулы для определения выхода продукта реакции выражаем 
Ответ: практическая масса нитрата аммония равна 14,4 кг.
Тип 3. Даны масса (объем, химическое количество) продукта и выход продукта. Требуется определить массу (объем, химическое количество) исходного вещества.
Пример:
Определите объем (н. у.) водорода, который понадобится для получения аммиака объемом 13,44 м3 (н. у.), если его практический выход равен 20 %.
Дано:

Решение
1. Рассчитаем теоретический объем аммиака:
2. Составим уравнение реакции синтеза аммиака и рассчитаем объем (х) водорода:
Ответ: для синтеза аммиака потребуется водород объемом 100,8 м3
Выход продукта реакции — это величина, равная отношению реально полученной в результате реакции массы (химического количества, объема) вещества к массе (химическому количеству, объему) этого вещества, рассчитанной по уравнению реакции
- Классы неорганических соединений
- Вещества и их свойства в химии
- Чистые вещества и смеси в химии
- Состав и строение веществ в химии
- Сера в химии
- Азот в химии
- Фосфор в химии
- Углерод в химии
Кремний

4.6
Средняя оценка: 4.6
Всего получено оценок: 412.
4.6
Средняя оценка: 4.6
Всего получено оценок: 412.
В состав песка, глины, горных пород входит простой неметалл – кремний. Это хрупкое кристаллическое вещество, вступающее в реакции с металлами и неметаллами и использующееся для изготовления стекла.
Электронное строение
Кремний (Si) – занимает 14 клетку в таблице Менделеева. Элемент располагается в IV группе, третьем периоде. Следовательно, вокруг положительно ядра с 14 протонами движется 14 отрицательно заряженных электронов на трёх энергетических уровнях.
Кремний относится к элементам p-семейства. Электронная формула кремния – 1s22s22p63s23p2. На внешнем энергетическом уровне располагается четыре электрона – пара электронов на 3s-уровне и два неспаренных электрона на 3p-уровне.
Благодаря распределению электронов атом проявляет степень окисления +2. За счёт свободной 3d-орбитали атом может переходить в возбуждённое состояние. Один электрон с s-уровня переходит на d-орбиталь. В этом случае атом проявляет степень +4. Однако в реакциях с металлами кремний является окислителем и проявляет степень окисления -4.
Простое вещество в чистом виде встречается редко за счёт активности элемента. Распространён в составе песка – диоксида кремния (SiO2). В зависимости от примесей (железо, марганец, медь) образует кварц, агат, аметист, кремень и другие горные породы.
Физическое описание
Кремний – хрупкий химический элемент тёмного серого цвета. Обладает металлическим блеском за счёт кубической гранецентрированной кристаллической решётки, напоминающей строением алмаз. Однако кремний в отличие от алмаза – менее прочное вещество из-за большей длины связи между атомами.
Основные физические свойства кремния:
- полупроводник;
- температура плавления – 1414°C;
- температура кипения – 3265°C;
- плотность при нормальных условиях – 2,33 г/см3;
- твёрдость по шкале Мооса – 7.
Кремний выделяют из песка путём прокаливания с углём или металлами. Например, с алюминием: 3SiO2 + 4Al → 3Si + 2Al2O3.
Химические свойства
Благодаря схеме строения атома кремний обладает свойствами окислителя и восстановителя. Взаимодействуя с металлами, кремний принимает электроны, т.е. является окислителем. В реакциях с неметаллами кремний отдаёт электроны и является восстановителем.
Основные химические свойства описаны в таблице.
|
Реакция |
Описание |
Уравнение |
|
С неметаллами |
Реагирует при нагревании |
– Si + O2 → SiO2; – Si + C → SiC; – 3Si + 2N2 → Si3N4 |
|
С галогенами |
Только с фтором реагирует без нагревания. Остальные реакции протекают при повышенных температурах |
– Si + 2F2 → SiF4; – Si + 2Cl2 → SiCl4 |
|
С галогеноводородами |
При обычных условиях реагирует только с плавиковой кислотой. С остальными веществами – при нагревании |
Si + 4HF → SiF4 + 2H2 |
|
С металлами |
Образуется силициды |
2Ca + Si → Ca2Si |
|
С кислотами |
Реагирует только со смесью азотной и плавиковой кислот |
3Si + 4HNO3 + 18HF → 3H2[SiF6] + 4NO + 8H2O |
|
Со щелочами |
Образуются силикат и водород |
Si + 2NaOH + H2O → Na2SiO3 + H2 |
Применение
Кремний в составе песка используется для изготовления стекла и цемента. Изготовлением материалов из кремния занимается силикатная промышленность, выпускающая:
- кирпич;
- кафельную плитку;
- фаянс;
- фарфор;
- силикатный клей.
В чистом виде кремний используется для производства:
- электронных приборов;
- микросхем;
- солнечных батарей;
- зеркал.
Что мы узнали?
Из урока 9 класса узнали, что кремний – неметалл, проявляющий в реакции свойства окислителя или восстановителя. Это твёрдый хрупкий неметалл, сходный по кристаллическому строению с алмазом. В чистом виде практически не встречается в природе. Взаимодействуя с кислородом, образует песок, являющийся основой всех горных пород. При нормальных условиях реагирует только с фтором, остальные реакции требуют нагревания. Кремний реагирует с металлами, неметаллами, щелочами. Из элемента изготавливают строительные материалы, микросхемы, стекло.
Тест по теме
Доска почёта

Чтобы попасть сюда — пройдите тест.
-
Руслан Карабаза
9/10
-
Сергей Ефремов
7/10
Оценка доклада
4.6
Средняя оценка: 4.6
Всего получено оценок: 412.
А какая ваша оценка?